| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Beata Tryba | + 3089 word(s) | 3089 | 2020-12-25 09:56:54 | | | |
| 2 | Vicky Zhou | -982 word(s) | 2107 | 2021-04-22 04:49:23 | | | | |
| 3 | Vicky Zhou | -982 word(s) | 2107 | 2021-04-22 04:50:27 | | |
Video Upload Options
Purification of air from the VOCs (Volatile Organic Compounds) by the photocatalytic process has been confirmed to be very perspective. Although many various photocatalysts have been prepared and studied so far, TiO2 is still the most commonly used, because of its advantageous properties such as non-toxicity, relatively low cost and high stability. Surface modifications of TiO2 were extensively proceeded in order to increase photocatalytic activity of the photocatalyst under both UV and visible light activations. High yield of VOCs decomposition can be achieved on TiO2, depending on its structure and preparation method. The contact time of reactant with the active sites of TiO2 surface will determinate the efficiency of the photocatalytic process. Although VOCs decomposition can occur under weak UV light, more intensive UV irradiation will guarante complete mineralisation process.
1. Introduction
According to classification provided by companies to ECHA in REACH registrations acetaldehyde was identified as a substance, which is suspected of causing cancer. Formaldehyde was also classified as a cancerogenic substance and its concentration in wooden products is regulated under TSCA (Toxic Substances Control Act) program in the United States. The concentration of VOCs in the indoor air is not high, however can be dangerous for human’s health. Therefore, some of air cleaning processes can be utilized to solve this problem [1]. Recently, one of the mostly explored method of VOCs degradation is photocatalysis with application of TiO2 as the photocatalyst [2].[4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30]
The schematic diagram of processes occurring during photocatalytic oxidation of acetaldehyde on an illuminated TiO2 particle was proposed by Fujishima et al. (Figure 1) [3].
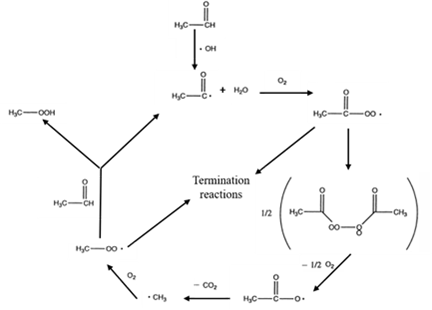
Figure 1. Schematic diagram of processes occurring during photocatalytic oxidation of acetaldehyde on an illuminated TiO2 particle [3].
In general, the first oxidation product of acetaldehyde conversion is acetic acid, which can be mineralised to CO2. The remaining methyl radical is transformed into formaldehyde, which can be further oxidised to formic acid and eventually to CO2. The scheme of the acetaldehyde transformation on the titania surface upon UV illumination has been already reported elsewhere [4]. Below in Figure 2 there is an outline of the path of acetaldehyde conversion by TiO2 under weak UV illumination:
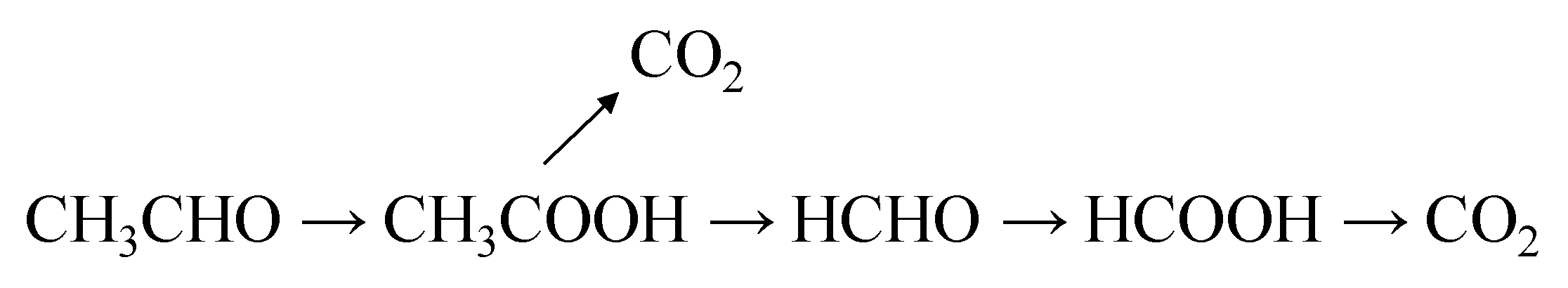
The photocatalytic conversion of acetaldehyde on TiO2-based materials depends on their structural parameters, phase composition and a way of chemical bounding to the surface [5][6][7][8].
2. Preparation of TiO2 and Impact of Its Structural Properties on Acetaldehyde Decomposition
For application of TiO2 in air purification, the nanosized material with high specific surface area will be preferable. For production of nanosized TiO2 following methods were developed: hydrothermal, sol-gel, chemical vapor deposition (CVD), solvothermal, flame spray pyrolysis, electrochemical, microemulsion, micelle and inverse micelle methods, sonochemical reactions and plasma evaporation. It was proved, that TiO2 prepared by a sol-gel method, which consisted of mixed phases anatase and brookite had enhanced photocatalytic activity towards acetaldehyde decomposition [9].
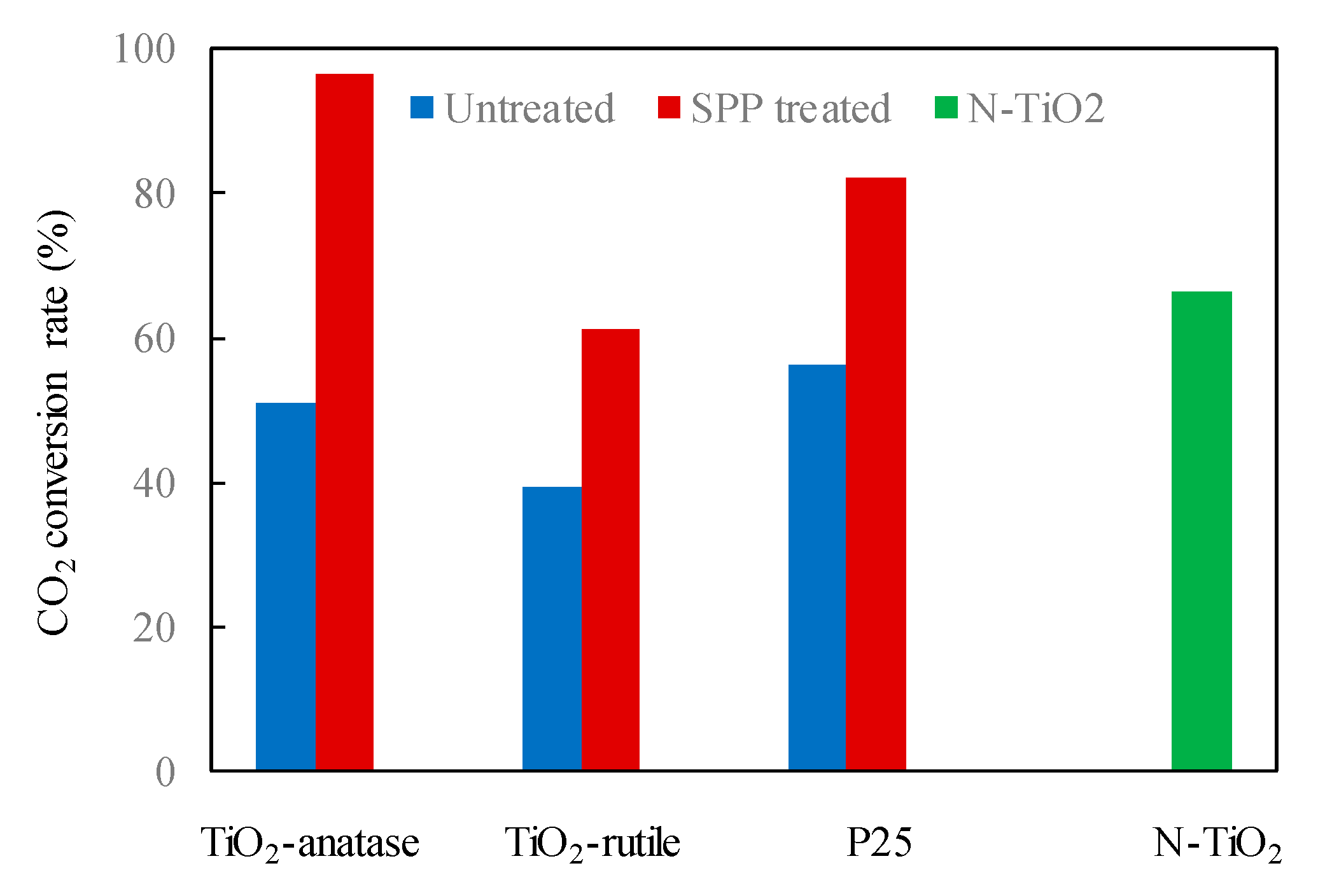
The solution plasma treatment of anatase can also lead to phase transformation of anatase to brookite [10]. Moreover, solution plasma treatment of TiO2 resulted in formation of some oxygen surface defects [10]. Both, oxygen surface defects and mixture of anatase and brookite improved charge separation in the titania sample [10]. Prepared in such way TiO2 showed high activity towards acetaldehyde decomposition under both, UV and visible light. Visible light activity of this TiO2 was even higher than that, prepared by nitrogen doping, as it was illustrating in Figure 3 [10].
Figure 3. Quantitative comparison of CO2 conversion rate (%) from the photocatalytic acetaldehyde degradation experiments using different photocatalyst samples [10].
It was widely reported in the literature, that for the efficient acetaldehyde decomposition, anatase-type TiO2 is more active, than rutile [11]. Anatase type TiO2 with mesoporous structure and large surface area (117 m2/g ) appeared to be the most active for acetaldehyde decomposition among the other prepared anatase samples [12]. The other titania structures can be also applied for air purification. The ordered titania nanotubes (TNT) revealed high activity towards acetaldehyde decomposition. This activity was enhanced with increasing their length. Found optimum length of TNT for acetaldehyde decomposition was around 17 µm [7]. These TNT were prepared by anodizing Ti foil at 20 V in formamide-based electrolyte for 6 h. Titania nanorods such as titania nanowires or nanofibres can be also applied for air purification, however their activity is more pronounced after modification with some species of metals such as Au or Fe [13][14]. Dependence of the exposed anatase crystal faces on the photocatalytic decomposition of acetaldehyde was also reported [8]. Titania structures with a large fraction of exposed anatase (001) facets exhibited higher activity towards acetaldehyde decomposition than these exposing predominantly (101) [8][15].
3. Adsorption of Acetaldehyde and Its Photocatalytic Transformation on TiO2
In the photocatalytic gas-phase reactions contact of the organic molecules with the photocatalyst surface is crucial. It was widely reported that acetaldehyde adsorbed on the anatase surface can undergo an aldol condensation to produce crotonaldehyde [4][5][16][17]. Performed in situ FTIR experiments of acetaldehyde adsorption showed, that acetaldehyde underwent both the aldol condensation and to a minor extent - oxidation [16]. The aldol condensation resulted in the formation of 3-hydroxybutanal and crotonaldehyde, while the oxidation processes led to formation of bidentate acetate, which was bounded to the titania surface [16]. It was also revealed, that upon illumination of TiO2 with UV light, the initially formed species were converted into several other intermediates, such as: acetic acid, formic acid, and formaldehyde [16]. Crotonaldehyde was photocatalytically converted to acetate and formate species. The formed formate species could be further oxidized to formic acid and eventually to CO2 [16].
Adsorption of acetaldehyde on the titania surface is essential, however, the way of acetaldehyde binding with the surface seems to be crucial for its conversion by mean of photocatalytic reactions. It was reported that acetaldehyde could be adsorbed either physically or chemically on the titania surface [4][5][17]. Under the humid conditions or on highly hydroxylated titania surface, acetaldehyde is mainly physisorbed with possible reversible desorption [4][5][17]. Contrary, the chemical binding of acetaldehyde on titania surface takes place on the low hydroxylated surface and then its further conversion occurs [5]. The stability of formed intermediates on the titania surface influences the efficiency of the acetaldehyde degradation.
Acetaldehyde is more favorable adsorbed on anatase, and undergoes an aldol condensation in the dark, whereas on rutile the oxidation of acetaldehyde to acetic and formate species takes place. Under illumination of a fluorescent light, both, acetic and formic acids are deposited on rutile and slow down the process of acetaldehyde decomposition. After photocatalytic process the amount of both, the acetic and formic acids adsorbed on titania surface was higher on rutile than anatase type samples, the most probably they were poorly mineralized on rutile. It can be concluded that rutile oxidizes acetaldehyde at the presence of oxygen easier than anatase. The products of acetaldehyde oxidation are deposited on TiO2 surface and slow down the process of its further decomposition. The scheme illustrated transformation of acetaldehyde on anatase and rutile surfaces under dark conditions and after illumination with weak UV light was shown in Figure 4. It was evidenced, that proceeding photocatalytic reactions on anatase could mineralize acetic acid more efficiently than these occurring on rutile.
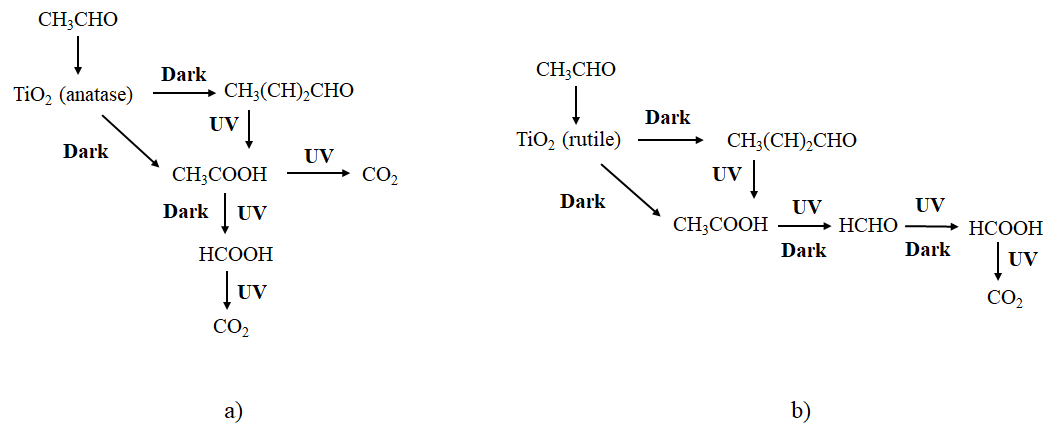
Figure 4. Scheme of the acetaldehyde conversion on titania surface during adsorption in the dark and photocatalytic decomposition under weak UV irradiation (a) anatase; (b) rutile.
4. The Role of Reactive Oxygen Radicals in the Photocatalytic Conversion of Acetaldehyde
Various potential pathways for the photocatalytic degradation by TiO2 have been proposed, including action of reactive species such as: hydroxyl radicals, carbonyl radicals, superoxide radicals, and hydrogen peroxide [3][10].
Transformation of acetaldehyde to crotonaldehyde and its partial oxidation to acetic acid on anatase surface was confirmed by many researchers [4][16]. Impact of oxygen on the oxidation and decomposition of acetaldehyde was also recognized and reported [3][4]. The mechanism of acetaldehyde decomposition via radical-initiated chain reactions consuming oxygen was also proposed [3]. It was evidenced [6], that acetaldehyde adsorbed on rutile type TiO2 significantly limited extent of hydroxyl radicals formation, but at the same time was recognized as neutral for formation of oxygen superoxide species. In addition, a great role of superoxide radicals in the photocatalytic decomposition of acetaldehyde on rutile was reported [6]. Photogenerated electrons take part in the following reaction:
|
e- + O2 → O2-• |
(1) |
whereas formed holes (h+) mediate in the reaction of carbonyl radicals formation:
|
3 CH3CHO + O2 + h+ → 2 CH3COOH + CH3CO• + H+ |
(2) |
The carbonyl radicals (CH3CO•) are capable to react with O2, which mediates the chain reactions of acetaldehyde oxidation. In these multiple reactions, acetic acid is the main intermediate in the transformation of acetaldehyde to CO2, according to the reaction [10]:
|
CH3COOH + •OH → CO2 + H2O + CH3• |
(3) |
Therefore, the improved separation of free radicals formed in TiO2 can conduct to enhanced photocatalytic conversion of acetaldehyde. Formation of both types of radicals, O2-• and •OH, is demanded for complete decomposition of acetaldehyde.
It is assumed, that poor photocatalytic activity of rutile in comparison with anatase, could be caused by high transformation of acetaldehyde to both acetic and formic acids, and by the high stability of the acetate species on rutile surface. Contrary to that, adsorption of acetaldehyde on anatase TiO2 was proceeded greatly through an aldol condensation to crotonaldehyde. Under UV irradiation crotonaldehyde was either oxidized to acetate and formate species or directly mineralized to CO2. Such pathway of acetaldehyde decomposition on anatase was more efficient in formation of CO2 than that on rutile.
5. The Impact of Light Wavelength Region on the Photocatalytic Decomposition of Acetaldehyde
The studies conducted by different teams indicated, that acetaldehyde was successfully undergoing photocatalytic decomposition on TiO2 surface under light emitted by the fluorescent lamps. However, efficiency of this process was lower than under UV-A light generated by a typical UV lamp [10]. To increase utilization of solar light, a lot of recent studies have been focused on the preparation and examination of visible-light active photocatalysts. One of the first works on the activity of nitrogen- doped TiO2 towards acetaldehyde decomposition under visible light was reported by Asahi et al. in Science journal [18]. They prepared TiO2-x Nx films by sputtering the TiO2 target in an N2 (40%)/Ar gas mixture followed by annealing at 550°C in N2 gas. The obtained nitrogen - doped TiO2 film showed absorption of visible light of wavelength below 500 nm and the photocatalytic degradation of acetaldehyde for l = 435 nm. The activities of both TiO2 films, doped with nitrogen and undoped, under UV light were comparable and higher than under visible light irradiation. irradiation. The results were illustrated in Figure 5 [18].
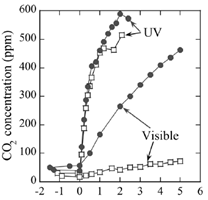 Figure 5. CO2 evolution as a function of irradiation time (light on at zero) during the photodegradation of acetaldehyde gas (with an initial concentration of 485 ppm) under UV and visible light irradiation; TiO2-xNx - solid circles, TiO2 - open squares.
Figure 5. CO2 evolution as a function of irradiation time (light on at zero) during the photodegradation of acetaldehyde gas (with an initial concentration of 485 ppm) under UV and visible light irradiation; TiO2-xNx - solid circles, TiO2 - open squares.
After publishing Asahi’s findings [18], numerous research works were targeted to obtain the visible light active TiO2 through doping of different anionic species, such as N, C, S, F as single doping or codoped [19][20][21][22], also with some metallic species such as Au, Ag, Cu, Co, Pt, Mn, Fe, and others [22][23][24][25][26][27]. The composites of TiO2 with other metal oxides such as WO3 or CeO2 were also prepared and showed photocatalytic activity under visible light irradiation towards acetaldehyde decomposition [28][29]. An activity of doped TiO2 under visible light was mainly obtained due to either the narrowing of the band gap in TiO2 [18][19][20][21] or by the introduction of an impurity level of dopant inside the titania structure and formation of intra band gap energy levels [26]. Formation of surface defects in TiO2 was also reported [10] as resulting in increasing the activity of titania under visible light.
Different oxidation states of metals are responsible for charge trapping and improved charge separation [22][23][24]. Thermo-photocatalytic decomposition was observed for titania samples loaded with platinum nanoparticles [27][30]. The extremely high rate of formic acid oxidation over Pt-TiO2-xNx was found to be due to a combined effect of both photocatalysis and thermal catalysis at room temperature facilitated by nanosized(1-2 nm) Pt [27]. The other researchers noted increased decomposition rate of acetaldehyde by decreasing distance between photocatalyst (Pt-TiO2/SiO2) and the source of light used in the process [30]. They observed, that in the presence of platinum by-products and coke precursors were easily burned. For that reason, the combination of the catalytic properties of some metals with the photocatalytic activity of TiO2 seems to be very interesting and promising solution for the complete decomposition of VOCs compounds.
Doping some of noble metals like Ag, Au, Pt or Pd to TiO2 results in obtaining material revealing enhanced photocatalytic activity under visible light. The observed enhancement is due to efficient transport of electrons generated over the doped nanoparticles by local surface plasmon resonance (LSPR), across the metal/semiconductor interface via the defect states of TiO2 [25].
References
- Xiao, R.; Mo, J.; Zhang, Y.; Gao, D. An in-situ thermally regenerated air purifier for indoor formaldehyde removal. Indoor Air 2018, 28, 266-275, doi:10.1111/ina.12441.
- Haghighatmamaghani, A.; Haghighat, F.; Lee, C.-S. Performance of various commercial TiO2 in photocatalytic degradation of a mixture of indoor air pollutants: Effect of photocatalyst and operating parameters. Sci. Technol. Built Environ. 2019, 25, 600-614, doi:10.1080/23744731.2018.1556051.
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1-21, doi:10.1016/S1389-5567(00)00002-2.
- Melchers, S.; Schneider, J.; Emeline, A.; Bahnemann, D. Effect of H2O and O2 on the Adsorption and Degradation of Acetaldehyde on Anatase Surfaces-An In Situ ATR-FTIR Study. Catalysts 2018, 8, 417, doi:10.3390/catal8100417.
- Tryba, B.; Rychtowski, P.; Srenscek-Nazzal, J.; Przepiorski, J. The inflence of TiO2 structure on the complete decomposition of acetaldehyde gas. Mater. Res. Bull. 2020, 126, 110816, doi:10.1016/j.materresbull.2020.110816.
- Zeng, Q.; Wang, X.; Xie, X.; Mahmood, A.; Lu, G.; Wang, Y.; Sun, J. Band bending of TiO2 induced by O-xylene and acetaldehyde adsorption and its effect on the generation of active radicals. J. Colloid Interface Sci. 2020, 572, 374-383, doi:10.1016/j.jcis.2020.03.114.
- Liu, Z.; Zhang, X.; Nishimoto, S.; Murakami, T.; Fujishima, A. Efficient Photocatalytic Degradation of Gaseous Acetaldehyde by Highly Ordered TiO2 Nanotube Arrays. Environ. Sci. Technol. 2008, 42, 8547-8551, doi:10.1021/es8016842.
- Stefanov, B.I.; Niklasson, G.A.; Granqvist, C.G.; Österlund, L. Gas-phase photocatalytic activity of sputter-deposited anatase TiO2 films: Effect of 〈0 0 1〉preferential orientation, surface temperature and humidity. J. Catal. 2016, 335, 187-196, doi:10.1016/j.jcat.2015.12.002.
- Tryba, B.; Jafari, S.; Sillanpää, M.; Nitta, A.; Ohtani, B.; Morawski, A.W. Influence of TiO2 structure on its photocatalytic activity towards acetaldehyde decomposition. Appl. Surf. Sci. 2019, 470, 376-385, doi:10.1016/j.apsusc.2018.11.137.
- Pitchaimuthu, S.; Honda, K.; Suzuki, S.; Naito, A.; Suzuki, N.; Katsumata, K.; Nakata, K.; Ishida, N.; Kitamura, N.; Idemoto, Y.; et al. Solution Plasma Process-Derived Defect-Induced Heterophase Anatase/Brookite TiO2 Nanocrystals for Enhanced Gaseous Photocatalytic Performance. ACS Omega 2018, 3, 898-905, doi:10.1021/acsomega.7b01698.
- Sopyan, I. Kinetic analysis on photocatalytic degradation of gaseous acetaldehyde, ammonia and hydrogen sulfide on nanosized porous TiO2 films. Sci. Technol. Adv. Mater. 2007, 8, 33-39, doi:10.1016/j.stam.2006.10.004.
- Fan, X.; Yu, T.; Zhang, L.; Chen, X.; Zou, Z. Photocatalytic Degradation of Acetaldehyde on Mesoporous TiO2 : Effects of Surface Area and Crystallinity on the Photocatalytic Activity. Chin. J. Chem. Phys. 2007, 20, 733-738, doi:10.1088/1674-0068/20/06/733-738.
- Pan, C.; Dong, L. Fabrication of Gold-Doped Titanium Dioxide (TiO2:Au) Nanofibers Photocatalyst by Vacuum Ion Sputter Coating. J. Macromol. Sci. Part B 2009, 48, 919-926, doi:10.1080/00222340903028662.
- Ohno, T.; Higo, T.; Saito, H.; Yuajn, S.; Jin, Z.; Yang, Y.; Tsubota, T. Dependence of photocatalytic activity on aspect ratio of a brookite TiO2 nanorod and drastic improvement in visible light responsibility of a brookite TiO2 nanorod by site-selective modification of Fe3+ on exposed faces. J. Mol. Catal. Chem. 2015, 396, 261-267, doi:10.1016/j.molcata.2014.09.036.
- Mahmood, A.; Shi, G.; Xie, X.; Sun, J. Assessing the adsorption and photocatalytic activity of TiO2 nanoparticles for the gas phase acetaldehyde: A computational and experimental study. J. Alloys Compd. 2020, 819, 153055, doi:10.1016/j.jallcom.2019.153055.
- Hauchecorne, B.; Terrens, D.; Verbruggen, S.; Martens, J.A.; Van Langenhove, H.; Demeestere, K.; Lenaerts, S. Elucidating the photocatalytic degradation pathway of acetaldehyde: An FTIR in situ study under atmospheric conditions. Appl. Catal. B Environ. 2011, 106, 630-638, doi:10.1016/j.apcatb.2011.06.026.
- Batault, F.; Thevenet, F.; Hequet, V.; Rillard, C.; Le Coq, L.; Locoge, N. Acetaldehyde and acetic acid adsorption on TiO2 under dry and humid conditions. Chem. Eng. J. 2015, 264, 197-210, doi:10.1016/j.cej.2014.10.089.
- Asahi, R. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269-271, doi:10.1126/science.1061051.
- Meroni, D.; Ardizzone, S.; Cappelletti, G.; Oliva, C.; Ceotto, M.; Poelman, D.; Poelman, H. Photocatalytic removal of ethanol and acetaldehyde by N-promoted TiO2 films: The role of the different nitrogen sources. Catal. Today 2011, 161, 169-174, doi:10.1016/j.cattod.2010.08.013.
- Nishijima, K.; Naitoh, H.; Tsubota, T.; Ohno, T. Visible-Light-Induced Hydrophilic Conversion of an S-Doped TiO2 Thin Film and Its Photocatalytic Activity for Decomposition of Acetaldehyde in Gas Phase. J. Ceram. Soc. Jpn. 2007, 115, 310-314, doi:10.2109/jcersj.115.310.
- Khalilzadeh, A.; Fatemi, S. Modification of nano-TiO2 by doping with nitrogen and fluorine and study acetaldehyde removal under visible light irradiation. Clean Technol. Environ. Policy 2014, 16, 629-636, doi:10.1007/s10098-013-0666-7.
- Hamal, D.B.; Klabunde, K.J. Synthesis, characterization, and visible light activity of new nanoparticle photocatalysts based on silver, carbon, and sulfur-doped TiO2. J. Colloid Interface Sci. 2007, 311, 514-522, doi:10.1016/j.jcis.2007.03.001.
- Morikawa, T.; Irokawa, Y.; Ohwaki, T. Enhanced photocatalytic activity of TiO2-xNx loaded with copper ions under visible light irradiation. Appl. Catal. Gen. 2006, 314, 123–127, doi:10.1016/j.apcata.2006.08.011.
- Kim, W.; Tachikawa, T.; Kim, H.; Lakshminarasimhan, N.; Murugan, P.; Park, H.; Majima, T.; Choi, W. Visible light photocatalytic activities of nitrogen and platinum-doped TiO2: Synergistic effects of co-dopants. Appl. Catal. B Environ. 2014, 147, 642-650, doi:10.1016/j.apcatb.2013.09.034.
- Song, H.; Yu, Y.-T.; Norby, P. Efficient Complete Oxidation of Acetaldehyde into CO2 Over Au/TiO2 Core-Shell Nano Catalyst Under UV and Visible Light Irradiation. J. Nanosci. Nanotechnol. 2009, 9, 5891-5897, doi:10.1166/jnn.2009.1263.
- Saqlain, S.; Cha, B.J.; Kim, S.Y.; Ahn, T.K.; Park, C.; Oh, J.-M.; Jeong, E.C.; Seo, H.O.; Kim, Y.D. Visible light-responsive Fe-loaded TiO2 photocatalysts for total oxidation of acetaldehyde: Fundamental studies towards large-scale production and applications. Appl. Surf. Sci. 2020, 505, 144160, doi:10.1016/j.apsusc.2019.144160.
- Morikawa, T.; Ohwaki, T.; Suzuki, K.; Moribe, S.; Tero-Kubota, S. Visible-light-induced photocatalytic oxidation of carboxylic acids and aldehydes over N-doped TiO2 loaded with Fe, Cu or Pt. Appl. Catal. B Environ. 2008, 83, 56-62, doi:10.1016/j.apcatb.2008.01.034.
- Muñoz-Batista, M.J.; de los Milagros Ballari, M.; Kubacka, A.; Cassano, A.E.; Alfano, O.M.; Fernández-García, M. Acetaldehyde degradation under UV and visible irradiation using CeO2-TiO2 composite systems: Evaluation of the photocatalytic efficiencies. Chem. Eng. J. 2014, 255, 297–306, doi:10.1016/j.cej.2014.06.056.
- Yamaguchi, Y.; Liu, B.; Terashima, C.; Katsumata, K.; Suzuki, N.; Fujishima, A.; Sakai, H.; Nakata, K. Fabrication of Efficient Visible-light-responsive TiO2-WO3 Hollow Particle Photocatalyst by Electrospray Method. Chem. Lett. 2017, 46, 122-124, doi:10.1246/cl.160851.
- Nakano, K.; Obuchi, E.; Nanri, M. Thermo-Photocatalytic Decomposition of Acetaldehyde Over Pt-TiO2/SiO2. Chem. Eng. Res. Des. 2004, 82, 297-301, doi:10.1205/026387604772992909.