
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jacek Szymański | + 1260 word(s) | 1260 | 2021-04-12 05:50:51 | | | |
| 2 | Dean Liu | Meta information modification | 1260 | 2021-04-22 03:47:08 | | |
Video Upload Options
Fowler’s syndrome is characterized by a large bladder capacity, reduced sensation, increased maximal urethral closure pressure, and detrusor underactivity.
1. Introduction
Urinary retention in young women is a relatively rare clinical problem. Epidemiological studies estimate that its incidence in young women ranges from 3 cases per 100,000 per year to 0.3% after the exclusion of other causes, such as postoperative, postpartum, gynecological, urological, rectal, and psychiatric causes [1][2][3]. The incidence of Fowler’s syndrome is rated at 0.2 cases per 100,000 per year [4].
Urinary retention could be related to bladder outlet obstruction (BOO) or detrusor underactivity (DUA), or it may constitute a combination of these two causes. The underlying pathology could be associated with anatomical, neurogenic, and myogenic factors, or it may result from pharmacotherapy or functional reasons where no organic cause is identified. [5]. The anatomical (mechanical) factors include, among others, pelvic organ tumors, stenosis of the bladder neck, urethral diverticulum, pelvic organ prolapse, and previous pelvic surgery. The functional causes are a result of the pathological changes in the contraction of the periurethral muscles (dysfunctional voiding and detrusor sphincter dyssynergia) or impaired urethral relaxation (Fowler’s syndrome) [6]. Urinary retention caused by mechanical BOO is usually successfully treated with medication or surgery, whereas the management of urinary retention resulting from detrusor underactivity or functional BOO remains a challenge. However, urinary retention caused by DUA as a consequence of pharmacotherapy is usually transient and reversible [5].
2. Pathophysiology of Fowler’s Syndrome
The etiology of Fowler’s syndrome remains unclear. One of the contributing factors is possibly the specific hyperactivity of the external urethral sphincter, which can be diagnosed using a concentric needle (electromyography) EMG electrode. Detailed EMG analysis shows that there are two components to sphincter activity: complex repetitive discharges (CRDs) and decelerating bursts [7]. CRDs appear as the direct spread of electrical activity from one muscle to another. They do not originate from neuromuscular transmission but are transmitted via adjacent membranes in parallel waves [7]. In this way, the CRDs create autonomous circuitous excitatory activity, leading to impaired relaxation of the urethral sphincter during voiding [5]. On the other hand, abnormal EMG activity has been found in 30–53% of healthy, asymptomatic women [8][9]. It is difficult to quantify the “amount” of abnormal EMG activity due to the small diameter of the sphincter muscle fibers and the limitations of the measuring techniques. Based on the observation that women with urinary retention often demonstrate polycystic ovaries, Fowler linked the increased sphincter activity to a hormonal disorder. This hypothesis explains the impaired transmission of the electrical impulses throughout the muscle by the improper muscle membrane stability as a consequence of ion channel dysfunction (hormonal channelopathy). In another study, over 80% of patients with Fowler’s syndrome had one or more gynecological disorders. The most prevalent were endometriosis, polycystic ovarian syndrome (PCOS), and subfertility. Although the incidence of these pathologies reached statistical significance, their occurrence in the FS group and the controls stayed within the population range, making the theory of the increased concomitance of FS with other gynecological pathologies tenuous [10][11]. Another theory assumes that the primary failure of relaxation of the striated muscle of the urethral sphincter leads to a raised urethral pressure profile. An ultrasound study carried out by Noble et al. [12] in women with obstructed voiding and abnormal sphincter EMG activity found evidence of local muscle hypertrophy. These findings were not confirmed by endoscopic ultrasound biopsy, suggesting that increased sphincter volume could be caused by components other than the skeletal muscle [13].
Large bladder capacity, reduced sensation, increased maximal urethral closure pressure (MUCP), and detrusor underactivity constitute the urodynamic characteristics of Fowler’s syndrome [14]. Increased urethral afferent activity most likely inhibits the bladder afferent signals from reaching the brain by potentiating a spinal mechanism of urinary continence. During the filling phase, strong urethral afferent signals are generated. They reduce the signal transmission to the periaqueductal grey (PAG) and higher centers by inhibiting bladder afferent activity in the sacral cord. Thus, the increased activity of the striated urethral sphincter leads to halted voiding [15] (Figure 2).
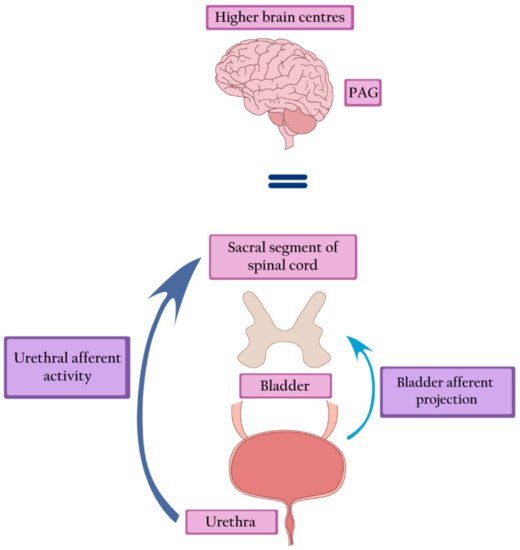
Figure 2. Fowler’s syndrome—strong afferent signals generated by the urethra inhibit the afferent activity of the bladder, leading to inactivation of the PAG and higher brain centers. PAG—periaqueductal grey.
3. Treatment
Sacral neuromodulation is defined as the permanent electrical stimulation of the sacral segment of the spinal cord that controls the functioning of the bladder and pelvic floor. It restores the balance in the transmission of signals between the pelvic organs, the spinal cord, and the higher centers of the central nervous system [16]. Sacral neuromodulation is the only intervention that can restore a typical voiding pattern in women with Fowler’s syndrome. Brain imaging studies show the restoration of the midbrain and pontine activity, as well as the normalization of the signal transfer between the brainstem and the cortex. Moreover, SNM attenuates anterior cingulate activity, thus restoring the desire and ability to void [17]. Presumably, by stimulating neurons in the spinal cords, SNM blocks the inhibition of spinal information transfer from the bladder [15] (Figure 3).
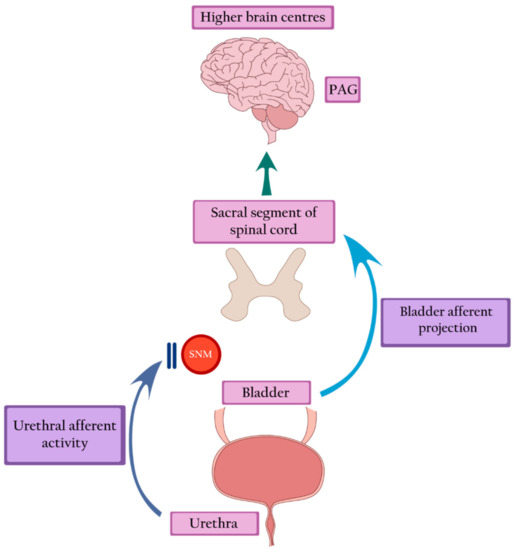
Figure 3. Sacral neuromodulation blocks urethral inhibition and restores bladder afferent projection, leading to the return of bladder sensation and activation of the PAG. PAG—periaqueductal grey, SNM—sacral neuromodulation.
However, the urodynamic evaluation of SNM therapy raises questions concerning a direct afferent effect. Although SNM restores normal voiding, it does not have a direct relaxant effect on the EUS. No changes have been demonstrated in EMG and MUCP before and after implantation (MUCP 92.9 cm H2O pre-SNM vs. 84.9 after stimulation, p = 0.06). As the abnormality persists, many of the patients void in an obstructed manner. Uroflowmetry shows an interrupted stream. Surprisingly, cystometry fails to reveal significantly elevated detrusor pressure, as could be expected due to a chronically unrelaxed sphincter. A modest increase in detrusor pressure is most likely sufficient in overcoming urethral resistance. The impact of SNM on detrusor contraction is consistent with its inhibiting effect on urethral afferent activation [14]. This hypothesis was confirmed by an animal study which showed that sacral neuromodulation could partially suppress or completely block the increase in bladder capacity induced by pudendal nerve stimulation [18]. In this context, voiding restoration seems to be achieved through a combination of spinal and supraspinal mechanisms. The clinical efficacy of SNM in the treatment of urinary retention was confirmed in several trials. Swinn et al. (2000) showed a 68% success rate, yet a relatively high reoperation rate of 21% [19]. Another study demonstrated the efficacy of SNM in 77% of patients at a 5-year follow-up. Shortly after implantation, 96% of subjects (25 patients) voided. However, two had their stimulator deactivated within five years due to pregnancy, and a further three reported loss of efficacy. The revision rate was 54%, and the most common complications included loss of effectiveness, implant-related discomfort, and leg pain [14]. The next study reported twelve years of experience in SNM therapy offered to the women with nonobstructive urinary retention, based on 27 individuals with a median follow-up of 5.7 years. A success rate of 70.83% was reflected in the return of spontaneous voiding and reduction of mean postvoid residual (PVR) urine from 402.2 to 28.1 mL (p < 0.0001) [20]. Some hopes in the treatment of Fowler’s syndrome have been linked to the injection of botulinum toxin A (BoNT-A) into the external urethral sphincter as a less invasive procedure than SNM. The first transperitoneal 200-U BoNT-A injections performed by Fowler in 1992 were unsuccessful [21]. In the following years, transurethral or periurethral injections of 50–100 U BoNT-A were attempted several times, resulting in an improvement of 37%–43% of female patients. The limitations of the trials were the small size of the study groups, the lack of control groups, various injection techniques, and different doses of BoNT-A [22].
References
- Doran, J.M.; Roberts, M. Acute Urinary Retention in the Female. Br. J. Urol. 1975, 47, 793–796.
- Klarskov, P.; Andersen, J.T.; Asmussen, C.F.; Brenøe, J.; Jensen, S.K.; Jensen, I.L.; Lund, P.; Schultz, A.; Vedel, T. Acute Urinary Retention in Women: A Prospective Study of 18 Consecutive Cases. Scand. J. Urol. Nephrol. 1987, 21, 29–31.
- Özveren, B.; Keskin, S. Presentation and prognosis of female acute urinary retention: Analysis of an unusual clinical condition in outpatients. Urol. Ann. 2016, 8, 444–448.
- Podnar, S.; Barbić, M. Non-neurogenic urinary retention (Fowler’s syndrome) in Two Sisters. Neurourol. Urodyn. 2006, 25, 739–741.
- Osman, N.I.; Chapple, C.R. Fowler’s syndrome—A cause of unexplained urinary retention in young women? Nat. Rev. Urol. 2014, 11, 87–98.
- Kessler, T.M.; Fowler, C.J. Sacral neuromodulation for urinary retention. Nat. Clin. Pract. Urol. 2008, 5, 657–666.
- Fowler, C.J.; Kirby, R.S.; Harrison, M.J. Decelerating burst and complex repetitive discharges in the striated muscle of the urethral sphincter, associated with urinary retention in women. J. Neurol. Neurosurg. Psychiatry 1985, 48, 1004–1009.
- Tawadros, C.; Burnett, K.; Derbyshire, L.F.; Tawadros, T.; Clarke, N.W.; Betts, C.D. External urethral sphincter electromyography in asymptomatic women and the influence of the menstrual cycle. BJU Int. 2015, 116, 423–431.
- Ramm, O.; Mueller, E.R.; Brubaker, L.; Lowenstein, L.; Kenton, K. Complex Repetitive Discharges—A Feature of the Urethral Continence Mechanism or a Pathological Finding? J. Urol. 2012, 187, 2140–2143.
- Goodwin, R.J.; Swinn, M.J.; Fowler, C.J. The neurophysiology of urinary retention in young women and its treatment by neuromodulation. World J. Urol. 1998, 15, 305–307.
- Karmarkar, R.; Abtahi, B.; Saber-Khalaf, M.; Gonzales, G.; Elneil, S. Gynaecological pathology in women with Fowler’s syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 194, 54–57.
- Noble, J.; Dixon, P.; Rickards, D.; Fowler, C. Urethral sphincter volumes in women with obstructed voiding and abnormal sphincter electromyographic activity. Br. J. Urol. 1995, 76, 741–746.
- Andrich, D.; Rickards, D.; Landon, D.; Fowler, C.; Mundy, A. Structural Assessment of the Urethral Sphincter in Women with Urinary Retention. J. Urol. 2005, 173, 1246–1251.
- DasGupta, R.; Fowler, C.J. Urodynamic Study of Women in Urinary Retention Treated with Sacral Neuromodulation. J. Urol. 2004, 171, 1161–1164.
- Kavia, R.; Dasgupta, R.; Critchley, H.; Fowler, C.; Griffiths, D. A functional magnetic resonance imaging study of the effect of sacral neuromodulation on brain responses in women with Fowler’s syndrome. BJU Int. 2010, 105, 366–372.
- Szymański, J.K.; Słabuszewska-Jóźwiak, A.; Zaręba, K.; Jakiel, G. Neuromodulation—A therapeutic option for refractory overactive bladder. A recent literature review. Videosurg. Miniinvasive Tech. 2019.
- Dasgupta, R.; Critchley, H.D.; Dolan, R.J.; Fowler, C.J. Changes in Brain Activity following Sacral Neuromodulation for Urinary Retention. J. Urol. 2005, 174, 2268–2272.
- Li, X.; Uy, J.; Yu, M.; Li, S.; Theisen, K.; Browning, J.; Shen, B.; Wang, J.; Roppolo, J.R.; De Groat, W.C.; et al. Sacral neuromodulation blocks pudendal inhibition of reflex bladder activity in cats: Insight into the efficacy of sacral neuromodulation in Fowler’s syndrome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R34–R42.
- Swinn, M.J.; Kitchen, N.D.; Goodwin, R.J.; Fowler, C.J. Sacral Neuromodulation for Women with Fowler’s Syndrome. Eur. Urol. 2000, 38, 439–443.
- Mehmood, S.; Altaweel, W. Long-term outcome of sacral neuromodulation in patients with idiopathic nonobstructive urinary retention: Single-center experience. Urol. Ann. 2017, 9, 244–248.
- Fowler, C.J.; Betts, C.D.; Christmas, T.J.; Swash, M. Botulinum toxin in the treatment of chronic urinary retention in women. Br. J. Urol. 1992, 70, 387–389.
- Kao, Y.-L.; Huang, K.-H.; Kuo, H.-C.; Ou, Y.-C. The therapeutic effects and pathophysiology of Botulinum Toxin A on voiding dysfunction due to urethral sphincter dysfunction. Toxins 2019, 11, 728.





