
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Oscar Vicente | + 1431 word(s) | 1431 | 2021-04-21 11:23:15 | | | |
| 2 | Catherine Yang | Meta information modification | 1431 | 2021-04-22 03:24:00 | | |
Video Upload Options
Limonium is one of the most interesting and biodiverse genera of halophytes, with many species adapted to saline environments. Limonium species have a promising potential as cultivated minor crops as many have ornamental value, or are already used as medicinal plants.
1. Introduction
The genus Limonium is the most biodiverse within the Plumbaginaceae family, distributed throughout the world. The number of its species was estimated to be around 400 [1], but including the numerous microspecies recently described, endemic to small territories, the total number can reach ca. 600 species [2]. In the Mediterranean region, Limonium is extremely rich in endemic taxa, with 70% of the total number of species endemic [2]; it is the richest genus in endemic species in the vascular floras of Italy, Spain, and Greece [3]. The genus includes many threatened taxa; 159 species are catalogued worldwide in red lists, red books, or lists of protected species at the national and regional levels [4]. However, the highest concentration of endemic and threatened species is found mainly in the Mediterranean coastal regions [5]. Many of these species grow in plant communities that in Europe fall within Directive 92/43 EEC as priority habitats Chritmo-Limonietalia ’1240 Vegetated sea cliffs of the Mediterranean coasts with endemic Limonium’ and to Limonietalia ‘1510 Mediterranean salt steppes’ [6]. The former habitats are specific saline environments, where only highly specialised halophytes can thrive, whereas the latter are found along the Mediterranean coastline and on the edges of the inland marshlands [7]. The exceptional biodiversity within this genus is related to the combination of sexual reproduction with apomixis, and a high frequency of polyploidisation, hybridisation and introgression [8][9]. Due to its complex diversity, Limonium has been the subject of many taxonomical studies, starting with the first infrageneric classification by Boissier [10][11] to molecular phylogenetic analyses during the last two decades [2][9][12][13][14].
Limonium species are mostly C3 perennial herbs or shrubs, with rosulate leaves and ascendant floral scapes, simple or ramified with abundant spikelets of small flowers grouped in panicles, which make them attractive as ornamentals. Limonium species are found on sandy beaches, cliffs, and salt marshes in coastal areas, but also in continental areas in lagoons, meadows, steppes, and deserts [2].
Limonium belongs to a specific category of halophytes, the so-called ’recretohalophytes’, which includes around 370 species [15], able to secrete salt from their leaves through salt bladders and salt glands. Salt bladders consist of single epidermal cells or modified trichomes that accumulate salt on the leaf surface; salt glands, on the other hand, are stable structures formed by two or more cells, often sunken into the epidermis, that continuously secrete toxic ions to the outside of the plant [15][16][17]. The salt glands are specific to several genera (including Limonium) of a few families, such as Plumbaginaceae, Acanthaceae, Tamaricaceae, Frankeniaceae, Amaranthaceae or Gramineae [18]. Salt glands play an essential role in maintaining the ion balance, contributing to the stability of osmotic pressure and enhancing salinity tolerance [19][20]. Salt glands also act regulating the internal ionic composition of the leaves, which, together with efficient osmotic adjustments, help avoid dehydration of leaf cells [16]. One of the main mechanisms ensuring osmotic balance under stress is the synthesis and accumulation in the cytoplasm of compatible solutes, the so-called osmolytes. These are diverse organic compounds that, apart from their fundamental function in osmotic adjustment, play additional roles in stress tolerance mechanisms; for example, increasing the thermodynamic stability of folded proteins and directly protecting macromolecular structures—in their role as low-molecular-weight chaperons—and also as scavengers of ‘reactive oxygen species’ (ROS), or as signalling molecules [21][22][23][24][25]. However, osmolyte biosynthesis represents a high cost for the plants since the same cellular osmolarity can be reached by ion uptake and transport with much lower energy consumption [26][27]. In dicotyledonous halophytes, osmotic adjustment can be provided at lower costs by ion uptake and accumulation, especially Na+ and Cl–, which are sequestered in the vacuoles to avoid toxicity effects in the cytosol [28][29][30].
2. Morpho-Anatomical Adaptations in Limonium Species
The morphology and anatomy of vegetative organs in Limonium species are generally well known and have been recently reviewed [20][31]. Studies on Limonium’s biology have been mostly linked to the presence of salt glands (‘Mettenius’ or ‘Licopoli’ glands/organs—see [32]), located on the stem and especially on the leaf surface. In fact, there are also some anatomical features found in Limonium that attest the xeromorphic nature of these halophytic species. Overall, taking into account the saline and arid environments where Limonium species grow, they could be considered a special case of xerophytes. This genus could serve as a model for this kind of morphological and anatomical studies [33].
Description of the morphology of the underground organs of Limonium in the botanical literature is sometimes confusing and contradictory. Although Limonium species are clearly recognised and described as perennial species (thus, the plants must possess rhizomes), there is no mention of this modified underground stem in the Romanian flora. Instead, the species are characterised as having ’tap, thick roots’ (L. gmelinii), ’thick, with nodes’ (L. vulgare Mill.), ‘cylindrical root, unbranched or rarely branched in its upper part’ (L. latifolium), or ’tap root more or less thick’ (L. caspia Willd.) [34]. The same is true for Flora of the USSR, where there is no data about the rhizome, and the underground system is referred to as ‘taproot’, with a single exception in the case of L. otolepis (Schrenk) Kuntze, which has a ‘taproot (or rootstock) fairly stout’ [35]. The species L. brasiliense (Boiss.) Kuntze represents another example of this error in terming the rhizome as a root (see discussions in [36]).
Nevertheless, the anatomical investigation of underground organs clarifies this confusion, as the microscopic structure reveals the typical configuration of a stem (rhizome) or a root [37]. In any case, even with available anatomical data on the structure of underground organs in different Limonium species, comparisons and extrapolations must be carried out with caution. Sometimes, the anatomical description of the rhizome and the root can be accurate for a particular species; however, this does not necessarily provide information on the organs’ morphology, unless it is clearly specified at which level the cross-sections were performed within an organ. Thus, an organ can be morphologically and anatomically identified as a root or a rhizome for a Limonium species but, in the absence of anatomical data, these results may not be morphologically applicable to other Limonium species. Some research papers contain anatomical information on the main root, lateral roots and rhizome, for example in Limonium gmelinii [38]; in this case, the rhizome is defined as ‘the underground part located between the collet and the rosette of leaves that forms in its terminal side, on soil surface’. For this reason, in the absence of precise anatomical data regarding underground organs, in ecophysiological studies on Limonium, it is highly recommended to use the term ’underground system’ or ‘rhizomatous root system’ [39].
A common anatomical feature for the underground organs (rhizome, root) of Limonium species is the extensive development of sclerenchyma tissue [37][38][40][41]. This feature has been evidenced in several Mediterranean Limonium species [33]. In the rhizome of Limonium furfuraceum Kuntze, there is a sinuous ring of sclerenchymatous fibres that surrounds the central cylinder, thus acting as a pericycle (Figure 1a); a similar sclerenchymatous ring occurs in the case of the aerial (flowering) stem (data not shown).
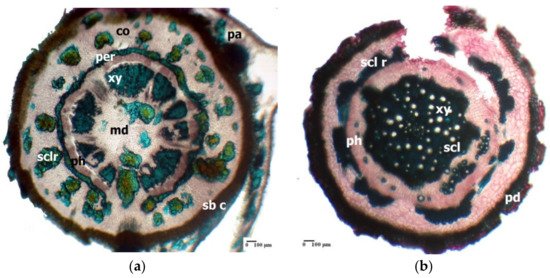
Figure 1. Cross-section through the rhizome of Limonium furfuraceum (a) and through the root of Limonium girardianum (b); co—cortex; md—medulla; pa—parenchyma; per—pericycle; ph—phloem; xy—xylem vessel; sb c—suberified cells; pd—periderm; scl r—sclerenchyma ring; scl—sclerenchyma; sclr—sclereid.
In the central cylinder of the L. girardianum (Guss.) Fourr. root, at its periphery, there is a discontinuous mechanical ring, consisting of several convex arches of sclerenchymatous fibres (Figure 1b).
The central cylinder in the rhizome of L. girardianum (Figure 2a) presents at its periphery a thin ring of sclerenchyma, consisting of cords of periphloemic fibres of vascular bundles; the secondary xylem forms a very thick ring, with a large amount of libriform, and many vessels scattered in the interior. In L. girardianum, the central cylinder of the aerial (flowering) stem has at its exterior an extremely thick ring of sclerenchyma fibres, with very thick and intensely lignified walls (Figure 2b). A similar situation is found in the aerial stem of L. narbonense Mill. (Figure 2c) and L. gmelinii subsp. hungaricum (Klokov) Soó [42].
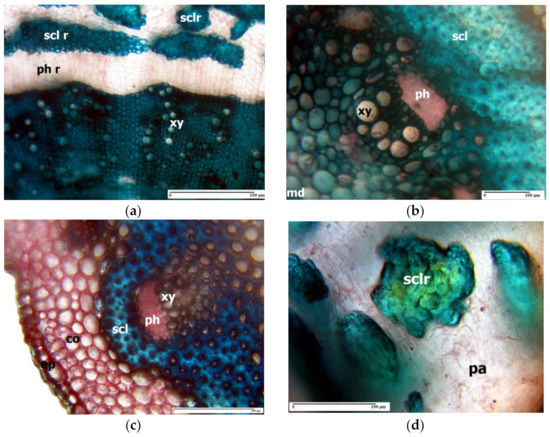
Figure 2. Cross-section through the rhizome of Limonium girardianum (a), aerial stem of Limonium girardianum (b), aerial stem of Limonium narbonense (c), and groups of sclereids located in the rhizome’ cortex of Limonium girardianum (Guss.) Fourr. (d); co—cortex; ep—epidermis; pa—parenchyma; ph r—phloemic ring; scl r—sclerenchyma ring; xy—xylem vessel; sclr—sclereid; md—medulla; ph—phloem; scl—sclerenchyma; xy—xylem vessel; ph—phloem.
References
- Erben, M. Die Gattung Limonium im südwestmediterranen Raum. Mitt. Bot. Staatssamml. München. 1978, 14, 361–631.
- Koutroumpa, K.; Theodoridis, S.; Warren, B.H.; Jiménez, A.; Celep, F.; Doğan, M.; Romeiras, M.M.; Santos-Guerra, A.; Fernández-Palacios, J.M.; Caujapé-Castells, J.; et al. An expanded molecular phylogeny of Plumbaginaceae, with emphasis on Limonium (sea lavenders): Taxonomic implications and biogeographic considerations. Ecol. Evol. 2018, 1–8.
- Buira, A.; Aedo, C.; Medina, L. Spatial patterns of the Iberian and Balearic endemic vascular flora. Biodivers. Conserv. 2017, 26, 479–508.
- The IUCN National Red List Working Group (NRLWG). IUCN/NRLWG National Red List Database. Available online: (accessed on 20 April 2020).
- Laguna, E.; Fos, S.; Ferrando-Pardo, I.; Ferrer-Gallego, P.P. Endangered halophytes and their conservations: Lessons from Eastern Spain. In From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland; Heidelberg, Germany, 2020; pp. 1–64.
- European Commission. Council Directive 92/43 CEE on the Conservation of Natural Habitats and of Wild Fauna and Flora. OJL 1992, 206, 1–50.
- European Commission DG Environment. Interpretation Manual of European Union Habitats (Version EUR27); European Commission DG Environment: Brussels, Belgium, 2007.
- Ferrer-Gallego, P.P.; Navarro, A.; Pérez-Rovira, P.; Roselló, R.; Rosselló, J.A.; Rosato, M.; Laguna, E. A new polyploid species of Limonium (Plumbaginaceae) from the Western Mediterranean basin. Phytotaxa 2015, 234, 263–270.
- Palacios, C.; Rosselló, J.A.; González-Candelas, F. Study of the evolutionary relationships among Limonium species (Plumbaginaceae) using nuclear and cytoplasmic molecular markers. Mol. Phylogen. Evol. 2000, 14, 232–249.
- Boissier, E. Plumbaginaceae. In Prodromus Systematis Naturalis Regni Vegetabilis; de Candolle, A.P., Ed.; Treuttel et Wurz: Paris, France, 1848; pp. 617–696.
- Boissier, E. Diagnoses Plantarum Orientalium Novarum; Baillière: Paris, France, 1859; pp. 61–71.
- Akhani, H.; Malekmohammadi, M.; Mahdavi, P.; Gharibiyan, A.; Chase, M.W. Phylogenetics of the Irano-Turanian taxa of Limonium (Plumbaginaceae) based on ITS nrDNA sequences and leaf anatomy provides evidence for species delimitation and relationships of lineages. Bot. J. Linn. Soc. 2013, 171, 519–550.
- Lledó, M.D.; Crespo, M.B.; Fay, M.F.; Chase, M.W. Molecular phylogenetics of Limonium and related genera (Plumbaginaceae): Biogeographical and systematic implications. Am. J. Bot. 2005, 92, 1189–1198.
- Malekmohammadi, M.; Akhani, H.; Borsch, T. Phylogenetic relationships of Limonium (Plumbaginaceae) inferred from multiple chloroplast and nuclear loci. Taxon 2017, 66, 1128–1146.
- Yuan, F.; Leng, B.; Wang, B. Progress in studying salt secretion from the salt glands in recretohalophytes: How do plants secrete salt? Front. Plant Sci. 2016, 7, 977.
- Shabala, S.; Bose, Y.; Hedrich, R. Salt bladders: Do they matter? Trends Plant Sci. 2014, 19, 687–691.
- Yuan, F.; Lyv, M.J.; Leng, B.Y.; Zheng, G.Y.; Feng, Z.T.; Li, P.H.; Wang, B.S. Comparative transcriptome analysis of developmental stages of the Limonium bicolor leaf generates insights into salt gland differentiation. Plant Cell. Environ. 2015, 38, 1637–1657.
- Lipschitz, N.; Waisel, Y. Existence of salt glands in various genera of the Gramineae. New Phytol. 1974, 73, 507–513.
- Zhang, D.Y.; Yin, L.K.; Pan, B.R. A review on the study of salt glands of Tamarix. Acta Bot. Bor. Occid. Sin 2003, 23, 190–194.
- Grigore, M.N.; Toma, C. Anatomical Adaptations of Halophytes. A Review of Classic Literature and Recent Findings; Springer: Cham, Switzerland, 2017.
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New. Phytol. 2008, 179, 945–963.
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681.
- Türkan, I.; Demiral, T. Recent developments in understanding salinity tolerance. Environ. Exp. Bot. 2009, 67, 2–9.
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97.
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447.
- Raven, J.A. Regulation of pH and generation of osmolarity in vascular plants: A cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytol. 1985, 101, 25–77.
- Shabala, S.; Shabala, L. Ion transport and osmotic adjustment in plants and bacteria. Biomol. Concepts 2011, 2, 407–419.
- Wyn Jones, R.; Storey, R.; Leigh, R.A.; Ahmad, N.; Pollard, A. A hypothesis on cytoplasmic osmoregulation. In Regulation of Cell membrane Activities in Plants; Marre, E., Ciferri, O., Eds.; Elsevier: Amsterdam, The Netherlands, 1977; pp. 121–136.
- Yeo, A.R. Salinity resistance: Physiologies and prices. Physiol. Plant. 1983, 58, 214–222.
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt tolerance and crop potential of halophytes. Crit. Rev. Plant Sci. 1999, 18, 227–255.
- Grigore, M.N.; Toma, C. Morphological and anatomical adaptations of halophytes: A review. In Handbook of Halophytes, From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020.
- Grigore, M.N.; Toma, C. Structure of salt glands of Plumbaginaceae. Rediscovering old findings from 19th century. ‘Mettenius’ or ‘Licopoli’ organs? J. Plant Dev. 2016, 23, 37–52.
- Grigore, M.N.; Toma, C. Integrative anatomy of halophytes from Mediterranean climate. In Handbook of Halophytes, From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020.
- Răvăruț, M. Plumbaginaceae. In Flora Republicii Populare Romîne; Săvulescu, T., Ed.; Editura Acdemiei Republicii Populare Romîne: Bucuresti, Romania, 1960; Volume 7, pp. 21–40.
- Linchevskii, L.A. Plumbaginaceae. In Flora of the U.S.S.R. (Series initiated by V. L. Komarov); Shishkin, B.K., Bobrov, E.G., Eds.; Translated from Russian; Israel Program for Scientific Translations: Jerusalem, Israel, 1967; pp. 216–348.
- Antonelli-Ushirobira, T.M.; Blainski, A.; Gancedo, N.C.; Gaburo, F.; Cardoso, K.A.K.; Leite-Melo, E.V.S.; Mello, J.C.P.; de Milaneze-Gutierre, M.A. Morpho-anatomical study of rhizome of Limonium brasiliense. Rev. Bras. Farmacogn. 2015, 25, 320–327.
- Grigore, M.N.; Ivănescu, L.; Toma, C. Halophytes. An Integrative Anatomical Study; Springer: Cham, Switzerland, 2014.
- Moțiu, T.; Toma, C.; Tiron, A.; Niță, M. Contribuții la cunoașterea structurii organelor vegetative de Limonium gmelini (Willd). Biol. Veget. 1987, 3, 11–14.
- González-Orenga, S.; Llinares, J.V.; Al Hassan, M.; Fita, A.; Collado, F.; Lisón, P.; Vicente, O.; Boscaiu, M. Physiological and morphological characterisation of Limonium species in their natural habitats: Insights into their abiotic stress responses. Plant Soil. 2020, 449, 267–284.
- Solereder, H. Systematic Anatomy of the Dicotyledons. A Handbook for Laboratories of Pure and Applied Botany; Clarendon: Oxford, UK, 1908; Volume 2.
- Colombo, P.; Trapani, S. Morpho-anatomical observations on three Limonium species endemic to the Pelagic Islands. Fl. Medit. 1992, 2, 77–90.
- Zoric, L.; Milic, D.; Karanovic, D.; Lukovic, J. Anatomical adaptations of halophytes within the Southern Pannonian Plain Region. In Handbook of Halophytes, From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020.




