
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Barbara Conway | + 2663 word(s) | 2663 | 2021-04-21 05:00:28 | | | |
| 2 | Rita Xu | -38 word(s) | 2625 | 2021-04-22 05:09:58 | | |
Video Upload Options
Skin and soft tissue infections are one of the most frequent types of infection, typically requiring medical intervention and contribute to morbidity and mortality in both primary care and hospitalised patients. Current antiseptics used in prevention are mainly formulated as traditional, simple dosage forms such as solutions and semi-solids. In recent years, there have been studies reporting the potential for nanotechnology ito improve the delivery of antiseptics.
1. Skin and Soft Tissue Infections
Skin and soft tissue infections (SSTIs) refer to acute conditions of inflammatory microbial occupation of the skin layers and underlying soft tissues [1][2]. The consequences have implications on healthcare, not only in low and middle income countries, but also globally [3]. SSTIs, is considered as one of the most frequent types of infection, typically require medical intervention and contribute to morbidity and mortality in both primary care and hospitalised patients [2]. It is estimated that 7–10% of hospital administrations in North America in 2005 were as a consequence of skin and soft tissue infections [4]. In the United States there was an increase of 65% in patients admitted with SSTIs in different hospital departments, from 32.1 visits per 1000 population in 1997 to 48.1 visits per 1000 population in 2005 [5]. Likewise, Lee and co-workers surveyed SSTIs occurrence in the US from 2000 to 2012 and reported that the total prevalence of SSTIs rose from 2.4 million to 3.3 million (an increase of nearly 40%) during this period [6]. In 2013, almost a third of the US population asked for medical advice related to skin conditions [7]. The incidence of SSTIs has increased, possibly as a result of an ageing population, the escalation of multidrug-resistant strains and the increasing numbers of immunocompromised patients as a consequence of immunosuppressive therapy, cancer, transplant interventions, or HIV⁄AIDS [2][8]. Global Health Metrics reported in 2017 regarding the prevalence, incidence, and years lived with disability (YLDs) covering 354 diseases in 195 countries; accordingly, there were nearly 4.2 billion new cases of skin and subcutaneous diseases worldwide. Around 50% of these were fungal skin diseases (accounting for more than 2.1 billion), whereas the incident cases linked with bacterial and viral pathogens were 0.27 and 0.12 billion, respectively [9].
Pathophysiology of SSTIs is related to an interruption in the balance between the immune barrier of the host and the pathogenicity of microbial population colonizing human skin [2]. Cellulitis, as an example, is caused by pathogens disrupting skin integrity, and is more prevalent in patients with comorbidities [10]. Disruption of the protective cutaneous layers can be caused by chemical and physical impacts such as ulceration, trauma, bites or surgical wounds, thermal injury, or previous inflammation [2][10]. Both the patient and the environment are key factors contributing to the risk of developing an SSTI. Older people or those with long-term conditions such as critical illness, obesity, cardiovascular diseases, and chronic kidney disease failure will be at higher risk of skin breakdown. Patients with spinal cord injury and paralysis that result in the alteration of skin perfusion and temperature control are also considered to be at higher risk. External factors which are likely to impair the skin barrier function can be scratching, pressure, shear and friction, UV exposure, or radiation contact in cancer patients [2][11]. Additionally, biofilm formation, the development of which enables microbes to survive and adapt to unfavourable conditions, has become a severe problem in the healthcare fields, responsible for 65% of nosocomial infections [12]. Biofilm is produced following a cell attaching to a surface, multiplying, maturating, and then creating an extracellular polymeric matrix which resists environmental impacts such as mechanical forces and antibiotics. This structure is detachable, affording opportunities for microorganisms to transmit into new sites and spread infections. Biofilms have been observed in medical devices such as intravenous and urinary catheters, stents, implants, ventilator tubes, or heart valves, contributing to the growing challenge of antimicrobial resistance [13].
In children, bacterial skin infections are more prevalent than fungal, parasitic and viral infections [14]. The major causative pathogens associated with skin and soft tissue infections are Gram-positive microorganisms, typically Staphylococcus aureus (including methicillin-resistant S. aureus/MRSA strains) and beta-hemolytic streptococci [1]. The most frequent Gram-negative strain isolated was Klebsiella sp. [15]. S. aureus was responsible for more than 40% of total SSTIs cases in 2003, and was a frequent cause of cellulitis, abscesses and wound infections [2]. The incidence of S. aureus-related skin and soft tissue infections increased two-fold from 2001 to 2009 in the US [16]. However, it was reported that the proportion of hospital administrations caused by MRSA-related skin and soft tissue infections (SSTIs) declined by 29% over the next five years [17].
Patients with dermatologic conditions often encounter physiological, psychological, as well as financial issues; not only that, many cutaneous concerns can lead to systemic diseases [18]. Moreover, comorbidity factors, such as diabetes, immuno-compromisation, obesity, liver and kidney failure, and cardiovascular diseases, have repercussions on treatment costs and prolong the length of stay in hospital [19]. Suaya et al. determined that the cost of SSTI hospitalizations due to S. aureus in 2009 was $4.50 billion, which was 34% higher than in 2001 [16]. According to the Global Burden of Disease Study, 15 different dermatologic concerns accounted for 1.79% of the total global burden of disease in 2013. This was calculated using disability-adjusted life years (DALYs) index, of which cellulitis, viral skin diseases and fungal skin diseases accounted for 0.04%, 0.16%, and 0.15%, respectively. Skin and subcutaneous conditions, next to iron deficiency anaemia, tuberculosis, and sensory organ diseases were the leading reasons inducing disability in the world [9][18].
2. Antiseptics
Antiseptics are biocidal products that can kill or impact the growth of disease-causing bacteria in, or on, living tissue, e.g., on the skin. Ideal properties for antiseptics include widespread and rapid bioactivity against bacteria, fungi and viruses, no toxicity or damage to the healthy tissue, and insignificant absorption into the systemic circulation following external application [20]. Antiseptic products may contain one or more active ingredients and are presented in various formulations and preparations, for example, antimicrobial hand washes, surgical scrubs, preoperative preparations, tinctures, ointments, creams, mouth-rinses, and toothpaste. They are commonly used as pre-operative skin preparations for prevention of surgical site infections [21], as routine skin hygiene such as hand-washes and hand rub products or for treating skin and wound diseases [20]. For skin and wound infections in deeper skin layers, antibiotics are more normally prescribed; in contrast, topical antiseptics are preferred for infections at the outermost surface. In such cases, the aim is to minimize any microbial colonization in a wound or on the skin surface without causing any deleterious effects on the living tissue or impeding the healing process [20][22]. Chemical structures of commonly used antiseptics are depicted in Figure 1.
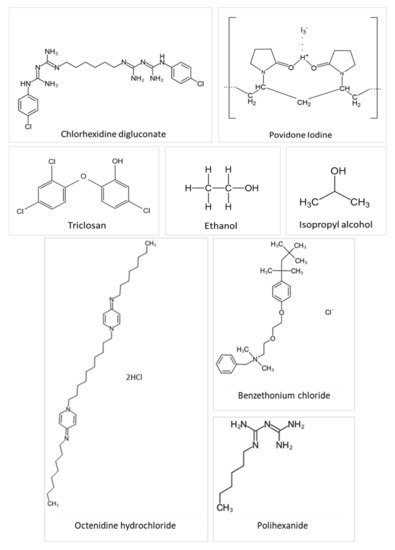
Figure 1. Chemical structures of several antiseptic agents.
2.1. Chlorhexidine
Chlorhexidine is a cationic polybiguanide (bisbiguanide) [23]. It primarily used as salt forms because of its insolubility in water. Chlorhexidine gluconate (CHG) and other salts like chlorhexidine diacetate, dihydrochloride, and dihydrobromide are used as surficial disinfectants (disinfection of the skin and hands), in cosmetics (in creams, toothpaste, hair care products, deodorants, and antiperspirants), and pharmaceutical products (e.g., preservative in eye drops, wound dressings, and antiseptic mouth-rinse) [20]. Chlorhexidine is supplied typically in solution from 0.5 to 4% w/v. Chlorhexidine gluconate 2% w/v (CHG) in 70% v/v isopropyl alcohol (IPA) is particularly recommended for pre-operative skin cleansing by several organizations, such as Health Protection Scotland (2013), the Centre for Disease Prevention and Control (2017), National Institute for Health and Clinical Excellence (2013) and the World Health Organization (2017) [24][25]. Chlorhexidine solutions at concentrations of 0.5% w/v and above, with alcohol, are employed to prepare skin prior to peripheral venous catheter insertion to prevent catheter-related bloodstream infections [26]. Chlorhexidine is a broad-spectrum antibacterial, active against both Gram-positive and Gram-negative bacteria, while exhibiting some activity on yeasts, dermatophytes, and some lipid-enveloped viruses [20]. Furthermore, Macias et al. concluded that CHG in IPA is preferred to 1% w/v triclosan in 70% IPA when a prolonged antisepsis is required due to its longer-lasting residual effect, [27]. Alcoholic CHG solutions at both 0.5% and 1.0% w/v concentrations were better than 10% w/v aqueous povidone-iodine (PVP-I) in minimizing microbial colony formation related to intravascular catheters [28]. Gels containing 2% w/v CHG also demonstrated a higher fungicidal activity than a comparative nanosilver gel against C. albicans [29].
The mechanism of antimicrobial activity of chlorhexidine is that the positively charged molecule binds to the negatively charged lipid bacterial cell surface, thus weakening the cell membrane integrity, followed by leakage of cytoplasm and precipitation of proteins and nucleic acids at lower concentrations and membrane disruption at higher concentrations [20][30]. Due to this non-specific mechanism of action, chlorhexidine use is widespread. However, there are some issues with its use, such as potential toxicity in the eyes, ears and brain, it can become inactivated in the presence of non-ionic surfactants, and it may cause dry skin [20][31]. Recently, the Food and Drug Administration (FDA) released a warning regarding the increasing occurrence of rare but severe allergic reactions to CHG. According to the FDA, healthcare specialists should take into account the patient’s allergy history prior to prescribing CHG-based products [32]. Furthermore, some recent studies have indicated that the increased use of CHG may be responsible for cross-resistance to colistin and daptomycin and the reduced susceptibility (manifested by higher CHG minimum inhibitory concentrations) against several skin pathogens such as Klebsiella pneumoniae, multidrug-resistant Acinetobacter baumannii, S. epidermidis, S. aureus, and vancomycin-resistant enterococci (VRE) [33][34][35][36].
2.2. Triclosan
Triclosan (2,4,4′-trichloro-2′-hydroxydiphenyl ether) is a phenoxyphenol compound that has been principally considered as an antibacterial and antifungal agent [20]. Triclosan has a very low aqueous solubility of 0.012 g/L at 20 °C [37]. It is a common ingredient in various antiseptic products, especially in antimicrobial soap, body and hand washes and toothpaste. It is typically used at concentrations of 0.1 to 2% w/v, with or without other active antimicrobials such as alcohols, to bring about long-lasting activity on the skin. Triclosan is active against Gram-positive bacteria, including Staphylococcus species. Moreover, it may also have an effect on Gram-negative bacteria and yeast, with some weaker activity against enveloped viruses, pseudomonads, and fungi [20]. Originally, triclosan was thought to target the cell membrane in a non-specific mechanism, however, recent studies have found a specific bacteriostatic action for triclosan on bacteria through inhibition of the bacterial fatty acid biosynthetic pathway. At the higher concentrations found in antiseptic preparations (2–20 mg/mL), there is a hypothesis that triclosan acts as a biocide with multiple actions on lipid, RNA and protein synthesis, leading to cell lysis [36][38]. The antimicrobial activity of triclosan-containing antiseptics can be influenced by formulation effects, for example, there is a synergistic activity with chelating agents (e.g., EDTA) in destroying the Gram-negative bacterial cell wall thereby improving uptake into cells. Triclosan shows negligible irritation and allergic skin reactions and it can retain persistent on the skin surface [20]. However, because of the lack of the scientific literature regarding the safety and effectiveness of triclosan for human health, in December 2017, the FDA issued a final rule prohibiting the use of triclosan in certain over-the-counter antiseptic preparations [39].
2.3. Povidone-Iodine (PVP-I)
Povidone-iodine (PVP-I), which is a complex of elemental iodine loosely bound to the carrier polyvinylpyrrolidone, is used as a broad-spectrum antimicrobial agent against bacteria, viruses, fungi, and protozoa at relatively low concentrations [20][40]. Typically, PVP-I is widely used as a topical antiseptic and disinfectant for skin and wound infections, mostly in solution, dry powder and lotion formulations. Application as an iodophor improves both solubility and stability while releasing the active iodine gradually from the polymer network over time. Therefore, its residual antimicrobial activity is maintained stably while side effects associated with iodine such as irritation and brown staining on the skin and mucous membranes are reduced. Its precise mechanism of action is still unknown, but it is believed that the active iodine species acts as an oxidizing agent which reacts with cell walls, membranes, and cytoplasm by exchanging and inactivating functional groups of amino acids (e.g., lysine, histidine, cysteine, and arginine). The consequence is the loss of cell structure and function [20].
2.4. Alcohol
Alcohols offer rapid and broad-ranging activity against bacteria, fungi, and viruses although less is known about their activity against protozoa and bacterial spores, but they are sporistatic. Isopropanol (isopropyl alcohol), ethanol and n-propanol are the most popular alcohols used as antiseptics and disinfectants. Their exact mechanism of action is not clear but they are able to cause denaturation and precipitation of proteins thus destroying cell membranes and leading to cell lysis. Concentrations ranging from 60% to 80% v/v are recommended for maximum antimicrobial activity because, in more concentrated solutions, alcohol quickly coagulates protein-based molecules present externally on the cell wall and interferes with penetration into the cell, therefore limiting further effects on protein-based inner cell compositions. Other potential attributes are relative stability, and low toxicity, odour and cost. Alcohols are also used as preservatives and common solvents for other biocides such as chlorhexidine [20].
2.5. Essential Oils
Essential oils are secondary metabolic products found in various parts of plants (such as flowers, seeds, leaves, peels, buds, barks, wood, or roots), and can be extracted by hydro-distillation and steam distillation, mechanical processes, or by “dry” distillation from some woods [20][41]. They are complex mixtures containing hundreds of compounds and their exact chemical composition depends on extraction processes and specific conditions. For example, dry vapour steam distillation is used when there is a requirement to minimize ester hydrolysis (e.g., linalyl acetate), or cohobating is proposed to improve the quantity of particular compounds such as sulfur compounds [41]. Essential oils and their components have been used in a wide range of products, from fragrances, toothpastes, cosmetics, to aromatherapy and phytomedicine, with tea tree oil and eugenol, being combined in many commercial antiseptic preparations, such as Ord River Tea Tree Antiseptic Cream®, Australian Tea Tree Antiseptic Cream® or Manuka Doctor ApiRevive Manuka and Tea Tree Antiseptic Gel® [20]. In dermatology, essential oils are primarily used for treating skin infections (62% of total cases), followed by skin inflammation and general skin maintenance at 20% and 18%, respectively [42]. Relative bioactivity varies between the different oils. In particular, tea tree oil demonstrates bactericidal activity (at 0.25 to 0.5% v/v), fungicidal activity (at 0.06–1% v/v), fungistatic activity (within 0.03–0.5% v/v) as well as activity against yeasts and dermatophytes (including Candida and Trichophyton). Tea tree oil, amongst others, presents persistent and long-lasting activity on the skin after application. Despite most essential oils presenting antimicrobial effectiveness at low concentrations, they have been reported to generate irritancy and allergenicity following application to skin and mucous membranes [20][42]. Almost 1.8% of patients tested with 5% and 10% tea tree oil patches experienced allergic contact dermatitis [43].
2.6. Silver Compounds
The active element is the silver ion (Ag2+) in silver nitrate (AgNO3) and silver sulfadiazine (AgSD). Generally, topical silver antiseptics are applied for prevention of skin and wound infections mostly caused by S. aureus and Pseudomonas in cream or solution forms and used in eye drop preparations for bacterial infections in neonates [20]. There are a number of studies indicating the valuable role of silver in wound care [44]. Additionally, silver compounds are also commonly used to cover surfaces prone to bacterial colonization such as catheters or dental instruments. Many commercial silver-based products are now available in many forms such as Atrauman Ag® Wound Dressing, Urgotul® SSD Antibacterial Contact Layer, Flamazine® Antibacterial Cream, Colloidal Silver Spray®, Silver Solution® Antimicrobial Wound Gel, MSM+Silver® Water Drops, or Natural Sense Colloidal Silver ® Eye Drops.
Silver compounds exhibit bacteriostatic and bactericidal activity at fairly low concentrations, especially on Gram-positive bacteria. Regarding the mechanism of action, active silver ions bind to sulfhydryl, amino, and carboxyl groups of amino acids on microorganism surfaces, thus denaturing proteins, and disrupting the cell wall and membrane functions. Silver also specifically inhibits cell wall metabolism and electron transport as well as the respiration chain [20][45]. Following application of topical antiseptic, respiratory sprays, implanted medical devices or wound dressings, silver has been shown to be absorbed into the systemic circulation, mostly in conjugation with protein and then deposited in human tissues, with higher levels in skin, kidneys, eyes, brain, liver, and bone marrow [46]. Argyria is a rare cutaneous condition resulted by excessive or chronic use of preparations containing silver, with the most characteristic symptom being the discolouration of skin into blue or blue-grey, especially in sunlight-exposed areas [47].
References
- Dryden, M.S. Skin and soft tissue infection: Microbiology and epidemiology. Int. J. Antimicrob. Agents 2009, 34, 2–7.
- Tognetti, L.; Martinelli, C.; Berti, S.; Hercogova, J.; Lotti, T.; Leoncini, F.; Moretti, S. Bacterial skin and soft tissue infections: Review of the epidemiology, microbiology, aetiopathogenesis and treatment. J. Eur. Acad. Dermatol. 2012, 26, 931–941.
- Selcuk, M.; Aysegul, U.; Demet, K.; Kalih, L.C. Bacterial skin infections: Epidemiology and latest research. Turk. J. Fam. Med. Prim. Care 2015, 9, 65–74.
- Vinh, D.C.; Embil, J.M. Rapidly progressive soft tissue infections. Lancet Infect. Dis. 2005, 5, 501–513.
- Hersh, A.L.; Chambers, H.F.; Maselli, J.H.; Gonzales, R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch. Intern. Med. 2008, 168, 1585–1591.
- Lee, G.C.; Boyd, N.K.; Lawson, K.A.; Frei, C.R. Incidence and cost of skin and soft tissue infections in the United States. Value Health 2015, 18, A245.
- Lim, H.W.; Collins, S.A.B.; Resneck, J.S., Jr.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A.; et al. The burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017, 76, 958–972.
- Esposito, S.; Noviello, S.; Leone, S. Epidemiology and microbiology of skin and soft tissue infections. Curr. Opin. Infect. Dis. 2016, 29, 109–115.
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet 2018, 392, 1789–1858.
- Moffarah, A.S.; Al Mohajer, M.; Hurwitz, B.L.; Armstrong, D.G. Skin and soft tissue infections. Microbiol. Immunocompromised Host 2016, 4, 691–708.
- Wounds UK. Best Practice Statement: Maintaining Skin Integrity. Available online: (accessed on 19 December 2019).
- Malheiro, J.; Simões, M. Antimicrobial resistance of biofilms in medical devices. In Biofilms and Implantable Medical Devices; Deng, Y., Lv, W., Eds.; Woodhead Publishing: Cambridge, UK, 2017; Chapter 4; pp. 97–113.
- English, J.S.C. General Dermatology: An Atlas of Diagnosis and Management; Clinical Publishing: Oxford, UK, 2007.
- World Health Organization. Epidemiology and Management of Common Skin Diseases in Children in Developing Countries. Available online: (accessed on 15 December 2019).
- Poulakou, G.; Lagou, S.; Tsiodras, S. What’s new in the epidemiology of skin and soft tissue infections in 2018? Curr. Opin. Infect. Dis. 2019, 32, 77–86.
- Suaya, J.A.; Mera, R.M.; Cassidy, A.; O’Hara, P.; Amrine-Madsen, H.; Burstin, S.; Miller, L.G. Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 through 2009. BMC Infect. Dis. 2014, 14, 296.
- Klein, E.Y.; Mojica, N.; Jiang, W.; Cosgrove, S.E.; Septimus, E.; Morgan, D.J.; Laxminarayan, R. Trends in methicillin-resistant Staphylococcus aureus hospitalizations in the United States, 2010–2014. Clin. Infect. Dis. 2017, 65, 1921–1923.
- Karimkhani, C.; Dellavalle, R.P.; Coffeng, L.E.; Flohr, C.; Hay, R.J.; Langan, S.M.; Nsoesie, E.O.; Ferrari, A.J.; Erskine, H.E.; Silverberg, J.I.; et al. Global skin disease morbidity and mortality: An update from the Global Burden of Disease Study 2013. JAMA Dermatol. 2017, 153, 406–412.
- Kaye, K.S.; Petty, L.A.; Shorr, A.F.; Zilberberg, M.D. Current epidemiology, etiology, and burden of acute skin infections in the United States. Clin. Infect. Dis. 2019, 68, S193–S199.
- McDonnell, G.E. Antisepsis, Disinfection, and Sterilization: Types, Action, and Resistance; ASM Press: Washington, DC, USA, 2007.
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surgery 2017, 152, 784–791.
- Claesen, J. Topical antiseptics and the skin microbiota. J. Investig. Dermatol. 2018, 138, 2106–2107.
- Tanzer, J.M.; Slee, A.M.; Kamay, B.A. Structural requirements of guanide, biguanide, and bisbiguanide agents for antiplaque activity. Antimicrob. Agents Chemother. 1977, 12, 721–729.
- World Health Organization. Global Guidelines on the Prevention of Surgical Site Infection. Available online: (accessed on 11 December 2019).
- Privitera, G.P.; Costa, A.L.; Brusaferro, S.; Chirletti, P.; Crosasso, P.; Massimetti, G.; Nespoli, A.; Petrosillo, N.; Pittiruti, M.; Scoppettuolo, G.; et al. Skin antisepsis with chlorhexidine versus iodine for the prevention of surgical site infection: A systematic review and meta-analysis. Am. J. Infect. Control. 2017, 45, 180–189.
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the prevention of intravascular catheter-related infections. Clin. Infect. Dis. 2011, 52, e162–e193.
- Macias, J.H.; Alvarez, M.F.; Arreguin, V.; Muñoz, J.M.; Macias, A.E.; Alvarez, J.A. Chlorhexidine avoids skin bacteria recolonization more than triclosan. Am. J. Infect. Control. 2016, 44, 1530–1534.
- Yasuda, H.; Sanui, M.; Abe, T.; Shime, N.; Komuro, T.; Hatakeyama, J.; Matsukubo, S.; Kawano, S.; Yamamoto, H.; Andoh, K.; et al. Comparison of the efficacy of three topical antiseptic solutions for the prevention of catheter colonization: A multicenter randomized controlled study. Crit. Care 2017, 21, 320.
- Mozayeni, M.A.; Hadian, A.; Bakhshaei, P.; Dianat, O. Comparison of antifungal activity of 2% chlorhexidine, calcium hydroxide, and nanosilver gels against Candida albicans. J. Dent. 2015, 12, 109–117.
- Auda, S.H.; Mahrous, G.M.; Ibrahim, M.A.; Shazly, G.A.; Salem-Bekhit, M.M. Novel chlorhexidine dermal patches, preparation characterization and antimicrobial evaluation. Polym. Bull. 2017, 74, 3995–4007.
- Zinn, J.; Jenkins, J.B.; Swofford, V.; Harrelson, B.; McCarter, S. Intraoperative patient skin prep agents: Is there a difference? AORN J. 2010, 92, 662–674.
- Boyce, J.M. Best products for skin antisepsis. Am. J. Infect. Control. 2019, 47, 17–22.
- Hayashi, M.; Kawamura, K.; Matsui, M.; Suzuki, M.; Suzuki, S.; Shibayama, K.; Arakawa, Y. Reduction in chlorhexidine efficacy against multi-drug-resistant Acinetobacter baumannii international clone II. Hosp. Infect. 2017, 95, 318–323.
- Bhardwaj, P.; Ziegler, E.; Palmer, K.L. Chlorhexidine Induces VanA-type vancomycin resistance genes in Enterococci. Antimicrob. Agents Chemother. 2016, 60, 2209.
- Wand, M.E.; Bock, L.J.; Bonney, L.C.; Sutton, J.M. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob. Agents Chemother. 2016, 61, e01162-16.
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and emerging topical antibacterials and antiseptics: Agents, action, and resistance patterns. Clin. Microbiol. Rev. 2017, 30, 827–860.
- Lee, J.; Kwack, S.; Shin, C.; Jang, H.-J.; Kim, H.; Kim, M.; Seo, D.-W.; Lee, B.; Kim, K.-B. Risk assessment of triclosan, a cosmetic preservative. Toxicol. Res. 2019, 35, 137–154.
- Schweizer, H.P. Triclosan: A widely used biocide and its link to antibiotics. FEMS Microbiol. Lett. 2001, 202, 1–7.
- The U.S. Food and Drug Administration (FDA). Q&A for Consumers: Health Care Antiseptics. Available online: (accessed on 21 December 2019).
- Günther Hierholzer, E.R.W.; Reimer, K. Topische Infektions therapie und Prophylaxe. Aktueller Stellenwert von PVP-Jod; Stuttgart Thieme: Stuttgart, Germany, 1996.
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. Trends Anal. Chem. 2015, 66, 146–157.
- Orchard, A.; van Vuuren, S. commercial essential oils as potential antimicrobials to treat skin diseases. Evid. Based Complement. Alternat. Med. 2017, 2017, 4517971.
- Rutherford, T.; Nixon, R.; Tam, M.; Tate, B. Allergy to tea tree oil: Retrospective review of 41 cases with positive patch tests over 4.5 years. Aust. J. Dermatol. 2007, 48, 83–87.
- Graham, C. The role of silver in wound healing. Br. J. Nurs. 2005, 14, S22–S28.
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178.
- Lansdown, A. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34.
- Weedon, D. Cutaneous deposits. In Weedon’s Skin Pathology, 3rd ed.; Weedon, D., Ed.; Churchill Livingstone: Edinburgh, UK, 2010; Chapter 14; pp. 369–396.




