
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chiara Porro | + 1948 word(s) | 1948 | 2021-04-21 06:18:52 | | | |
| 2 | Dean Liu | -10 word(s) | 1938 | 2021-04-21 11:33:59 | | |
Video Upload Options
Quercetin possess multiple pharmacological applications including anti-inflammatory, antitumoral, antiapoptotic and anti-thrombotic activities, widely demonstrated in both in vitro and in vivo studies.
1. Introduction
The search for novel natural therapeutic agents to prevent or slow the progression of neurodegenerative diseases is gaining great attention. Indeed, recent evidence has affirmed that the consumption of fruits and vegetables is strictly linked to a decreased risk of developing a large variety of age-related and neurodegenerative diseases.
Quercetin is a natural bioflavonoid found abundantly in fruit and vegetables such as apples, berries, onions and capers. It was firstly isolated by the physiologist Albert Szent- gyorgyi de Nagyrapolt, a Nobel Prize recipient for Physiology/Medicine in 1936 [1].
Quercetin has multiple pharmacological properties, including neuroprotective, anti- cancer, cardioprotective, antioxidant, antiviral, antimicrobial, antithrombotic, antiapoptotic, anti-inflammatory and hepatoprotective. It is also used as potential treatment for severe inflammation [2].
The glial cells of the central nervous system (CNS) consist of oligodendrocytes, astrocytes, microglia and ependymal glial cells. However, the peripheral nervous system (PNS) is composed of other glial cells such as Schwann cells, and satellite glial cells, which provide nutrients and structural support to neurons. Glial cells, an essential part of the CNS and immune system, provide homeostatic support, protection, defense against pathogens, and neuronal maintenance. Upon activation by tissue injury and inflammation, they release inflammatory mediators and induce neuroinflammatory diseases. Also, the blood–brain barrier (BBB) is maintained by the homeostasis of CNS microenvironment, and acts as a physical and metabolic barrier for limiting the movement of substances into the brain.
Quercetin is known for its multiple proven health benefits including its protective role in neurodegenerative diseases due to its capacity, among other, to modulate microRNA (miRNA) expression. miRNAs have been shown to regulate critical processes, including inflammation, differentiation, proliferation, apoptosis and immune responses as well as neurodegeneration. To improve the bioavailability and stability of quercetin many researchers have developed nano-formulations to increase bioavailability of quercetin.
2. Quercetin and Its Dietary Sources
“Quercetin” is derived from the Latin word “quercetum,” and literally means “oak forest”. Quercetin is a natural and bioactive flavonoid which is not produced in the human body [3][4]. Most common forms of quercetin described in the literature are quercetin glycoside, quercetin sulfate, quercetin glucuronide and methylated quercetin [5].
Quercetin is the most abundant flavonoid in vegetables and fruits such as onions, broccoli, berries, grapefruit, apple, black and green tea, red grapes, citrus fruits, green leafy vegetables and beans [6][7]. The required human dietary intake of all flavonoids is estimated between 200 and 350 mg/day, while the normal daily ingestion of flavanols varies from 20 to 35 mg, of which quercetin accounts for nearly 50%, with a daily consumption of about 10 mg/day [8][9]. However, the ingested amount with the diet is subject to many variables essentially the plant composition, dietary habits, age and other factors.
Like other flavanols, after ingestion, the primary site of quercetin absorption is the small intestine and only minor proportion of quercetin is absorbed in the stomach. The conjugated quercetin is transported and modified in the liver before re-entering the systemic circulation and transported to the muscles and brain. Quercetin produced metabolites after undergoing phase I and II metabolism in the liver then transported to the body tissues through the circulating blood [10][11]. The main metabolites found in urine after ingestion of quercetin are quercetin—diglucuronide, -3′-glucuronide, isorhamnetin-glucuronide, glucuronide sulfate, and -methyl quercetin diglucuronide. In total, 23 metabolites were identified, with twelve being quantified in the urine and only five in plasma [12]. However, quercetin-3-O-β-D-glucuronide, is the most abundant metabolite of quercetin, which contributes to the activation of many physiological functions with beneficial effects when distributed in the tissues [13].
Quercetin has been proven to possess various biological properties including its antioxidant and anti-inflammatory roles in many inflammatory, metabolic and neurodegenerative diseases [5]. This may ameliorate the overall health and contributes to diseases prevention [14]. In addition, quercetin exhibits other physiological properties, including antithrombotic, anti-ischemic, antiapoptotic, antitumoral, antibacterial and antiviral activities [15][16][17].
More interestingly, a growing body of evidence has shown that quercetin has a broad therapeutic potential for the treatment and prevention of various diseases including cancer, cardiovascular and neurodegenerative diseases. Hence, it is considered as neurohormetic phytochemical with potential neuroprotective effects associated with reduced levels of oxidative stress [2].
In general, the oral administration of quercetin in humans was well tolerated and safe; adverse effects have been rarely reported [14][18][19]. However, the biosafety assessment of the long-term use of high doses of quercetin in human requires further investigation.
3. Broad Mechanisms of Action of Quercetin
Quercetin has benzo-(γ)-pyrone skeletal structure with a C6-C3-C6 carbon framework, which consists of two benzene rings linked through a heterocyclic pyrone ring. Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is known for having five hydroxyl (OH) groups that may undergo glycosylation to quercetin glycosides, which constitutes the major quercetin derivatives.
This modification can change the absorption, solubility, and the in vivo effects of quercetin. It is critical to note that there is a strong relationship between the structural activities of quercetin and its derivatives on the anti-inflammatory and antioxidant activities [20][21].
The protective effects of quercetin are exerted by the chelating activity of divalent cations, scavenging free radicals, and protecting DNA from damage, in addition to their preventive effects against lipid peroxidation and free radical-mediated cytotoxicity [22]. Quercetin not only lowers the serum triglyceride level but also shows protective potential on digestive enzyme activity, antioxidant effect in the hepatopancreas and improve growth performance in freshwater fish (Tilapia) [23][24] and in dietary-induced obese mice [25]. Interestingly, it has been reported that quercetin reduced the triglyceride level via the PPARα cascade in cultured hepatocytes from a chicken, leading to a decreased level of lipid deposition and may reduce the chronic inflammation associated with lipid accumulation [26].
The chemical structure of quercetin guarantees potent antioxidant activity. Indeed, being an excellent scavenger of reactive oxygen species (ROS) and reactive nitrogen species (RNS), quercetin becomes promising candidate to reduce endoplasmic reticulum stress (ER-stress), i.e., an important contributor to inflammation. Furthermore, quercetin attenuated NF–kB activity, a key mediator of inflammation, thereby directly decreasing the cytokine production via this transcription factor. Another study highlighted that quercetin has a profound effect on the activation of the Nuclear factor erythroid 2-related factor 2 (Nrf2) antioxidant signaling pathway and the expression of its associated downstream effector phase II detoxification enzyme glutathione-S-transferase (GST)P1 in skin HaCaT keratinocytes and the human foreskin fibroblasts (BJ cells) [27].
Another study by Liu X et al. [28] reported that quercetin had significantly improved cardiac function by reducing myocardial injury and the infarct size. In addition, the in vivo and in vitro quercetin treatment induced a remarkable improvement of myocardium oxidative damage and apoptosis. Quercetin effects involved the reduction of NF–kB activation cascade as a consequence of myocardial ischemia-reperfusion damage [27].
The investigation of the molecular pathways underlying the neuroprotection mechanisms of quercetin in in vitro and in the mouse model of Parkinson’s Disease (PD) has shown that the activation of PKD1–Akt pathway via the upregulation of PPAR-gamma coactivator 1-alpha (PGC-1a) and the transcription factor A, mitochondrial (TFAM) represents a mechanism to restore mitochondrial function and decrease the progression of dopaminergic neurodegeneration [29][30]. Moreover, during endotoxic stress, quercetin increase heme oxygenase (HO-1) expression via mitogen-activated protein kinase (MAPKs). Taken together, this evidence supports the central function of quercetin in regulating inflammation in microglial cells.
Furthermore, quercetin exerts physiological functions on many organs including liver, brain, kidney, blood vessels, muscle, skin, intestine and bone. Mounting evidence suggests that quercetin can influence neurodegenerative diseases, mood disorders, atherosclerosis, and metabolic syndrome [13]. In addition, quercetin treatments of neurodegenerative diseases are able to modulate the expression levels of pro-inflammatory, anti-inflammatory cytokines and chemokines [31].
Studies on cellular, human, and animal models have demonstrated that the strengthened antioxidant, anti-inflammatory, anticancer and neuroprotective activities of quercetin can be achieved through targeting multiple signaling pathways and the downstream effectors gene expression involved in these processes [32]. This includes antiapoptotic effects of quercetin against cervical cancer cells growth [33], anti-oxidative, anti-ER-stress effects associated with diabetic encephalopathy and atherosclerosis [34][35]. Other potential neuroprotective effects of quercetin were shown in various study models such as: diabetes-induced nerve injury [36], mouse model of neurotoxicity [37][38] and CPF-induced neurotoxicity in animal models.
The modulatory effect of quercetin on inflammatory processes involved a variety of signaling pathways, including its interaction with phosphatidylinositol-3-phosphate kinase (PI3K), MAPK, extracellular signal regulated kinase (ERK), kinase (MEK1), and others [29]. It may also inhibit the activity of PI3K by shifting ATP binding from PI3K and activate AMP-activated protein kinase (AMPK), which triggers antitumoral and anti-inflammatory responses [13]. Inflammation commonly seen in an obesity context is known to induce meta-inflammation described as chronic and low-grade inflammation [39][40][41]. This chronic inflammation induced a release of proinflammatory cytokines and infiltration of macrophages into adipose tissue that is in close association with the progression of insulin resistance via the crosstalk of the insulin-signaling pathway in adipose tissue and skeletal muscle. Importantly, it has been suggested that the quercetin inhibited the synthesis and secretion of proinflammatory mediators and consequently it improves insulin resistance in this context.
Furthermore, another pathway involved in obesity inflammation is those triggered by toll-like receptor 4 (TLR4) [42]. Indeed, TLR4 plays a modulatory role in immune responses, stimulates the release of pro-inflammatory chemokines and cytokines, [43] and the activation of NF–kB signaling pathways [44][45][46]. This deleterious signaling pathway could be modulated by quercetin, which possesses the ability to decrease inflammation by acting on the TLR4/NF–kB pathway [47].
Given the fact that CNS functions are strongly influenced by the gastrointestinal microbiota, it increasingly becoming obvious that the gut microbiota is strongly connected to the CNS through various bidirectional pathways involving immune, endocrine and neuronal pathways. This bidirectional communication can be achieved via the microbiota production of a large number of mediators/substances that can act either locally or at distant and regulating the CNS functions [48]. More interestingly, a study by Xie et al. [49] reported that quercetin treatment is able to influence gut microbiota in diabetic peripheral neuropathy rats by protecting axon and myelin damage from oxidative stress caused by chronic hyperglycemia [49]. Taken together, this suggests that quercetin may exert its indirect neuroprotective role via microbiota involving ROS pathways suppression and other unknown pathways that need further investigation [49].
Quercetin has an interesting inhibitory effect on inflammatory responses: from one side, it inhibits the expression of different components of NLRP3 inflammasome, including the pro-IL-1β; from the other side, it impedes the activation of other signaling pathways, particularly NF–kB [2]. All triggered mechanisms converge to suppress inflammation.
In addition to its regulatory roles in energy metabolism and mitochondrial function [50][51], it is widely believed that the Nrf2 cascade exerts antioxidant effects and participates in cell protection and maintaining the redox homeostasis through the subsequent induction of cytoprotective protein expression [22]. Indeed, a great number of genes involved in antioxidant responses, including HO-1, GST, catalase, superoxide dismutase (SOD), sulfidorexin, thioredoxin reductase-1, glutamate cysteine ligase, NADPH, quinone oxidoreductase-1, are under Nrf2 controls [52][53]. Moreover, the interaction of Nrf2 with other signaling molecules modulate the efficiency of the cellular stress response. Beyond its fundamental function in the regulation of the inflammatory responses in various diseases, the activation of Nrf2-ARE has shown neuroprotective effects against neurodegenerative diseases [54]. Figure 1 summarizes the protective effects of quercetin through modulating microbiota and ROS levels for the prevention of neurological disorders.
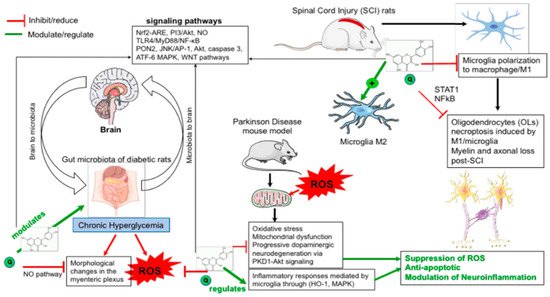
Figure 1. Protective effects of quercetin through modulating microbiota and reactive oxygen species levels to prevent neurological disorders. Quercetin exerts its indirect neuroprotective effect through microbiota involving reactive oxygen species (ROS) pathways suppression in diabetic peripheral neuropathy rats. It regulates cytoprotective protein expression against ROS-induced oxidative stress and suppresses neuroinflammatory responses induced by ROS. Quercetin inhibited inflammatory responses in microglial cells and up-regulate heme oxygenase-1 (HO-1) against endotoxic stress through MAPKs.
References
- Suganthy, N.; Devi, K.P.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. Biomed. Pharmacother. 2016, 84, 892–908.
- Martel, J.; Ojcius, D.M.; Ko, Y.-F.; Young, J.D. Phytochemicals as Prebiotics and Biological Stress Inducers. Trends Biochem. Sci. 2020, 45, 462–471.
- Lakhanpal, P.; Rai, D.K. Quercetin: A Versatile Flavonoid. IJMU 2007, 2, 22–37.
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Di Pierro, F. A role for quercetin in coronavirus disease 2019 (COVID-19). Phytotherapy Res. 2021, 35, 1230–1236.
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, in-flammation and immunity. Nutrients 2016, 8, 167.
- Shankar, G.M.; Antony, J.; Anto, R.J. Quercetin and tryptanthrin: Two broad spectrum anti-cancer agents for future chemotherapeutic interventions. Enzymes 2015, 37, 43–72.
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major up-date of the Phenol-Explorer database to incorporate data on the effects of food processing on pol-yphenol content. Database 2013, 2013, bat070.
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417.
- García-Barrado, M.J.; Iglesias-Osma, M.C.; Pérez-García, E.; Carrero, S.; Blanco, E.J.; Carretero-Hernández, M.; Carretero, J. Role of Flavonoids in the Interactions among Obesity, Inflammation, and Autophagy. Pharmaceuticals 2020, 13, 342.
- Manach, C.; Regerat, F.; Texier, O.; Agullo, G.; Demigne, C.; Remesy, C. Bioavailability, metabolism and physiological impact of 4-oxo-flavonoids. Nutr. Res. 1996, 16, 517–544.
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288.
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731.
- Nabavi, S.F.; Russo, G.L.; Daglia, M. Role of quercetin as an alternative for obesity treatment: You are what you eat! Food Chem. 2015, 179, 305–310.
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62.
- Dajas, F.; Rivera-Megret, F.; Blasina, F.; Arredondo, F.; Abin-Carriquiry, J.; Costa, G.; Echeverry, C.; Lafon, L.; Heizen, H.; Ferreira, M.; et al. Neuroprotection by flavonoids. Braz. J. Med Biol. Res. 2003, 36, 1613–1620.
- Zhang, M.; Swarts, S.G.; Yin, L.; Liu, C.; Tian, Y.; Cao, Y.; Swarts, M.; Yang, S.; Zhang, S.B.; Zhang, K.; et al. Anti-Oxidant Properties of Quercetin. In Oxygen Transport to Tissue XXXII. Advances in Experimental Medicine and Biology; LaManna, J., Puchowicz, M., Xu, K., Harrison, D., Bruley, D., Eds.; Springer: Boston, MA, USA, 2011; Volume 701.
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38.
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.; Flamm, G.; Williams, G.; Lines, T. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205.
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5.
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75.
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Al-gammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Phar-macokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374.
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxidative Med. Cell. Longev. 2016, 2016, 1–10.
- Zhai, S.-W.; Liu, S.-L. Effects of Dietary Quercetin on Growth Performance, Serum Lipids Level and Body Composition of Tilapia (Oreochromis Niloticus). Ital. J. Anim. Sci. 2013, 12, e85.
- Zhai, S.W.; Liu, S.L. Effects of dietary quercetin on the growth performance, digestive en-zymes and antioxidant potential in the hepatopancreas of tilapia (Oreochromis niloticus). Isr. J. Aquacult. Bamid. 2014, 66, 1–7.
- Kuipers, E.N.; Van Dam, A.D.; Held, N.M.; Mol, I.M.; Houtkooper, R.H.; Rensen, P.C.; Boon, M.R. Quercetin Lowers Plasma Triglycerides Accompanied by White Adipose Tissue Browning in Diet-Induced Obese Mice. Int. J. Mol. Sci. 2018, 19, 1786.
- Wang, M.; Xiao, F.L.; Mao, Y.J.; Ying, L.L.; Zhou, B.; Li, Y. Quercetin decreases the triglycer-ide content through the PPAR signalling pathway in primary hepatocytes of broiler chickens. Biotechnol. Biotechnol. Equip. 2019, 33, 1000–1010.
- Schadich, E.; Hlaváč, J.; Volná, T.; Varanasi, L.; Hajdúch, M.; Džubák, P. Effects of Ginger Phenylpropanoids and Quercetin on Nrf2-ARE Pathway in Human BJ Fibroblasts and HaCaT Keratinocytes. BioMed Res. Int. 2016, 2016, 1–6.
- Liu, X.; Yu, Z.; Huang, X.; Gao, Y.; Wang, X.; Gu, J.; Xue, S. Peroxisome proliferator- acti-vated receptor γ (PPARγ) mediates the protective effect of quercetin against myocardial ische-mia-reperfusion injury via suppressing the NF-κB pathway. Am. J. Transl. Res. 2016, 8, 5169–5186.
- Ay, M.; Luo, J.; Langley, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunc-tion and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson’s Disease. J. Neurochem. 2017, 141, 766–782.
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59.
- Jeong, E.; Lee, J.Y. Intrinsic and Extrinsic Regulation of Innate Immune Receptors. Yonsei Med. J. 2011, 52, 379–392.
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271.
- Sundaram, M.K.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; Haque, S.; Hussain, A. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci. Rep. 2019, 39, BSR20190720.
- Zhang, F.; Feng, J.; Zhang, J.; Kang, X.; Qian, D. Quercetin modulates AMPK/SIRT1/NF‑κB signaling to inhibit inflammatory/oxidative stress responses in diabetic high fat diet‑induced atherosclerosis in the rat carotid artery. Exp. Ther. Med. 2020, 20, 1.
- Hu, T.; Shi, J.J.; Fang, J.; Wang, Q.; Chen, Y.B.; Zhang, S.J. Quercetin ameliorates diabetic encephalopathy through SIRT1/ER stress pathway in db/db mice. Aging 2020, 12, 7015–7029.
- Martins-Perles, J.V.C.; Bossolani, G.D.P.; Zignani, I.; de Souza, S.R.G.; Frez, F.C.V.; de Souza Melo, C.G.; Barili, E.; de Souza Neto, F.P.; Guarnier, F.A.; Armani, A.L.C.; et al. Quercetin increases bioavailability of nitric oxide in the jejunum of euglycemic and diabetic rats and induces neuronal plasticity in the myenteric plexus. Auton. Neurosci. 2020, 227, 102675.
- Dong, F.; Wang, S.; Wang, Y.; Yang, X.; Jiang, J.; Wu, D.; Qu, X.; Fan, H.; Yao, R. Quercetin ameliorates learning and memory via the Nrf2-ARE signaling pathway in d-galactose-induced neurotoxicity in mice. Biochem. Biophys. Res. Commun. 2017, 491, 636–641.
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.S.; Niaz, K. Molecular mechanisms un-derlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019, 224, 109–119.
- Kyaw, M.; Yoshizumi, M.; Tsuchiya, K.; Izawa, Y.; Kanematsu, Y.; Tamaki, T. Atheroprotective effects of antioxidants through inhibition of mitogen-activated protein kinases. Acta Pharmacol. Sin. 2004, 25, 977–985.
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445.
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185.
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432.
- Schnare, M.; Barton, G.M.; Holt, A.C.; Takeda, K.; Akira, S.; Medzhitov, R. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2001, 2, 947–950.
- Jagannathan, M.; Hasturk, H.; Liang, Y.; Shin, H.; Hetzel, J.T.; Kantarci, A.; Rubin, D.; McDonnell, M.E.; Van Dyke, T.E.; Ganley-Leal, L.M.; et al. TLR Cross-Talk Specifically Regulates Cytokine Production by B Cells from Chronic Inflammatory Disease Patients. J. Immunol. 2009, 183, 7461–7470.
- Kawai, T.; Akira, S. Signaling to NF-kappaB by toll-like receptors. Trends Mol Med. 2007, 13, 460–469.
- Panaro, M.A.; Corrado, A.; Benameur, T.; Cantatore, F.P.; Cici, D.; Porro, C. The emerging role of curcumin in the modulation of TLR-4 signaling pathway: Focus on neuroprotective and an-ti-rheumatic properties. Int. J. Mol. Sci. 2020, 21, 2299.
- Wu, M.; Liu, F.; Guo, Q. Quercetin attenuates hypoxia-ischemia induced brain injury in ne-onatal rats by inhibiting TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2019, 74, 105704.
- Cox, L.M.; Weiner, H.L. Microbiota signaling pathways that influence neurologic dis-ease. Neurotherapeutics 2018, 15, 135–145.
- Xie, J.; Song, W.; Liang, X.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X. Protective effect of quercetin on streptozotocin-induced diabetic peripheral neuropathy rats through modulating gut microbiota and reactive oxygen species level. Biomed. Pharmacother. 2020, 127, 110147.
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426.
- Vomhof-Dekrey, E.E.; Picklo Sr, M.J. The Nrf2-antioxidant response element pathway: A target for regulating energy metabolism. J. Nutr. Biochem. 2012, 23, 1201–1206.
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295.
- Sun, Y.; Yang, T.; Leak, R.K.; Chen, J.; Zhang, F. Preventive and Protective Roles of Dietary Nrf2 Activators against Central Nervous System Diseases. CNS Neurol. Disord. Drug Targets 2017, 16, 326–338.
- Gan, L.; Johnson, J.A. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim. Biophys. Acta 2014, 1842, 1208–1218.




