
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Miguel A. González-Fuentes | + 1530 word(s) | 1530 | 2021-04-09 04:56:51 | | | |
| 2 | Camila Xu | Meta information modification | 1530 | 2021-04-21 08:33:31 | | |
Video Upload Options
SERS (Surface-enhanced Raman spectroscopy) is based on the amplification of the Raman response of an analyte interacting with the surface plasmon of metals such as Au, Ag, or Cu; in some cases, the response results enough to achieve the single–molecule detection
1. Mechanisms of SERS
SERS is based on the amplification of the Raman response of an analyte interacting with the surface plasmon of metals such as Au, Ag, or Cu; in some cases, the response results enough to achieve the single–molecule detection [1]. Paradoxically, after 46 years since the discovery of SERS, the exact mechanism in the signal enhancement is still a matter of debate, but it is generally accepted to be driven mainly by two principles: the electromagnetic (EM) and the chemical (CHEM) effects.
The EM mechanism is the most understood; it comes from the substrate and is originated when a free–electron–like metal is irradiated with a laser whose frequency is resonant with the frequency resulting from the collective oscillation of conduction band electrons. This phenomenon is known as surface plasmon resonance (SPR). Especially in some regions called hot spots, an intense local field enhancement is produced around the interface of the metal by the concentration of light, which creates an oscillating dipole on the molecules in close proximity with the nanoparticles. This results in an oscillating dipole that enhances the radiation efficiency (Figure 1) [2]. In this situation, the magnitude of the electromagnetic field between the nanoparticle center and the analyte decays with the distance (R) as ~ (r/R)12, where r is the nanoparticle radius[3]. This expression indicates a narrow optimum distance (no more than 2 nm) where the maximum enhancement is obtained [4]. Therefore, to obtain the maximum enhancement by this principle (about eight orders of magnitude), the physical interaction between the analyte and the substrate or the specific adsorption of a small size analyte is required[5] Considering the analyte–substrate affinity, the EM enhancement is the common denominator in the literature to address the sensitivity of the SERS experiments, by either increasing the highly localized regions of large field enhancement (hot spots), changing the morphology and size of the nanoparticle, or controlling the interparticle spacing or aggregation [6]. Despite the great advances in this area, the substrates present low uniformity due to the lack of control in the generation of hot spots, which occurs even at the same substrate. Likewise, they present low stability over time because of the inherent oxidation of the most common plasmonic metals. The above circumstances result in a serious impediment for the practical applications of SERS[7][8].
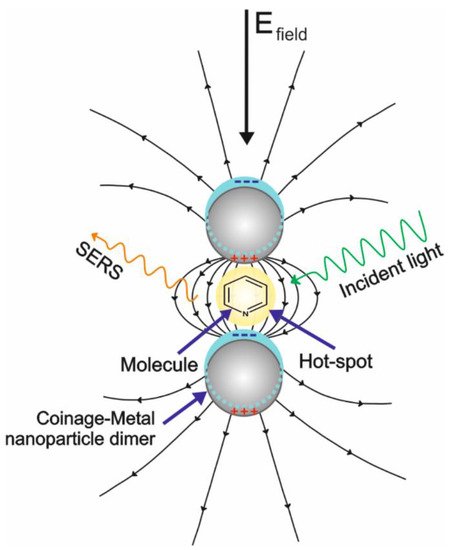
Figure 1. Schematic representation of the surface-enhanced Raman scattering (SERS) from the electromagnetic (EM) effect.
The chemical mechanism (CHEM) involves the intrinsic features of the adsorbate and the new properties arising when the adsorbate is combined with the substrate (adsorbate–metal nanostructure complex) under the effect of the incident light. The plasmonic nature, substrate roughness or nanoparticulate structure is not mandatory for the CHEM to occur, or the or as; instead, the experimental conditions as well as the adsorbate orientation and symmetry on the surface have influence [8]. Thus, the CHEM mechanism comes from different origins, some of those scarcely understood, and it is limited to occur within the first layer of absorbed molecules (short–range interactions) [9]. So far, the main known contributions of the CHEM mechanism result from the charge transfer (CT) between the metal and the target molecule, or vice versa, with a Raman enhancement of about four orders of magnitude. CT is categorized as a resonant effect and can be driven by the imposition of an electrical potential [10]. During chemisorption, new electronic states are generated due to the overlap of the wave functions of the molecule and the plasmonic metal, allowing resonant intermediate states that arise from the introduction of new mixed charge–transfer states in the ligand–metal complex. If the energy gap between the Fermi level of the metal (Ef) and the electronic level of the molecule (Em) matches the energy of the photon (Ef) coming from the laser source, the transfer of electrons occurs and a maximum Raman intensity is observed in particular modes [11]. Other source for CHEM comes from a non–resonant effect (static charge transfer) by the adsorption process of the molecule in its electronic ground state. Under this situation, the formation of a new electronic state due to the adsorbate–adsorbent interaction does not occur [1]; instead, only a renormalization of the molecular orbitals is produced, causing modifications on the molecular polarizability [12].
A special assisted contribution to the total enhancement of the Raman signal is the resonance Raman scattering on SERS substrates (SERRS) that comprises the SERS–CT contribution. This occurs when the excitation laser is tuned into an electronic absorption energy of the adsorbed molecule. Raman bands related to the chromophore can be enhanced up to six orders of magnitude compared to the EM mechanism [13].
2. SERS Substrates
SERS substrates can be made of rough surface metals, as well as nanoparticles in suspension or deposited on solid substrates [14]. The use of colloids as SERS substrates is widely spread because of their ease synthesis with good size and morphology control, in addition to the possibility of studying absorption phenomena in aqueous environments [15]. However, potential applications are restricted by the poor stability and reproducibility due to the Brownian motion of particles in the suspension that causes a permanent change in the measurement scenario. Immobilization of the nanoparticles on a solid substrate, usually silicon, provides a significant increase in the reproducibility of the enhancement effect; however, the current research in this area consists in the reproduction of the same substrate surface to increase the repeatability of the spectroscopic measurements [16]. Among the most efficient techniques to obtain organized metallic nanostructure arrays on solid substrates for SERS we can find lithography and its indirect techniques (electron beam lithography, laser interference lithography, UV photolithography, electro–oxidative lithography) as well as block copolymer self–assembly [17].
3. SERS Measurements on Solid Substrates
SERS measurements on solid substrates can be made in the dry or wet forms [8]. The dry form is more common and is carried out after evaporation of the sample on the substrate. Evaporation process can be preceded by the immersion of the substrate into the sample for a certain period (incubation process) [18]. Wet experiments can be made using sophisticated microfluidic systems (LoC–SERS), or on a drop of the liquid sample placed on the SERS substrate which has been covered with a thin coverslip. It can be also carried out by using the “drop technique”, which consists on the signal acquisition on the periphery of a drop of sample placed on the SERS substrate [19]. Nevertheless, these methodologies present drawbacks that influence the final SERS results. In the case of the evaporative procedure, the adsorption process can be abruptly interrupted during the sample evaporation, inducing the heterogeneity in the formed layer or the “coffee–ring” effect [20]. In addition, oxidation or dissolution of the metallic nanoparticles may occur during the incubation processes, together with the loss of molecular perpendicular arrangements during the Raman measurements. On liquid media, the use of another interface or the influence of different refractive indexes when using a coverslip gives an inefficient Raman signal. Concerning the “drop technique”, it has the advantage that a proper change in the substrate hydrophobicity can lead to the analyte pre-concentration, thus avoiding the diffusion limitation and enhancing the SERS signal [21][22]. However, a camera in the Raman spectrometer is necessary to identify the optimal distance from the periphery to the center of the drop where the measurement must be recorded. This distance must be sufficient to avoid the effects from evaporation of the sample at the chosen spot during the experiment but short enough to obtain a good response from the substrates.
4. Detection of Analytes
Detection of analytes by SERS can be conducted by the direct or indirect approaches[2]. The direct measurement is accomplished with the analyte adsorbed on the substrate, or when the analyte is retained close enough to the substrate using molecular linkers or capture elements such as antibodies, aptamers, or related molecules immobilized onto nanostructured surfaces. This method is recommended for analytes that present high Raman scattering cross–section. It has the advantages of a major control and accuracy of the quantification process and the possibility of the identification and chemical characterization of the analyte from the study of its vibrational features. The indirect detection correlates the SERS spectrum changes of a metabolite, reaction product, or reporter molecule (RM) with the concentration of the target analyte [19]. With this methodology, analytes with low or null Raman vibration modes can be detected, and multiplexed detection has been achieved [ 23 ]. The use of reporter molecules is the most common way to address the indirect detection in biological samples. It consists in the functionalization of the substrates with one or several molecules (monoplexed or multiplexed detection, respectively), that present a change in their Raman cross–sections due to the interaction with the target analyte. RMs usually are small in size and present high Raman cross–sections. In addition, they are characterized by a narrow Raman spectrum or very few of their bands are superimposed with those from the matrix or the analyte spectra, and are photochemically stable [ 24 ]. .
References
- Demirel, G.; Usta, H.; Yilmaz, M.; Celik, M.; Alidagi, H.A.; Buyukserin, F. Surface–enhanced Raman spectroscopy (SERS): an adventure from plasmonic metals to organic semiconductors as SERS platforms. J. Mater. Chem. C 2018, 6, 5314–5335.
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface–enhanced Raman scattering. Biosensors 2019, 9, 57.
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Duyne, R.P.V. Surface–Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626.
- Moore, T.J.; Moody, A.S.; Payne, T.D.; Sarabia, G.M.; Daniel, A.R.; Sharma, B. In vitro and in vivo SERS biosensing for disease diagnosis. Biosensors 2018, 8, 46.
- Panneerselvam, R.; Liu, G.–K.; Wang, Y.–H.; Liu, J.–Y.; Ding, S.–Y.; Li, J.–F.; Wu, D.–Y.; Tian, Z.–Q. Surface–enhanced Raman spectroscopy: bottlenecks and future directions. Chem. Commun. 2018, 54, 10–25.
- Lin, X.–M.; Cui, Y.; Xu, Y.–H.; Ren, B.; Tian, Z.–Q. Surface–enhanced Raman spectroscopy: substrate–related issues. Anal. Bioanal. Chem. 2009, 394, 1729–1745.
- Zheng, Y.; Rosa, L.; Thibaut, T.; Ng, S.H.; Saulius, J.; Bach, U. Phase controlled SERS enhancement. Sci. Rep. 2019, 9, 1–9.
- Pérez–Jiménez, A. I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface–enhanced Raman spectroscopy: benefits, trade–offs and future developments. Chem. Sci. 2020, 11, 4563–4577.
- Kim, J.; Jang, Y.; Kim, N.–J.; Kim, H.; Yi, G.–C.; Shin, Y.; Kim, M.H.; Yoon, S. Study of chemical enhancement mechanism in nonplasmonic surface enhanced Raman spectroscopy (SERS). Front. Chem. 2019, 7, 582.
- Cui, L.; Wu, D.–Y.; Wang, A.; Ren, B.; Tian, Z.–Q. Charge–transfer enhancement involved in the SERS of adenine on Rh and Pd demonstrated by ultraviolet to visible laser excitation. J. Phys. Chem. C 2010, 114, 16588–16595.
- Aranda, D.; Valdivia, S.; Avila, F.J.; Soto, J.; Otero, J.C.; López–Tocón, I. Charge transfer at the nanoscale and the role of the out–of–plane vibrations in the selection rules of surface–enhanced Raman scattering. Phys. Chem. Chem. Phys. 2018, 20, 29430–29439.
- Morton, S.M.; Silverstein, D.W.; Jensen, L. Theoretical studies of plasmonics using electronic structure methods. Chem. Rev. 2011, 111, 3962–3994.
- Murgida, D.H.; Hildebrandt, P. Heterogeneous electron transfer of cytochrome C on coated silver electrodes. Electric field effects on structure and redox potential. J. Phys. Chem. B 2001, 105, 1578–1586.
- Mosier–Boss, P. A. Review of SERS substrates for Chem. sensing. Nanomaterials 2017, 7, 142.
- Zhao, X.; Deng, M.; Rao, G.; Yan, Y.; Wu, C.; Jiao, Y.; Deng, A.; Yan, C.; Huang, J.; Wu, S.; et al. High‐Performance SERS Substrate Based on Hierarchical 3D Cu Nanocrystals with Efficient Morphology Control. Small 2018, 14, 1802477.
- Yang, L.; Bao, Z.; Wu, Y.; Liu, J. Clean and reproducible SERS substrates for high sensitive detection by solid phase synthesis and fabrication of Ag‐coated Fe3O4 microspheres. J. Raman Spectrosc. 2012, 43, 848–856.
- Suresh, V.; Ding, L.; Chew, A. B.; Yap, F. L. Fabrication of large–area flexible SERS substrates by nanoimprint lithography. ACS Appl. Nano Mater. 2018, 1, 886–893.
- Martínez–Torres, P. G.; Martínez–García, M. M.; Cardoso–Ávila, P. E.; Pichardo–Molina, J. L. Facile nanostructured substrate preparation using gold nanocuboids for SERS. Nanomater. Nanotechnol. 2015, 5, 5–12.
- López–Castaños, K. A.; Ortiz–Frade, L. A.; Méndez, E.; Quiroga–González, E.; González–Fuentes, M. A.; Méndez–Albores, A. Indirect Quantification of Glyphosate by SERS Using an Incubation Process With Hemin as the Reporter Molecule: A Contribution to Signal Amplification Mechanism. Front. Chem. 8, 1209.
- Jahn, I.; Žukovskaja, O.; Zheng, X.–S.; Weber, K.; Bocklitz, T.; Cialla–May, D.; Popp, J. Surface–enhanced Raman spectroscopy and microfluidic platforms: challenges, solutions and potential applications. Analyst 2017, 142, 1022–1047.
- Grilli, S.; Miccio, L.; Gennari, O.; Coppola, S.; Vespini, V.; Battista, L.; Orlando, P.; Ferraro, P. Active accumulation of very diluted biomolecules by nano–dispensing for easy detection below the femtomolar range. Nat. Commun. 2014, 5, 5314.
- Kumar, S.; Cherukulappurath, S.; Johnson, T.W.; Oh, S.H. Milimeter–sized suspended plasmonic nanohole arrays for surface–tension–driven flow–through SERS. Chem. Mater. 2014, 26, 6523–6530.
- Xu, M.–L.; Gao, Y.; Li, Y.; Li, X.; Zhang, H.; Han, X.X.; Zhao, B.; Su, L. Indirect glyphosate detection based on ninhydrin reaction and surface–enhanced Raman scattering spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 197, 78–82.
- Kearns, H.; Shand, N.; Smith, W.; Faulds, K.; Graham, D. 1064 nm SERS of NIR active hollow gold nanotags. Phys. Chem. Chem. Phys. 2015, 17, 1980–1986.





