
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Keiichi Matsubara | + 2272 word(s) | 2272 | 2021-04-06 10:16:52 | | | |
| 2 | Lily Guo | Meta information modification | 2272 | 2021-04-20 05:10:54 | | |
Video Upload Options
The pathogenesis of preeclampsia begins when a fertilized egg infiltrates the decidua, resulting in implantation failure (e.g., due to extravillous trophoblast infiltration disturbance and abnormal spiral artery remodeling). Thereafter, large amounts of serum factors (e.g., soluble fms-like tyrosine kinase 1 and soluble endoglin) are released into the blood from the hypoplastic placenta, and preeclampsia characterized by multiorgan disorder caused by vascular disorders develops. Successful implantation and placentation require immune tolerance to the fertilized egg as a semi-allograft and the stimulation of extravillous trophoblast infiltration. Recently, exosomes with diameters of 50–100 nm have been recognized to be involved in cell–cell communication. Exosomes affect cell functions in autocrine and paracrine manners via their encapsulating microRNA/DNA and membrane-bound proteins. The microRNA profiles of blood exosomes have been demonstrated to be useful for the evaluation of preeclampsia pathophysiology and prediction of the disease. In addition, exosomes derived from mesenchymal stem cells have been found to have cancer-suppressing effects. These exosomes may repair the pathophysiology of preeclampsia through the suppression of extravillous trophoblast apoptosis and promotion of these cells’ invasive ability. Exosomes secreted by various cells have received much recent attention and may be involved in the maintenance of pregnancy and pathogenesis of preeclampsia.
1. Introduction
Preeclampsia (PE) is a hypertensive disorder of pregnancy associated with renal and/or liver dysfunction with poor perinatal outcomes, including maternal and neonatal mortality [1]. The etiology of PE has been explained with the “two-stage theory” (Figure 1) [2].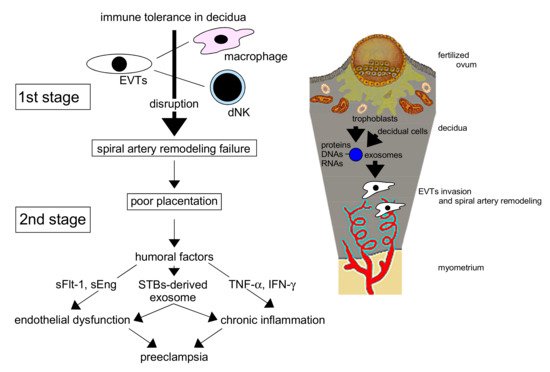 Figure 1. The interaction of decidual cell– and trophoblast-derived exosomes creates a favorable microenvironment for extravillous trophoblasts (EVTs) in the uterine endometrium, which promotes the invasion of EVTs and the remodeling of spiral arteries for adequate placentation in normal pregnancy. Disturbance of the remodeling in the first stage leads to poor placentation, resulting in preeclampsia pathophysiology in the second stage by placenta-derived humoral factors. dNK: decidual natural killer cell, sFlt-1: soluble fms-like tyrosine kinase 1, sEng: soluble endoglin, STBs: syncytiotrophoblasts, TNF-α: tumor necrosis factor-α, IFN-γ: interferon-γ.In normal pregnancy, appropriate placentation is critical. Extravillous trophoblasts (EVTs) from the cytotrophoblast (CBT) column of a fertile ovum invade the maternal uterine decidua and myometrium, resulting in spiral arterial remodeling to supply abundant maternal blood to the placental intervillous space for the maintenance of fetal growth. This remodeling results in the loss of vascular smooth muscle, leading to relaxation of the spiral artery and marked dilation of the vessel lumens to maintain abundant blood flow for the embryo.In PE, however, placental perfusion is reduced in early pregnancy when the invasion of EVTs is disturbed, resulting in reduced spiral artery remodeling. EVTs cannot sufficiently invade the decidua and myometrium, the spiral arteries remain narrow, and placental neovascularization is disturbed. As a result, placentation is shallow, leading to reduced placental perfusion (poor placentation). In mid–late pregnancy, the reduced perfusion with disturbed neovascularization [3], posited as secondary to failed remodeling of the maternal vessels supplying the intervillous space, is not sufficient to maintain normal pregnancy. Reduced placental perfusion and vascular endothelial injury result in the increased production of humoral factors that injure or activate endothelial cells (ECs), including inflammatory humoral factors, in the uteroplacental circulation [4]. These factors are introduced into the systemic circulation and affect many maternal organs. Trophoblast-derived products may cause PE through EC damage and dysfunction [5], the latter additionally resulting in the stimulation of vascular sensitivity to endogenous pressors and vascular permeability [6]. These products can damage the maternal liver, kidney, eyes, brain, blood vessels, and lungs, placing the woman in mortal danger due to potential multiorgan failure. Various humoral factors and modifiers in the peripheral blood have been reported to underlie the pathology of PE, and the clinical manifestation of this condition varies. The pathophysiology of PE is also modified by maternal genetic and environmental factors. To improve the perinatal prognosis for PE, study of its pathogenesis, prediction [4], prevention [7], and treatment is important.Rolnik et al. [7] reported that the measurement of blood flow in the uterine artery and the concentrations of pregnancy-associated plasma protein A and placental growth factor (PlGF) is useful for PE prediction, and that aspirin (150 mg/day) significantly reduced the incidence of early-onset PE relative to placebo (odds ratio = 0.38) in a high-risk group. However, the pathogenesis of PE needs to be evaluated in more detail to enable more accurate prediction and prevention of this disorder. The identification of women at high risk of PE before its onset is especially important. Recently, microRNAs (miRNAs) and proteins wrapped in an exosome, a subtype of extracellular small vesicle (20–130 nm), were found to be secreted from the placenta into the systemic circulation, resulting in multisystemic organ damage, in patients with PE [8]. As trophoblast-derived exosomes in preeclamptic placentas are thought to have high concentrations of PE-specific contents, the evaluation of exosomes in the peripheral blood of patients with PE is considered to be important. To improve the prognosis, prevention, and treatment of PE, humoral factors, including exosomes, produced from early pregnancy need to be investigated.
Figure 1. The interaction of decidual cell– and trophoblast-derived exosomes creates a favorable microenvironment for extravillous trophoblasts (EVTs) in the uterine endometrium, which promotes the invasion of EVTs and the remodeling of spiral arteries for adequate placentation in normal pregnancy. Disturbance of the remodeling in the first stage leads to poor placentation, resulting in preeclampsia pathophysiology in the second stage by placenta-derived humoral factors. dNK: decidual natural killer cell, sFlt-1: soluble fms-like tyrosine kinase 1, sEng: soluble endoglin, STBs: syncytiotrophoblasts, TNF-α: tumor necrosis factor-α, IFN-γ: interferon-γ.In normal pregnancy, appropriate placentation is critical. Extravillous trophoblasts (EVTs) from the cytotrophoblast (CBT) column of a fertile ovum invade the maternal uterine decidua and myometrium, resulting in spiral arterial remodeling to supply abundant maternal blood to the placental intervillous space for the maintenance of fetal growth. This remodeling results in the loss of vascular smooth muscle, leading to relaxation of the spiral artery and marked dilation of the vessel lumens to maintain abundant blood flow for the embryo.In PE, however, placental perfusion is reduced in early pregnancy when the invasion of EVTs is disturbed, resulting in reduced spiral artery remodeling. EVTs cannot sufficiently invade the decidua and myometrium, the spiral arteries remain narrow, and placental neovascularization is disturbed. As a result, placentation is shallow, leading to reduced placental perfusion (poor placentation). In mid–late pregnancy, the reduced perfusion with disturbed neovascularization [3], posited as secondary to failed remodeling of the maternal vessels supplying the intervillous space, is not sufficient to maintain normal pregnancy. Reduced placental perfusion and vascular endothelial injury result in the increased production of humoral factors that injure or activate endothelial cells (ECs), including inflammatory humoral factors, in the uteroplacental circulation [4]. These factors are introduced into the systemic circulation and affect many maternal organs. Trophoblast-derived products may cause PE through EC damage and dysfunction [5], the latter additionally resulting in the stimulation of vascular sensitivity to endogenous pressors and vascular permeability [6]. These products can damage the maternal liver, kidney, eyes, brain, blood vessels, and lungs, placing the woman in mortal danger due to potential multiorgan failure. Various humoral factors and modifiers in the peripheral blood have been reported to underlie the pathology of PE, and the clinical manifestation of this condition varies. The pathophysiology of PE is also modified by maternal genetic and environmental factors. To improve the perinatal prognosis for PE, study of its pathogenesis, prediction [4], prevention [7], and treatment is important.Rolnik et al. [7] reported that the measurement of blood flow in the uterine artery and the concentrations of pregnancy-associated plasma protein A and placental growth factor (PlGF) is useful for PE prediction, and that aspirin (150 mg/day) significantly reduced the incidence of early-onset PE relative to placebo (odds ratio = 0.38) in a high-risk group. However, the pathogenesis of PE needs to be evaluated in more detail to enable more accurate prediction and prevention of this disorder. The identification of women at high risk of PE before its onset is especially important. Recently, microRNAs (miRNAs) and proteins wrapped in an exosome, a subtype of extracellular small vesicle (20–130 nm), were found to be secreted from the placenta into the systemic circulation, resulting in multisystemic organ damage, in patients with PE [8]. As trophoblast-derived exosomes in preeclamptic placentas are thought to have high concentrations of PE-specific contents, the evaluation of exosomes in the peripheral blood of patients with PE is considered to be important. To improve the prognosis, prevention, and treatment of PE, humoral factors, including exosomes, produced from early pregnancy need to be investigated.
2. Humoral Factors Related to the Pathogenesis of PE
PE is characterized by chronic inflammation beginning early in pregnancy, with leukocyte activation and high serum levels of cytokines [9][10]. Tumor necrosis factor-α (TNF-α), a monocyte-derived cytotoxic protein, can induce vascular activation and dysfunction via the activation of leukocytes and the induction of vascular endothelial adhesion molecules. In the setting of chronic inflammation, increased TNF-α levels in early pregnancy may stimulate the expression of intercellular adhesion molecule-1 (ICAM-1) on vascular ECs and trophoblasts, thereby activating them. Essentially, ECs mediate vascular homeostasis and regulate the coagulation cascade [11] through the maintenance of vascular tone and permeability [12][13][14]. Chronic inflammation also activates lymphocyte function-associated antigen-1 on leukocytes, resulting in disturbed remodeling of spiral arteries with EC and trophoblast activation. Such activation of ECs leads to vascular dysfunction, resulting in PE (Figure 2).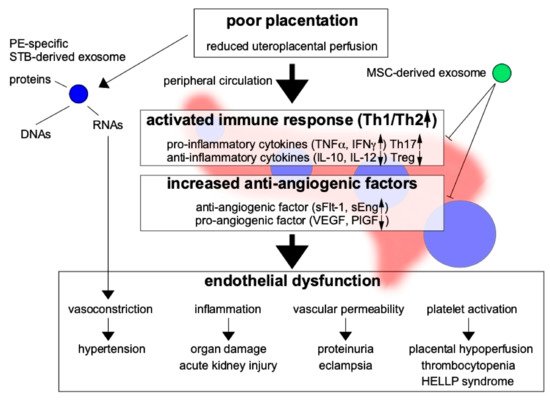 Figure 2. Poor placentation secrets exosomes and promotes immune reactivity and anti-angiogenic factors resulted in increased proinflammatory cytokines and anti-angiogenic factors. On the other hand, anti-inflammatory cytokines and pro-angiogenic factors are decreased. Preeclampsia (PE)-specific exosomes derived from damaged trophoblasts are secreted and may transport RNA, DNA, and proteins to distant maternal organs, causing multiple-organ failure, especially due to endothelial dysfunction. The pathogenesis of PE may be ameliorated by the immunosuppressive and anti-inflammatory effects of mesenchymal stem cell (MSC)-derived exosomes. Th17: type-17 T helper cell, Treg: regulatory T cell, VEGF: vascular endothelial growth factor, PlGF: placental growth factor. Blue circle: pathogenic exosomes, Green circle: exosomes with therapeutic potential.In normal pregnancy, the fertile ovum is protected by immune tolerance, resulting in no dysfunction of ECs, trophoblasts, and leukocytes, and no inflammation. For this reason, EVTs can invade the uterine decidua and myometrium, resulting in spiral arterial remodeling. To maintain immunotolerance at the fetal–maternal interface, interaction of human leukocyte antigen (HLA)-G with decidual natural killer (NK) cells [15][16][17], between dendritic cells (DCs) and regulatory T (Treg) cells [18], and of cytokines secreted by uterine NK cells [19] in the decidua through reduction of the maternal immune response is needed. However, immune tolerance to fetal antigens, such as trophoblasts, is impaired in PE. Impaired remodeling of the spiral artery could reduce subsequent placentation, resulting in impaired fetal growth due to poor uteroplacental circulation. In the beginning of PE pathogenesis, trophoblasts stimulate the maternal immune response, leading to chronic inflammation. In turn, serum levels of cytokines, especially proinflammatory cytokines such as TNF-α, are significantly increased in early pregnancy by the active immune response; such increases may have predictive value for PE development [20].Furthermore, in PE, type-1 T helper (Th1) cells are known to be dominant [21] and can secrete proinflammatory cytokines, such as TNF-α, interferon-γ (IFN-γ), and interleukin (IL)-6. Th17 cells also can secrete the proinflammatory cytokine IL-17. The serum TNF-α concentration is reportedly increased even before the onset of PE [20][22].Chronic inflammation is also related to oxidative stress, an important humoral factor generated by poor placental perfusion in PE [23]. Oxidative stress stimulates the adhesion of leukocytes to the vascular endothelium and the release of cytokines and antiangiogenic factors. Soluble adhesion molecules, such as soluble E-selectin and soluble ICAM-1, are increased in blood collected early in pregnancy from women who subsequently develop PE [20], suggesting that inflammation plays an important role in the first step of PE pathogenesis. The reactive oxygen species level, which is increased during inflammation, was also found to be increased in early pregnancy in women who subsequently developed PE [24].Abundant angiogenesis at the implantation site in early pregnancy is critical for placental development, with reduced uterine vascular resistance allowing the supply of plenty of maternal blood to the placenta. The disturbance of angiogenesis leads to vascular dysfunction, resulting in poor placentation, and is a main cause of PE [3][4]. Increased levels of antivascular growth factors (e.g., soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sEng)) in the maternal circulation are believed to be important in the pathogenesis of PE, as they reduce angiogenesis in the placenta, and are known to be present even before the onset of PE [25]. Such factors are also involved in the pathogenesis of PE, as they decrease the PlGF level, resulting in poor angiogenesis during placentation.As many humoral factors are known to be involved in the pathogenesis of PE, the measurement of these factors in the maternal peripheral blood might enable the prediction of PE occurrence. At present, quantification of the serum levels of the antiangiogenic factor sFlt-1 and the angiogenic factor PlGF is the most widespread means of predicting PE development. However, the many methods used currently to predict the occurrence of PE are not adequately sensitive. Recently, exosomes have been identified as important predictive factors for PE; they have been reported to transport various humoral factors (including RNA) to distant organs and are thought to play an important role in PE-related systemic organ damage [21].
Figure 2. Poor placentation secrets exosomes and promotes immune reactivity and anti-angiogenic factors resulted in increased proinflammatory cytokines and anti-angiogenic factors. On the other hand, anti-inflammatory cytokines and pro-angiogenic factors are decreased. Preeclampsia (PE)-specific exosomes derived from damaged trophoblasts are secreted and may transport RNA, DNA, and proteins to distant maternal organs, causing multiple-organ failure, especially due to endothelial dysfunction. The pathogenesis of PE may be ameliorated by the immunosuppressive and anti-inflammatory effects of mesenchymal stem cell (MSC)-derived exosomes. Th17: type-17 T helper cell, Treg: regulatory T cell, VEGF: vascular endothelial growth factor, PlGF: placental growth factor. Blue circle: pathogenic exosomes, Green circle: exosomes with therapeutic potential.In normal pregnancy, the fertile ovum is protected by immune tolerance, resulting in no dysfunction of ECs, trophoblasts, and leukocytes, and no inflammation. For this reason, EVTs can invade the uterine decidua and myometrium, resulting in spiral arterial remodeling. To maintain immunotolerance at the fetal–maternal interface, interaction of human leukocyte antigen (HLA)-G with decidual natural killer (NK) cells [15][16][17], between dendritic cells (DCs) and regulatory T (Treg) cells [18], and of cytokines secreted by uterine NK cells [19] in the decidua through reduction of the maternal immune response is needed. However, immune tolerance to fetal antigens, such as trophoblasts, is impaired in PE. Impaired remodeling of the spiral artery could reduce subsequent placentation, resulting in impaired fetal growth due to poor uteroplacental circulation. In the beginning of PE pathogenesis, trophoblasts stimulate the maternal immune response, leading to chronic inflammation. In turn, serum levels of cytokines, especially proinflammatory cytokines such as TNF-α, are significantly increased in early pregnancy by the active immune response; such increases may have predictive value for PE development [20].Furthermore, in PE, type-1 T helper (Th1) cells are known to be dominant [21] and can secrete proinflammatory cytokines, such as TNF-α, interferon-γ (IFN-γ), and interleukin (IL)-6. Th17 cells also can secrete the proinflammatory cytokine IL-17. The serum TNF-α concentration is reportedly increased even before the onset of PE [20][22].Chronic inflammation is also related to oxidative stress, an important humoral factor generated by poor placental perfusion in PE [23]. Oxidative stress stimulates the adhesion of leukocytes to the vascular endothelium and the release of cytokines and antiangiogenic factors. Soluble adhesion molecules, such as soluble E-selectin and soluble ICAM-1, are increased in blood collected early in pregnancy from women who subsequently develop PE [20], suggesting that inflammation plays an important role in the first step of PE pathogenesis. The reactive oxygen species level, which is increased during inflammation, was also found to be increased in early pregnancy in women who subsequently developed PE [24].Abundant angiogenesis at the implantation site in early pregnancy is critical for placental development, with reduced uterine vascular resistance allowing the supply of plenty of maternal blood to the placenta. The disturbance of angiogenesis leads to vascular dysfunction, resulting in poor placentation, and is a main cause of PE [3][4]. Increased levels of antivascular growth factors (e.g., soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sEng)) in the maternal circulation are believed to be important in the pathogenesis of PE, as they reduce angiogenesis in the placenta, and are known to be present even before the onset of PE [25]. Such factors are also involved in the pathogenesis of PE, as they decrease the PlGF level, resulting in poor angiogenesis during placentation.As many humoral factors are known to be involved in the pathogenesis of PE, the measurement of these factors in the maternal peripheral blood might enable the prediction of PE occurrence. At present, quantification of the serum levels of the antiangiogenic factor sFlt-1 and the angiogenic factor PlGF is the most widespread means of predicting PE development. However, the many methods used currently to predict the occurrence of PE are not adequately sensitive. Recently, exosomes have been identified as important predictive factors for PE; they have been reported to transport various humoral factors (including RNA) to distant organs and are thought to play an important role in PE-related systemic organ damage [21].
3. Exosomes
Extracellularly secreted vesicles are known to be involved in cell–cell communication [26], immunity regulation [26][27], and cancer growth [28]. Extracellular vesicles (EVs) have lipid bilayer membranes that surround specific cargos of biomolecules [29]. The membranous vesicles are categorized as exosomes, shedding microvesicles (SMVs), and apoptotic bodies (ABs) based on the modes of biogenesis and release. SMVs are large vesicles ranging from 100–1000 nm in diameter [30] that are released from the plasma membrane through processes such as budding. They are thought to contain high concentrations of matrix metalloproteinases (MMPs) [31], P-selectin [32], and Mac-1 [33], according to cell characteristics. ABs (50–5000 nm in diameter) are heterogeneous vesicles that are released from dying cells, causing apoptosis [34][35]. This process selectively removes aged, damaged, infected, and aberrant cells to maintain healthy tissues; it dismantles cells, and the cellular debris is packed into ABs. Thus, ABs may be involved in cell dismantling and recycling of biomolecular building blocks.Exosomes (50–100 nm in diameter) are secreted by most cells and are involved in antigen presentation and the transportation of various substances (e.g., messenger RNA (mRNA), miRNA [36], DNA [37], and proteins [38]) to distant organs while avoiding immune response stimulation. They are thought to modify the functions of various organs [39][40]. The exosome membrane is a lipid bilayer that contains cholesterol and sphingomyelin [41] and expresses many types of protein [42].Although EVTs do not metastasize in the way that malignant tumors do, their autonomous cell proliferation, invasion, and ability to form their own nutrient vessels are similar to those of cancer cells. Cancer cells are known to secrete more exosomes than normal cells [28]. Exosomes can be endocytosed in or interact with recipient cells [43], leading to their involvement in cancer growth and metastasis [44]. Cancer cell–derived exosomes are known to inhibit the function of immune cells that attack cancer cells by causing other cells to act on various substances [45], promoting cancer cell proliferation and vascular EC migration to attract blood into tumors. Yang et al. [45] reported that programmed death ligand-1 (PD-L1)–containing exosomes suppressed immune activity against tumor cells, which suggests that exosomes secreted by cancer cells create an environment conducive to cancer cell growth. The conjugation of PD-L1 and PD-L suppresses T-cell activity by decreasing the production of proinflammatory cytokines [46]. This immune checkpoint signal allows cancer cells to resist the host immune response. Such immune tolerance is also necessary for pregnancy establishment, and the same phenomenon may occur in trophoblasts from the beginning of pregnancy through placentation.The analysis of exosomes in blood, where they are abundant, is thus a less invasive and sensitive means of diagnosing cancer. This is also true for the analysis of trophoblasts in pregnancy, as these cells proliferate and migrate to create a suitable environment for the fetus. Embryo- and decidua-derived exosomes are thought to contribute to trophoblast proliferation and invasion for embryo implantation. The physiological functions of exosomes are also thought to be important for the maintenance of pregnancy. Placenta-derived exosomes have been detected in the maternal peripheral circulation, and their levels vary in patients with PE [47]. Exosome-based methods might be developed for biomarker analysis and as therapeutic tools for clinical practice in the future.
Exosomes may be involved in the pathogenesis of PE and have great potential for the treatment of this disease. PE pathogenesis could be ameliorated or prevented by inhibiting exosome effects or preventing their binding to target organs. We would first examine the effects of exosomes released from the preeclamptic placenta on various organs by searching for proteins, RNA, and DNA in the exosomes. Exosomes could be used as markers to predict the onset of PE and to follow the course of this disease [48]. Marleau et al. [49] suggested that the anticancer effects of molecular targeted drugs could be enhanced by removing exosomes from the circulating blood via hemodialysis. This approach may also be used to treat PE via the removal of STB EVs. The use of various exosome-based methods may aid the identification of the best solution for PE prevention and treatment.
References
- Chappell, L.C.; Enye, S.; Seed, P.; Briley, A.; Poston, L.; Shennan, A.H. Adverse perinatal outcomes and risk factors for preeclampsia in women with chronic hypertension: A prospective study. Hypertension 2008, 51, 1002–1009.
- Roberts, J.M.; Hubel, C.A. The two stage model of preeclampsia: Variations on the theme. Placenta 2009, 30 (Suppl. A), S32–S37.
- Roberts, J.M.; Taylor, R.N.; Musci, T.J.; Rodgers, G.M.; Hubel, C.A.; McLaughlin, M.K. Preeclampsia: An endothelial cell disorder. Am. J. Obstet. Gynecol. 1989, 161, 1200–1204.
- Lam, C.; Lim, K.; Karumanchi, S.A. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension 2005, 46, 1077–1085.
- Cockell, A.P.; Learmont, J.G.; Smárason, A.K.; Redman, C.W.; Sargent, I.L.; Poston, L. Human placental syncytiotrophoblast microvillous membranes impair maternal vascular endothelial function. Br. J. Obstet. Gynaecol. 1997, 104, 235–240.
- Smárason, A.K.; Sargent, I.L.; Starkey, P.M.; Redman, C.W. The effect of placental syncytiotrophoblast microvillous membranes from normal and pre-eclamptic women on the growth of endothelial cells in vitro. Br. J. Obstet. Gynaecol. 1993, 100, 943–949.
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco-Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N. Engl. J. Med. 2017, 17, 613–622.
- Pillay, P.; Moodley, K.; Moodley, J.; Mackraj, I. Placenta-derived exosomes: Potential biomarkers of preeclampsia. Int. J. Nanomed. 2017, 12, 8009–8023.
- Abe, E.; Matsubara, K.; Ochi, H.; Ito, M.; Oka, K.; Kameda, K. Elevated levels of adhesion molecules derived from leukocytes and endothelial cells in patients with pregnancy-induced hypertension. Hypertens. Preg. 2003, 22, 31–43.
- Abe, E.; Matsubara, K.; Oka, K.; Kusanagi, Y.; Ito, M. Cytokine regulation of intercellular adhesion molecule-1 expression on trophoblasts in preeclampsia. Gynecol. Obstet. Investig. 2008, 66, 27–33.
- Ballegeer, V.C.; Spitz, B.; de Baene, L.A.; van Asshe, A.F.; Hidajat, M.; Criel, A.M. Platelet activation and vascular damage in gestational hypertension. Am. J. Obstet. Gynecol. 1990, 166, 629–633.
- Palmer, R.M.J.; Ashton, D.S.; Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988, 333, 664–666.
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A Novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415.
- Roberts, J.M.; Taylor, R.N.; Goldfien, A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am. J. Hypertens. 1991, 4, 700–708.
- Darmochwal-Kolarz, D.; Kolarz, B.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The concentrations of soluble HLA-G protein are elevated during mid-gestation and decreased in pre-eclampsia. Folia Histochem. Cytobiol. 2012, 50, 286–291.
- Gregori, S.; Amodio, G.; Quattrone, F.; Panina-Bordignon, P. HLA-G Orchestrates the Early Interaction of Human Trophoblasts with the Maternal Niche. Front. Immunol. 2015, 6, 128.
- Xu, X.; Zhou, Y.; Wei, H. Roles of HLA-G in the Maternal-Fetal Immune Microenvironment. Front. Immunol. 2020, 11, 592010.
- Saito, S.; Shima, T.; Nakashima, A.; Inada, K.; Yoshino, O. Role of Paternal Antigen-Specific Treg Cells in Successful Implantation. Am. J. Reprod. Immunol. 2016, 75, 310–316.
- Murata, H.; Tanaka, S.; Tsuzuki-Nakao, T.; Kido, T.; Kakita-Kobayashi, M.; Kida, N.; Hisamatsu, Y.; Tsubokura, H.; Hashimoto, Y.; Kitada, M.; et al. The transcription factor HAND2 up-regulates transcription of the IL15 gene in human endometrial stromal cells. J. Biol. Chem. 2020, 295, 9596–9605.
- Matsubara, K.; Abe, E.; Ochi, H.; Kusanagi, Y.; Ito, M. Change of serum concentrations of tumor necrosis factor a and adhesion molecules in normal pregnant women and those with pregnancy-induced hypertension. J. Obstet. Gynaecol. Res. 2003, 29, 422–426.
- Saito, S.; Sakai, M. Th1/Th2 balance in preeclampsia. J. Reprod. Immunol. 2003, 59, 161–173.
- Vince, G.; Starkey, P.; Austgulen, R.; Kwiatkowski, D.; Redman, C.W. Interleukin-6, tumor necrosis factor and soluble tumor necrosis factor receptors in women with pre-eclampsia. Br. J. Obstet. Gynecol. 1995, 102, 20–25.
- Roberts, J.M.; Hubel, C.A. Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet 1999, 354, 788–789.
- Matsubara, K.; Matsubara, Y.; Hyodo, S.; Katayama, T.; Ito, M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. J. Obstet. Gynaecol. Res. 2010, 36, 239–247.
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683.
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383.
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668.
- Zhang, L.; Yu, D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 455–468.
- Kalra, H.; Drummen, G.P.C.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. IJMS 2016, 17, 170.
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 2009, 19, 1875–1885.
- Martínez de Lizarrondo, S.; Roncal, C.; Calvayrac, O.; Rodríguez, C.; Varo, N.; Purroy, A.; Lorente, L.; Rodríguez, J.A.; Doeuvre, L.; Hervás-Stubbs, S.; et al. Synergistic effect of thrombin and CD40 ligand on endothelial matrix metalloproteinase-10 expression and microparticle generation in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1477–1487.
- Mezouar, S.; Darbousset, R.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int. J. Cancer 2015, 136, 462–475.
- Pluskota, E.; Woody, N.M.; Szpak, D.; Ballantyne, C.M.; Soloviev, D.A.; Simon, D.I.; Plow, E.F. Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood 2008, 112, 2327–2335.
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920.
- Simpson, R.J.; Mathivanan, S. Extracellular microvesicles: The need for internationally recognised nomenclature and stringent purification criteria. J. Proteom. Bioinform. 2012, 5, 2.
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659.
- Konečná, B.; Tóthová, Ľ.; Repiská, G. Exosomes-associated DNA: New marker in pregnancy complications? Int. J. Mol. Sci. 2019, 20, 2890.
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Saffar, H.A.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A web-based compendium of exosomal cargo. J. Mol. Biol. 2016, 428, 688–692.
- Bowers, E.C.; Hassanin, A.A.I.; Ramos, K.S. In vitro models of exosome biology and toxicology: New frontiers in biomedical research. Toxicol. In Vitro 2020, 64, 104462.
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455.
- De Gassart, A.; Geminard, C.; Fevrier, B.; Raposo, G.; Vidal, M. Lipid raft-associated protein sorting in exosomes. Blood 2003, 102, 4336–4344.
- Toh, W.S.; Lai, R.C.; Zhang, B.; Lim, S.K. MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 2018, 46, 843–853.
- Kahroba, H.; Hejazi, M.S.; Samadi, N. Exosomes: From carcinogenesis and metastasis to diagnosis and treatment of gastric cancer. Cell. Mol. Life Sci. 2019, 76, 1747–1758.
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-mediated metastasis: Communication from a distance. Dev. Cell 2019, 49, 347–360.
- Yang, Y.; Li, C.W.; Chan, L.C.; Wei, Y.; Hsu, J.M.; Xia, W.; Cha, J.H.; Hou, J.; Hsu, J.L.; Sun, L.; et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018, 28, 862–864.
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264.
- Kurian, N.K.; Modi, D. Extracellular vesicle mediated embryo-endometrial cross talk during implantation and in pregnancy. J. Assist. Reprod. Genet. 2019, 36, 189–198.
- Cho, H.J.; Eun, J.W.; Baek, G.O.; Seo, C.W.; Ahn, H.R.; Kim, S.S.; Cho, S.W.; Cheong, J.Y. Serum exosomal microRNA, miR-10b-5p, as a potential diagnostic biomarker for early-stage hepatocellular carcinoma. J. Clin. Med. 2020, 9, 281.
- Marleau, A.M.; Chen, C.S.; Joyce, J.A.; Tullis, R.H. Exosome removal as a therapeutic adjuvant in cancer. J. Transl. Med. 2012, 10, 134.




