
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Guijin Liu | + 1793 word(s) | 1793 | 2021-04-16 08:09:06 | | | |
| 2 | Rita Xu | Meta information modification | 1793 | 2021-04-19 12:02:57 | | |
Video Upload Options
Supercritical anti-solvent process shows great potential in the scalable and continuous manufacturing of desired solid multicomponent systems.
1. Introduction
Active pharmaceutical ingredients (APIs) are usually formulated into designed dosage forms before being administered to patients. The performance of dosage forms is strongly influenced by the physicochemical properties of APIs, such as chemical stability, mechanical properties, hygroscopicity, solubility, and dissolution rate [1][2]. Unfortunately, there are many drug candidates with poorly physicochemical properties under development. Thus, it is essential to improve insufficiently physicochemical properties for these drug candidates capable of accessing the market.
The vast majority of drug products are supplied in solid dosage forms, such as tablets, capsules, powders, and dry syrups. The solid-state properties of APIs play an important role in modifying their physicochemical properties [2][3]. It is paramount for the pharmaceutical industries to control accurately the solid-state properties of APIs during their manufacture, formulation and shelf life. In recent years, solid multicomponent systems (SMS) have been of great interest to the pharmaceutical scientists. The SMS development mainly focuses on the manipulation of the solid-state properties of APIs, such as the formation of salts, amorphous formulations, solvates, cocrystals, liquid crystals, and nano-cocrystals, to overcome problems of the final drug form, such as poor solubility and dissolution rate, hygroscopicity, poor tabletability, instability, and bitter taste [4][5].
There are two major existing approaches for the production of solid particles, i.e., top-down approaches and bottom-up approaches [6]. Top-down approaches are essentially high energy processes where drug particles are generated through the comminution of bulk materials by the use of technologies such as jet milling, pearl-ball milling, and high-pressure homogenization [7]. On the contrary, the bottom-up approaches are broadly called precipitation processes where drug particles are created from molecules or nucleus by the use of technologies such as spray drying, anti-solvent precipitation, and hot melt extrusion [8]. In general, top-down approaches present several challenges, including a limited control over the solid-state properties of APIs, a long processing time to achieve the required size and the thermal/mechanical degradation of temperature-sensitive APIs. Bottom-up approaches through controlled crystallization or precipitation represent an emerging area in manipulating the solid-state properties of APIs. Among them, supercritical fluid (SCF) techniques have been investigated and applied by many researchers and companies for the solid-state pharmaceutical development [9][10][11][12]. SCFs exhibit properties in between a vapor and a liquid. That is, their density is similar to a liquid, allowing for a good solvation power, while viscosity and diffusivity are similar to that of a vapor, allowing for efficient mass transfer. SCFs, in particular supercritical carbon dioxide (scCO2), provide numerous opportunities for the development of ideal particle formation processes in the pharmaceutical industry. ScCO2 is safe, inexpensive, readily available, and an ideal substitute for many toxic solvents. ScCO2 was first applied to extraction of natural products at the end of the 1980s. Nowadays, scCO2 based extraction technology has become a mature and readily controlled process in the extraction industry, which has been utilized as a green approach for the productive extraction and recovery of valuable compounds, such as bioactive plant phytochemicals [13], nutrition and functional food ingredients [14], and other high added-value compounds [15]. However, the development of the scCO2 technology for the production of solid particles in the pharmaceutical industry is still in the early stages. ScCO2 can act as solvent, anti-solvent, or solute at different processes. Well-established scCO2 processes include rapid expansion of supercritical solution (RESS) process, particles from the gas-saturated solution (PGSS) process, and supercritical anti-solvent (SAS) process [16][17][18].
SAS process, by exploiting the anti-solvent effect of scCO2, is a potential alternative to conventional anti-solvent crystallization, which stands out for the production of solid particles of one or more compounds in a controlled manner [19][20][21][22]. By utilizing the tunable properties of scCO2 at different operating conditions, SAS process offers the possibility to control the crystalline form of APIs in the micron, sub-micron and nano ranges, then to produce nanoparticles, microparticles, expanded hollow microparticles, micro/nano crystals, amorphous particles, and others [23][24][25][26]. In our previous studies, SAS process has been applied to produce 10-hydroxycamptothecin proliposomes [27], 10-hydroxycamptothecin/poly (L-lactic acid) microspheres [28], camptothecin microcrystals [29], gefitinib polymorphs [30], paracetamol/trimethylglycine co-crystals [31], nimesulide amorphous solid dispersions [32], and itraconazole solid dispersions [33]. These studies have demonstrated that SAS process holds great promise for the manipulation of the solid-state properties of APIs.
Combining with research experiences in our research group, this paper aims to provide the reader with a comprehensive review of the applications of SAS process in the SMS preparation. An overview of the process is shown in Figure 1. The development of SAS process from batch to continuous is introduced firstly. Then, examples of pharmaceutical co-crystals and solid dispersions prepared via the SAS process are summarized. After that, the underlying mechanisms on the manipulation of solid-state properties of APIs are discussed. Finally, guidelines for future prospects are proposed.
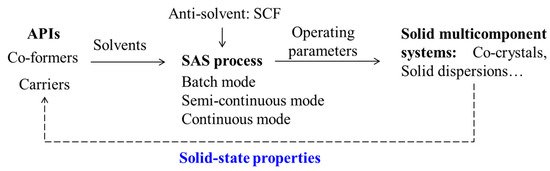
Figure 1. An overview of the SAS applications for solid multicomponent systems.
2. SAS Process: From Batch to Continuous
In the SAS process, SCF acts as an anti-solvent and causes the precipitation of APIs and/or excipients from organic solvents. Nowadays, the SAS technique has been developed in batch, in semi-continuous, and more recently in continuous mode.
2.1. Batch Mode
The batch mode is known as the gas anti-solvent (GAS) process, which has been proposed in 1989 by Gallagher et al. [34]. A simplified diagram of the GAS process is presented in Figure 2. In general, the liquid solution is prepared and poured into the precipitation vessel firstly. Then, compressed CO2 is pumped at a given flow rate into the vessel through valve 1 for pressurization, where valve 2 remains closed, and pressure inside the vessel is measured, and temperature is controlled. During the pressurization, the solvating power of solvent is reduced, resulting in the precipitation of the dissolved solute. After the formation of desired particles, valve 2 is opened for solvent removal and CO2 flushing. Finally, the obtained particles are collected from the filter located at the bottom of the vessel for further analyses. In particular, a stirrer is necessary to improve the mixing between the solution and the scCO2 when relatively large volumes of solution are processed. Additionally, a sapphire window on the base of the vessel with the fiber optic lighting is useful for visual observation of the process [35]. The GAS process is simple and particularly useful for the crystallization of pharmaceuticals. However, a clear disadvantage of the GAS process is its batch mode, resulting in a relatively small production capacity, which is limited by the capacity of the precipitation vessel.
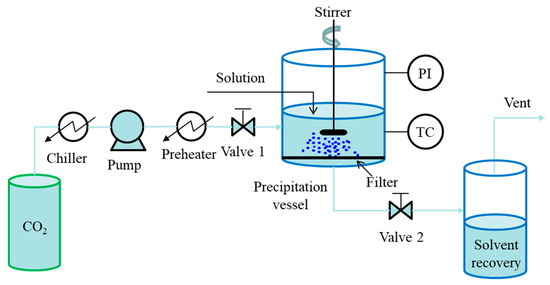
Figure 2. Schematic diagram of batch SAS process: GAS process.
2.2. Semi-Continuous Mode
The semi-continuous mode is known as aerosol spray extraction system (ASES) [36] or precipitation with compressed anti-solvents (PCA) [37]. A simplified diagram of the semi-continuous SAS process is presented in Figure 3. In general, liquefied CO2 is continuously introduced into the precipitation vessel via a high-pressure pump at a given flow rate, meanwhile pressure and temperature inside the vessel are controlled. When the desired temperature and pressure are reached, pure solvent is sprayed into the precipitation vessel through a nozzle for few minutes to obtain steady state composition conditions of the fluid phase during the solute precipitation. Then, the solution is injected instead of pure solvent into the precipitation vessel at a given flow rate. When in contact with the scCO2, the liquid solution including solute dissolves into the scCO2, so high supersaturation of solute in the mixed solution can be achieved, resulting in the precipitation of the dissolved solute. At the end of the solution delivery, scCO2 is kept flowing to remove the residual solvent. Finally, the obtained particles are collected from the precipitation vessel after the pressure relief.
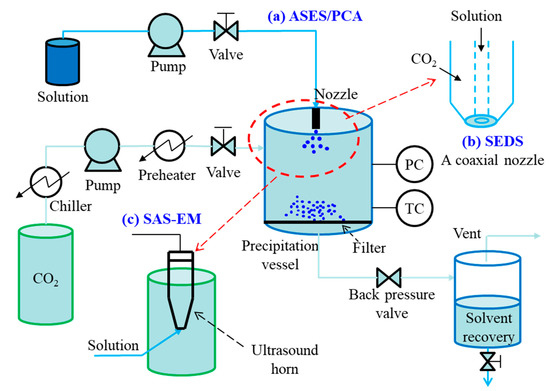
Figure 3. Schematic diagram of semi-continuous SAS process: (a) ASES/PCA, (b) SEDS process, (c) SAS-EM process.
In ASES or PCA, to minimize particle agglomeration frequently observed and to reduce or eliminate drying times, increased mass transfer rates are required. Some modified versions are proposed, such as solution enhanced dispersion by supercritical fluids (SEDS) process [38], and supercritical anti-solvent with enhanced mass transfer (SAS-EM) process [39]. The main difference among these processes is in the injection device. With respect to ASES/PCA, SEDS process consists of a two (or three) coaxial passages nozzle to provide a simultaneous introduction of the solution/suspension and different solvents; whereas, the SAS-EM process utilizes an ultrasound horn to provide a different methodology to create the jet break-up. The main advantage of semi-continuous SAS techniques over GAS technique is their continuous injection of solution and scCO2, which is a prerequisite for large scale mass production of particles. They are also better for the control of particle solid-state properties, such as size, morphology, crystal habits, and others.
2.3. Continuous Mode
The continuous mode is known as atomization and anti-solvent (AAS) process [40] or atomization of supercritical anti-solvent induced suspensions (ASAIS) process [41]. The AAS process is similar to SEDS process: for SEDS setup, the solution is atomized into the precipitation vessel pressurized with scCO2; but for AAS process, the solution is mixed with the scCO2 inside the coaxial nozzle to precipitate solids, and then the solids are collected in the precipitation vessel at near atmospheric pressure.
In the pharmaceutical industry, spray drying is a continuous unit operation capable of transforming solutions or suspensions into a solid product. The spray drying process consists of four basic stages: atomization of the liquid by a kinetic energy or pneumatic nozzle where the liquid stream is broken in small droplets by interaction with a second fluid, usually pressurized air; mixing of the droplets with the drying gas, often air or in some cases nitrogen; evaporative drying of the droplets into fine particles; and separation of the dried particles from the gas using a cyclone or a bag-filter [42][43]. The ASAIS technique is a combination of AAS and spray drying. A simple diagram of ASAIS process is presented in Figure 4. In ASAIS, the solution is mixed with scCO2 in a small volume mixer inside a coaxial nozzle to generate a suspension which is then immediately sprayed for solvent extraction by spray drying at normal pressure.
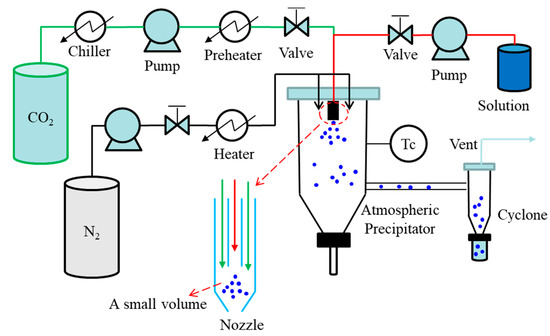
Figure 4. Schematic diagram of continuous SAS process: ASAIS process.
The transition of SAS process from batch to continuous is encouraged by most regulatory bodies for pharmaceutical manufacturing processes. In AAS and ASAIS methods, the supercritical conditions are restricted to a very small volume mixer, avoiding the large volume equipment at high pressure and complex particle harvesting in filters. These are compatible with the continuous regime operation and implement at the industrial scale. Moreover, their installations are simplified and become compatible with existing spray drying equipment. When compared to conventional spray drying methods, the continuous SAS techniques present their own irreplaceable advantages. For example, conventional spray drying methods typically generate amorphous APIs, while the continuous SAS techniques have the potential to generate and control the crystalline form of APIs by inducing nucleation inside the nozzle before the spray drying step [11].
References
- Tran, P.H.L.; Tran, T.T.D. Dosage form designs for the controlled drug release of solid dispersions. Int. J. Pharm. 2020, 581, 119274.
- Byrn, S.R.; Zografi, G.; Chen, X.S. Solid State Properties of Pharmaceutical Materials, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017.
- Zhong, L.; Gao, L.; Li, L.; Zang, H. Trends-process analytical technology in solid oral dosage manufacturing. Eur. J. Pharm. Biopharm. 2020, 153, 187–199.
- Sathisaran, I.; Dalvi, S.V. Engineering cocrystals of poorly water-soluble drugs to enhance dissolution in aqueous medium. Pharmaceutics 2018, 10, 108.
- Morissette, S.L.; Almarsson, Ö.; Peterson, M.L.; Remenar, J.F.; Read, M.J.; Lemmo, A.V.; Ellis, S.; Cima, M.J.; Gardner, C.R. High-throughput crystallization: Polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv. Drug Deliver. Rev. 2004, 56, 275–300.
- Rasenack, N.; Müller, B.W. Micron-size drug particles: Common and novel micronization techniques. Pharm. Dev. Technol. 2004, 9, 1–13.
- Van Eerdenbrugh, B.; Van den Mooter, G.; Augustijns, P. Top-down production of drug nanocrystals: Nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharm. 2008, 364, 64–75.
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharm. 2013, 453, 126–141.
- Girotra, P.; Singh, S.K.; Nagpal, K. Supercritical fluid technology: A promising approach in pharmaceutical research. Pharm. Dev. Technol. 2013, 18, 22–38.
- Campardelli, R.; Baldino, L.; Reverchon, E. Supercritical fluids applications in nanomedicine. J. Supercrit. Fluids 2015, 101, 193–214.
- Long, B.; Ryan, K.M.; Padrela, L. From batch to continuous—New opportunities for supercritical CO2 technology in pharmaceutical manufacturing. Eur. J. Pharm. Sci. 2019, 137, 104971.
- Soh, S.H.; Lee, L.Y. Microencapsulation and nanoencapsulation using supercritical fluid (SCF) techniques. Pharmaceutics 2019, 11, 21.
- Arumugham, T.K.R.; Hasan, S.W.; Show, P.L.; Rinklebe, J.; Banat, F. Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications—A review. Chemosphere 2021, 271, 129525.
- Galanakis, C.M. Functionality of food components and emerging technologies. Foods 2021, 10, 128.
- Marcus, Y. Some advances in supercritical fluid extraction for fuels, bio-materials and purification. Processes 2019, 7, 156.
- Liu, G.; Jiang, Y.; Wang, X. Tailoring particle microstructures via supercritical CO2 processes for particular drug delivery. Curr. Pharm. Des. 2015, 21, 2543–2562.
- Badens, E.; Masmoudi, Y.; Mouahid, A.; Crampon, C. Current situation and perspectives in drug formulation by using supercritical fluid technology. J. Supercrit. Fluids 2018, 134, 274–283.
- Padrela, L.; Rodrigues, M.A.; Duarte, A.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C. Supercritical carbon dioxide-based technologies for the production of drug nanoparticles/nanocrystals—A comprehensive review. Adv. Drug Deliver. Rev. 2018, 131, 22–78.
- De Marco, I.; Rossmann, M.; Prosapio, V.; Reverchon, E.; Braeuer, A. Control of particle size, at micrometric and nanometric range, using supercritical antisolvent precipitation from solvent mixtures: Application to pvp. Chem. Eng. J. 2015, 273, 344–352.
- Prosapio, V.; De Marco, I.; Reverchon, E. Supercritical antisolvent coprecipitation mechanisms. J. Supercrit. Fluids 2018, 138, 247–258.
- Kalani, M.; Yunus, R. Application of supercritical antisolvent method in drug encapsulation: A review. Int. J. Nanomed. 2011, 6, 1429–1442.
- MacEachern, L.; Kermanshahi-pour, A.; Mirmehrabi, M. Supercritical carbon dioxide for pharmaceutical co-crystal production. Cryst. Growth Des. 2020, 20, 6226–6244.
- Reverchon, E.; Adami, R.; Caputo, G.; De Marco, I. Spherical microparticles production by supercritical antisolvent precipitation: Interpretation of results. J. Supercrit. Fluids 2008, 47, 70–84.
- Reverchon, E.; De Marco, I.; Torino, E. Nanoparticles production by supercritical antisolvent precipitation: A general interpretation. J. Supercrit. Fluids 2007, 43, 126–138.
- Reverchon, E.; De Marco, I.; Adami, R.; Caputo, G. Expanded micro-particles by supercritical antisolvent precipitation: Interpretation of results. J. Supercrit. Fluids 2008, 44, 98–108.
- Rossmann, M.; Braeuer, A.; Dowy, S.; Gallinger, T.G.; Leipertz, A.; Schluecker, E. Solute solubility as criterion for the appearance of amorphous particle precipitation or crystallization in the supercritical antisolvent (SAS) process. J. Supercrit. Fluids 2012, 66, 350–358.
- Liu, G.; Wang, W.; Wang, H.; Jiang, Y. Preparation of 10-hydroxycamptothecin proliposomes by the supercritical CO2 anti-solvent process. Chem. Eng. J. 2014, 243, 289–296.
- Wang, W.; Liu, G.; Wu, J.; Jiang, Y. Co-precipitation of 10-hydroxycamptothecin and poly (l-lactic acid) by supercritical CO2 anti-solvent process using dichloromethane/ethanol co-solvent. J. Supercrit. Fluids 2013, 74, 137–144.
- Liu, G.; Wang, H.; Jiang, Y. Recrystallization and micronization of camptothecin by the supercritical antisolvent process: Influence of solvents. Ind. Eng. Chem. Res. 2013, 52, 15049–15056.
- Liu, G.; Lin, Q.; Huang, Y.; Guan, G.; Jiang, Y. Tailoring the particle microstructures of gefitinib by supercritical CO2 anti-solvent process. J. CO2 Util. 2017, 20, 43–51.
- Zhao, Z.; Liu, G.; Lin, Q.; Jiang, Y. Co-crystal of paracetamol and trimethylglycine prepared by a supercritical CO2 anti-solvent process. Chem. Eng. Technol. 2018, 41, 1122–1131.
- Liu, G.; Gong, L.; Zhang, J.; Wu, Z.; Deng, H.; Deng, S. Development of nimesulide amorphous solid dispersions via supercritical anti-solvent process for dissolution enhancement. Eur. J. Pharm. Sci. 2020, 152, 105457.
- Liu, G.; Wu, Z.; Zhang, J.; Gong, L.; Zhang, Y.; Deng, S. Particle design of itraconazole by supercritical anti-solvent technology: Processing-microstructure-solubility relationship. Chem. Eng. Process. 2020, 154, 108013.
- Gallagher, P.M.; Coffey, M.P.; Krukonis, V.J.; Klasutis, N. Gas antisolvent recrystallization: New process to recrystallize compounds insoluble in supercritical fluids. In Supercritical Fluid Science and Technology; American Chemical Society: Washington, DC, USA, 1989; Volume 406, pp. 334–354.
- Ober, C.A.; Montgomery, S.E.; Gupta, R.B. Formation of itraconazole/l-malic acid cocrystals by gas antisolvent cocrystallization. Powder Technol. 2013, 236, 122–131.
- Bleich, J.; Müller, B.W.; Waßmus, W. Aerosol solvent extraction system—A new microparticle production technique. Int. J. Pharm. 1993, 97, 111–117.
- Dixon, D.J.; Johnston, K.P.; Bodmeier, R.A. Polymeric materials formed by precipitation with a compressed fluid antisolvent. AIChE J. 1993, 39, 127–139.
- Palakodaty, S.; York, P.; Pritchard, J. Supercritical fluid processing of materials from aqueous solutions: The application of seds to lactose as a model substance. Pharm. Res. 1998, 15, 1835–1843.
- Chattopadhyay, P.; Gupta, R.B. Production of griseofulvin nanoparticles using supercritical CO2 antisolvent with enhanced mass transfer. Int. J. Pharm. 2001, 228, 19–31.
- Padrela, L.; Rodrigues, M.A.; Velaga, S.P.; Matos, H.A.; de Azevedo, E.G. Formation of indomethacin–saccharin cocrystals using supercritical fluid technology. Eur. J. Pharm. Sci. 2009, 38, 9–17.
- Rodrigues, M.A.; Padrela, L.; Geraldes, V.; Santos, J.; Matos, H.A.; Azevedo, E.G. Theophylline polymorphs by atomization of supercritical antisolvent induced suspensions. J. Supercrit. Fluids 2011, 58, 303–312.
- Paudel, A.; Worku, Z.A.; Meeus, J.; Guns, S.; Van den Mooter, G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2013, 453, 253–284.
- Boel, E.; Koekoekx, R.; Dedroog, S.; Babkin, I.; Vetrano, M.R.; Clasen, C.; Van den Mooter, G. Unraveling particle formation: From single droplet drying to spray drying and electrospraying. Pharmaceutics 2020, 12, 625.





