
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Evgeny Kopnin | + 1174 word(s) | 1174 | 2021-03-31 05:27:17 | | | |
| 2 | Rita Xu | Meta information modification | 1174 | 2021-04-19 12:01:00 | | |
Video Upload Options
High pressure applications is strongly required for the synthesis of these materials because it is only way to stabilize gold oxides which are thermally very unstable at ambient pressures.
1. Introduction
Numerous high-temperature superconducting cuprates have complex perovskite-based intergrowth crystal structures. In these structures, it is possible to select different blocks, for example, perovskite-like (CaTiO3), halite-like (NaCl), fluorite-like (CaF2) and/or other ones stacking along the four-fold axis of the perovskite sublattice [1]. The first member of series of Ruddlesden-Popper (RP) phases La2CuO4 has a simple intergrowth structure consisting of perovskite-like and halite-like fragments (K2NiF4 structural type). High-temperature superconductivity at Tc = 36 K was discovered in this material after hole doping by Ba2+ [2]. Tc was shown to increase up to 52.5 K when measured in situ under a hydrostatic pressure of 1.68 GPa [3].
The crystal structures of Hg-containing series of superconducting cuprates, HgBa2Can−1CunO2n+2+δ (n = 1–6), can be described as intergrowth ones [4][5][6][7][8][9]. Defect halite-like fragments and perovskite-like fragments are stacking along the four-fold axis, which coincides with the crystallographic c-axis (Figure 1). Hg2+ cations have typical dumbbell-like coordination with a significant range of oxygen stoichiometries in the HgOδ layers. In the crystal structures of compounds with n = 2–6, the corresponding amounts of CuO2 layers are separated by Ca2+ cations forming repeated perovskite-like blocks. It is important to note that the highest Tc (up to 138 K) was reported for the compound with n = 3. Moreover, at ultrahigh pressures above 20 GPa Tc increased remarkably and reached 166 K in fluorinated Hg-1223 [10][11][12].
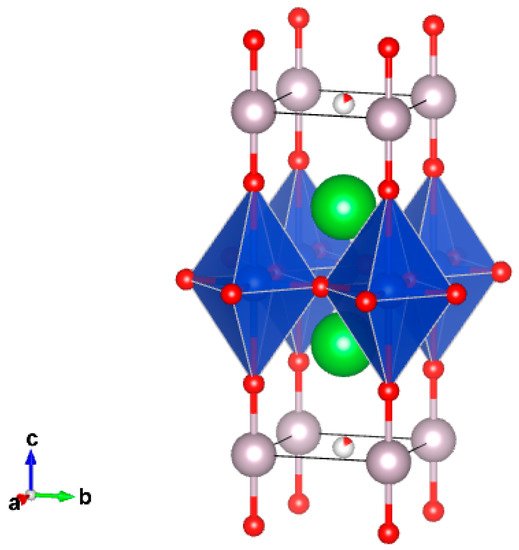
Figure 1. Crystal structure of Hg-1201. The coordination polyhedra of copper (distorted octahedron) and Hg (dumbbell) are shown.
Since the first high-pressure device was invented by Bridgeman in the 1940s, high-pressure has been extensively used in solid state sciences. It is established that ultrahigh pressure deals with the pressures greater than 1.01 GPa, and now the pressure scale is extended up to 500 GPa at temperatures exceeding 3000 °C. The most frequently used apparatus in high pressure research is the diamond-anvil cell (DAC) in a piston-cylinder design. It allows one to achieve pressures as high as 4 GPa or even more. The disadvantage of the Bridgeman device in a piston-cylinder design is the small sample size. That problem was solved by the belt-like apparatus invented in the 1960s, that can generate 10 GPa at temperatures up to 2000 °C. The modern devices are multianvil cells, where the anvils are usually made from materials with high hardness, preferably from diamond. Detailed descriptions and a comparison of different high-pressure apparatus are given in a recent review [13].
2. Au-Containing Cuprates: High-Pressure Synthesis and Characterization
High pressure applications is strongly required for the synthesis of these materials because it is only way to stabilize gold oxides which are thermally very unstable at ambient pressures. The general formula of the homologous series is as follows: AuBa2(Ca,Ln)n−1CunO2n+3 (n = 1–4; Ln = rare-earth trivalent cations). The second member AuBa2Y1−xCaxCu2O7 was synthesized in polycrystalline and single crystal form at 1.8 GPa/950 °C and at 6 GPa/1380 °C, respectively [14][15]. Its crystal structure was determined from single crystal X-ray diffraction data in space group Pmmm (Jana 2000 software; R, Rw (I >3 σ (I)) = 0.0260, 0.0219; R, Rw (all data) = 0.0443, 0.0225; the details are given in [15]); a = 3.8260(2) Å, b = 3.8501(2) Å, c = 12.075 (1) Å. The samples exhibited bulk superconductivity with Tc = 82–84 K. The crystal structure of Au-1212 compound is shown in Figure 2.
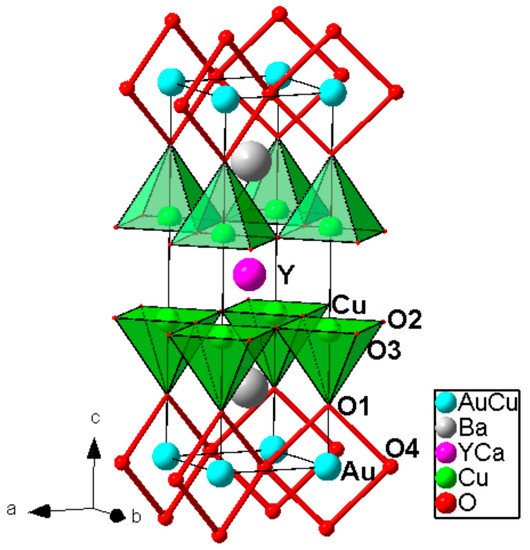
Figure 2. Crystal structure of the Au-1212 compound. Zigzag chain of AuO4 square-planar units is selected. Reproduced with permission from [9].
The cation stoichiometry Au: Ba: Y: Ca: Cu = 0.8(1): 1.9(1): 0.7(1): 0.4(1): 2.2(1) obtained from electron probe microanalysis (EPMA) data is in agreement with that obtained from X-ray single crystal diffraction, indicating ca. 20% substitution of Cu1+ cations on the Au3+ site, it was associated with approximately the same amount of oxygen vacancies in the corresponding plane. It should be noted that no superstructure related to possible Cu/Au ordering was revealed from the X-ray single crystal diffraction data.
Au3+ is located in square-planar coordination with Au-O distances 1.980 Å and 2.027 Å. AuO4 squares share corners and they are forming zigzag chains along the short a-axis, and that is different from YBa2Cu3O7 where CuO4 squares ran along the longer b-axis. The coordination of the in-plane Cu is square pyramidal with an apical distance of 2.41 Å, it is typical for layered cuprate superconductors.
The third and fourth members of the Au-series, Au1+xBa2Ca2Cu3−xO9 and AuBa2Ca3Cu4O11, were synthesized at 6 GPa and 1250–1300 °C [16]. They crystallize in primitive orthorhombic unit cells with a = 3.8182(4) Å, b = 3.8555(4) Å, c = 15.445(2) Å and a = 3.8266(3) Å, b = 3.8505(3) Å, c = 18.494(1) Å, for Au-1223 and Au-1234, respectively.
A HRTEM image of Au-1223 phase along the b-axis is shown in Figure 3. It is confirmed the 1223 stacking along the c-axis: BaO-AuO-BaO-CuO2-Ca-CuO2-Ca-CuO2. Attempts to synthesize next members (n = 5, 6) were not successful both at 1.8 GPa and at 6 GPa. AuBa2Ca3Cu4O11 showed bulk superconductivity with Tc ~ 99 K, but Au-1223 showed only a weak diamagnetic signal at Tc ~30 K. In order to understand the possible reason, crystal structure of Au-1223 was determined from single crystal X-ray diffraction data (SHELXL-97 software; S.G. Pmmm; R1, wR2 (I > 2 σ (I)) = 0.0188, 0.0437; R, Rw (all data) = 0.0301, 0.0484; the details are available in [17].
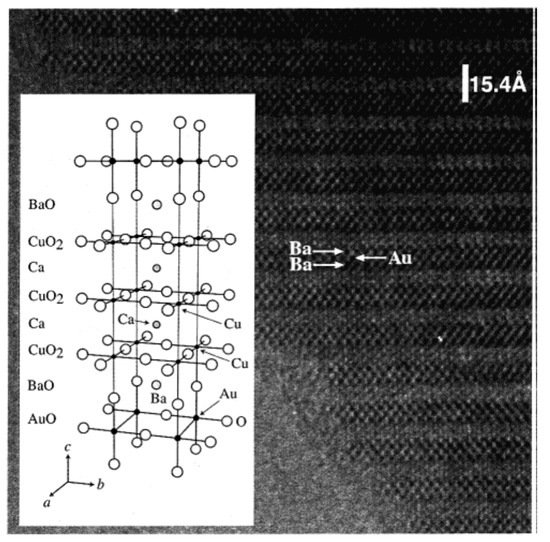
Figure 3. HRTEM image of Au-1223 phase taken along (100) zone axis. The structure model of Au-1223 is shown in the inset. Reproduced with permission from [10].
The cation stoichiometry Au: Ba: Ca: Cu = 0.9(1): 1.91(6): 2.10(6): 3.1(1) was obtained from EPMA. The crystal structure is similar to Hg-1223, but the cation stoichiometry is indicative of some possibility of Cu/Au disorder. The refinement of the occupation yielded 4.1% of Cu in Au site. Au-O in-plane distance is 2.055 Å and it is noticeably longer than typical Cu-O in-plane distances that correlate with their ionic radii difference.
Contrary to Hg-1223, oxygen in the AuO layer is located at (x,1/2,0), it leads to formation of AuO4 zigzag chains as in Au-1212. Electron diffraction revealed the weak superstructure with bs = 2b in Au-1223, however, the corresponding intensities in X-ray single crystal diffraction data were extremely weak. This suggests no ordering of zigzag-like chains and/or their irregularity.
Related to the suppression of superconductivity, some incorporation of Au for Cu in the CuO2 planes (6–8%) should be taken into consideration. Although the degree of the substitution is very moderate, even this level can lead to superconductivity suppression.
Au-1201 is the less known member of the Au-series. Au(Ba,La)2CuO5±δ was obtained as a principal phase in the polyphasic sample prepared at 6 GPa and 1250 °C [18]. It crystallizes in an orthorhombic primitive cell with the parameters a = 3.7976(3) Å, b = 3.8509(3) Å, c = 8.5749(9) Å. EPMA shown the cation stoichiometry Au: Ba: La: Cu = 0.75(8): 0.8(1): 1.2(1): 1.25(8), therefore, the approximate formula of Au-1201 can be expressed as Au0.75Ba0.8La1.2Cu1.25O5±δ. Therefore, it suggests that ca. 25% of Au positions are occupied by Cu. HRTEM indicated that this is a first member of AuBa2(Ca,Ln)n−1CunO2n+3 series (Figure 4). However, an ED study revealed a weak superstructure with as = 5a, bs = b, cs = 2c. It could be due to possible Au/Cu ordering. Samples of Au-1201 showed a weak diamagnetic signal with Tc = 19 K and low level of diamagnetic Meissner fraction.
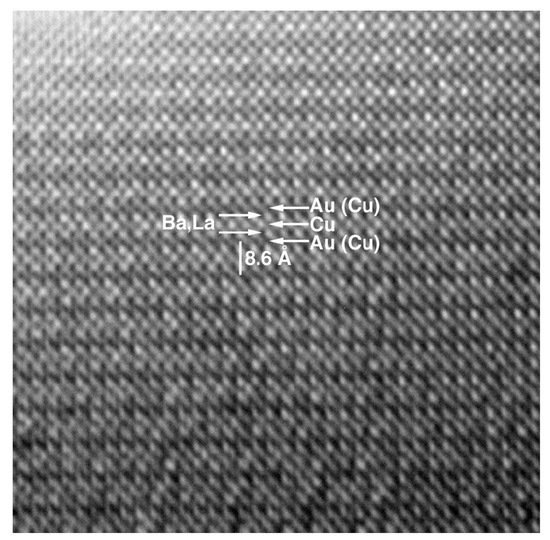
Figure 4. HRTEM image of Au-1201 compounds taken along (010) zone axis. Reproduced with permission from [12].
References
- Abakumov, A.M.; Antipov, E.V.; Kovba, L.M.; Kopnin, E.M.; Putilin, S.N.; Shpanchenko, R.V. Complex oxides with coherent intergrowth structures. Russ. Chem. Rev. 1995, 64, 719–729.
- Bednorz, J.G.; Müller, K.A. Possible high Tc superconductivity in the Ba-La-Cu-O system. Z. Phys. B 1986, 64, 189.
- Chu, C.W.; Hor, P.H.; Meng, R.L.; Gao, L.; Huang, Z.J. Superconductivity at 52.5 K in the Lanthanum-Barium-Copper-Oxide System. Science 1987, 235, 567–569.
- Putilin, S.N.; Antipov, E.V.; Chmaissem, O.; Marezio, M. Superconductivity at 94 K in HgBa2Cu04+δ. Nature 1993, 362, 226.
- Schilling, A.; Cantoni, M.; Guo, J.D.; Ott, H.R. Superconductivity above 130 K in the Hg–Ba–Ca–Cu–O system. Nature 1993, 363, 58.
- Antipov, E.; Loureiro, S.; Chaillout, C.; Capponi, J.; Bordet, P.; Tholence, J.; Putilin, S.; Marezio, M. The synthesis and characterization of the HgBa2Ca2Cu3O8+δ and HgBa2Ca3Cu4O10+δ phases. Phys. C Supercond. 1993, 215, 1–10.
- Capponi, J.; Kopnin, E.; Loureiro, S.; Antipov, E.; Gautier, E.; Chaillout, C.; Souletie, B.; Brunner, M.; Tholence, J.; Marezio, M. High-pressure synthesis and heat treatments of the HgBa2Ca4Cu5O12+δ and HgBa2Ca5Cu6O14+δ phases. Phys. C Supercond. 1996, 256, 1–7.
- Karpinski, J.; Schwer, H.; Conder, K.; Meijer, G.I.; Kopnin, E.; Molinski, R. High pressure crystal growth of Y2Ba4Cu6+nO14+n and HgBa2Can-1CunO2n+2+δ superconductors. Solid State Ionics 1997, 101–103, 985.
- Schwer, H.; Molinski, R.; Kopnin, E.; Meijer, G.I.; Karpinski, J. Structure and Properties of 1256 and 1267 Type Hg1-xRexBa2Can-1CunO2n+2+4x+d single crystals. J. Solid State Chem. 1999, 43, 277.
- Núñez-Regueiro, M.; Tholence, J.-L.; Antipov, E.V.; Capponi, J.-J.; Marezio, M.; Anderson, P.W. Pressure-Induced Enhancement of Tc Above 150 K in Hg-1223. Science 1993, 262, 97–99.
- Monteverde, M.; Acha, C.; Nuňez-Regueiro, M.; Pavlov, D.A.; Lokshin, K.A.; Putilin, S.N.; Antipov, E.V. High-pressure effects in fluorinated HgBa2Ca2Cu3O8+δ. Europhys. Lett. 2005, 72, 458.
- Yamamoto, A.; Takeshita, N.; Terakura, C.; Tokura, Y. High-pressure effects revisited for the cuprate superconductor family with highest critical temperature. Nat. Commons 2015, 6, 8990.
- Liu, X.Y. High pressure synthesis and preparation of inorganic materials. In Modern Inorganic Synthetic Chemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 5; pp. 105–141.
- Bordet, P.; Lefloch, S.; Chaillout, C.; Duc, F.; Gorius, M.; Perroux, M.; Capponi, J.; Toulemonde, P.; Tholence, J. AuBa2(Y1−x, Cax)Cu2O7: A new superconducting gold cuprate with Tc above 80 K. Phys. C Supercond. 1997, 276, 237–244.
- Bordet, P.; Kopnin, E.M.; Sato, A.; Takayama-Muromachi, E. Structure analysis of superconducting Au-1212 cuprate. Supercond. Sci. Technol. 2003, 16, 685–689.
- Kopnin, E.M.; Loureiro, S.M.; Asaka, T.; Anan, Y.; Matsui, Y.; Takayama-Muromachi, E. New family of Au-based supercon-ductors AuBa2Can-1CunO2n+3 (n = 3, 4). Chem. Mater. 2001, 13, 2905.
- Kopnin, E.; Sato, A.; Asaka, T.; Matsui, Y.; Takayama-Muromachi, E. Structure analysis of Au-containing cuprate of Au1+xBa2Ca2Cu3−xO9 (Au-1223). Phys. C Supercond. 2003, 387, 406–410.
- Kopnin, E.; Sato, A.; Asaka, T.; Matsui, Y.; Takayama-Muromachi, E. High-pressure synthesis and characterization of the Au-1201 phase. J. Alloy. Compd. 2003, 361, 28–31.




