
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Le Minh Tu Phan | + 1420 word(s) | 1420 | 2021-04-16 11:37:43 | | | |
| 2 | Rita Xu | Meta information modification | 1420 | 2021-04-19 08:33:20 | | |
Video Upload Options
Recently, photothermal therapy (PTT) has emerged as one of the most promising biomedical strategies for different areas in the biomedical field owing to its superior advantages, such as being noninvasive, target-specific and having fewer side effects. Graphene-based hydrogels (GGels), which have excellent mechanical and optical properties, high light-to-heat conversion efficiency, and good biocompatibility, have been intensively exploited as potential photothermal conversion materials.
1. Introduction
Photothermal therapy (PTT) has emerged in the last few decades as a potential medical tool in the area of various diseases and medical therapy, such as cancer therapy [1][2], bacterial infection [3], bone regeneration and tissue engineering [4] and drug delivery [5]. PTT is one of the main types of phototherapeutic methods for disease treatment because of its localized ablation of the tissue of interest and minimal heating damage to normal tissues near the targeted tissues. The core principle of PTT is the application of external near-infrared (NIR) light, which is absorbed by highly efficient photothermal agents (PTAs) accumulated noninvasively within the targeted tissues to convert absorbed light into thermal energy, thereby increasing the kinetic energy to provide local overheating. Because the hypothermia effect occurs only in the presence of PTAs in the targeted tissues, PTT offers highly efficient and unique methods for disease treatment owing to its precise spatial-temporal effect, high sensitivity, high pharmacokinetics, few side effects, ease of manipulation, low invasive burden, speed and efficacy of treatment and low cost [6][7]. The primary prerequisite for an effective PTT is the efficient delivery of PTAs to the tissue of interest and to precisely direct the external light into the tissues where PTAs are located. To meet this requirement, the dominant strategy has been to create highly efficient and multifunctional nanomaterials as PTAs with sufficient photothermal conversion efficacy and high biocompatibility [8][9][10]. PTAs with high light absorption capacity, high photostability, minimal cytotoxicity and good biocompatibility are more favorable. It is also important to note that the PTA components, structures and size remarkably affect the pharmacokinetics of PTT, including drug absorption, distribution, metabolism and release [11][12]. Hence, highly integrated nanomaterials are crucial for enhancing the optical properties, particle stability and biocompatibility, while reducing undesirable effects or toxicity, especially for drug delivery and tumor therapy.
To date, a variety of NIR-responsive nanomaterials, including both organic [13][14] and inorganic agents [15][16] have been explored for PTA preparation. Among them, biomaterials with adequate biocompatibility are highly preferred. Hydrogels that possess the obligatory characteristics for biomedical applications, such as good biocompatibility and low toxicity, have been envisioned as a new promising class of PTAs [17][18]. A hydrogel is a group of polymeric materials that can form a three-dimensional (3D) network of hydrophilic soft polymers with several unique features, including hydrophilicity, viscosity, elasticity, high water content and tunable stiffness, making them able to be fashioned into specific level through manipulation with different crosslinker types [19][20]. Other excellent characteristics of hydrogels include their biodegradability [21] and ability to mimic the compositions and physicochemical properties of the natural extracellular matrix [22], making hydrogels a highly biocompatible material with negligible cytotoxicity [23]. Owing to these unique properties, significant research attention has been given to hydrogels in recent years for many applications in the field of biomedicine, such as tissue engineering [24], drug carriers [25], anticancer [26] and antibacterial therapies [27] and biosensors [28][29]. Notably, hydrogel-based systems have shown distinct advantages in PTT and intensive studies have been conducted to fabricate hydrogel-modified composites to enhance the efficacy of photothermal therapeutics [30][31]. Nevertheless, a major limitation of hydrogels is the lack of mechanical strength, making it a great challenge for therapeutic applications. One innovative strategy to overcome this issue is to incorporate other materials into hydrogels to precisely mimic the extracellular matrix and improve the composite stiffness [32]. Among the different nanomaterials, graphene and graphene-based nanocomposites have been proposed as a new promising class of photothermal materials and the incorporation of graphene into hydrogel networks has significantly improved the capacity of hydrogels owing to their superior mechanical, electrical and optical properties [33].
Graphene and its chemical derivatives graphene oxide (GO) and reduced GO (rGO) are two-dimensional carbon single layers that possess abundant functional groups on their surface (carboxyl and hydroxyl groups), novel physical properties (photothermal properties, photoluminescent properties and large specific surface area) and good biocompatibility [34]. Owing to these superior properties, graphene and graphene derivatives have sparked great attention for different applications in the field of biosensors, drug delivery and bioimaging [35][36]. Among different types of graphene, GO is a useful material for photothermal application and is commonly used as a basic building material for the fabrication of other graphene-based nanomaterials. GO nanosheets have been used as effective building blocks for improving the chemical and physical properties of new integrated nanocomposites, such as light absorption, thermal and electrical conductivity and flexibility [37]. Furthermore, graphene possesses strong optical absorption in the NIR regions [38], making it a promising candidate for photothermal applications compared to existing conventional photothermal materials. Owing to the aforementioned features of graphene and hydrogel, there have been increasing studies to explore graphene-based hydrogel (GGel) nanocomposites for NIR-mediated PTT. The existing GGels have exhibited improved physical, chemical and biological properties, making them great candidates as potential PTAs [39][40].
2. Thermal Property of Graphene-Integrated Hydrogels
Based on an inherently extraordinary structure, graphene can strongly interact with low-frequency photons and generate heat under specific wavelengths such as NIR light through plasmonic photothermal conversion [41]. Upon NIR irradiation, graphene surface plasmons are stimulated and induce random resonance and dipole transmission, which is required for the conversion of thermal photon energy output. The absorption spectra of graphene and GO show that absorbance of GO falls from UV to NIR region while graphene keeps constant absorbance (Figure 3a). To validate the higher thermal conductivity of graphene-based materials for photothermal application, photothermal conversion efficiencies of graphene, GO and Au nanorods were measured under illumination at different NIR wavelength (980, 808, 650 nm) using time constant and integrating sphere methods, respectively, exhibiting the higher photothermal efficiency of graphene and GO about 58–67% under 808 nm NIR irradiation compared to 52% of Au nanorods (Figure 3b) [42]. Hence, GGels also exhibit noticeable photon absorption under NIR irradiation, facilitating local temperature enhancement surrounding injected materials and killing cancerous cells.
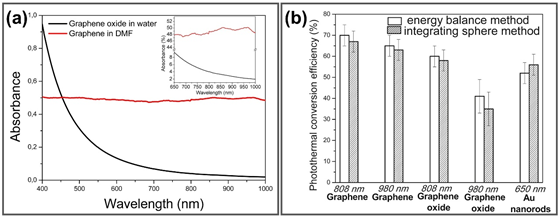
Figure 3. (a) Absorption spectra of graphene in dimethylformamide and GO in water in both visible and NIR range, inset is magnification of spectra from 650–1000 nm. (b) Photothermal conversion efficiencies of graphene, GO and Au nanorods determined by time constant and integrating sphere methods. Adapted with permission from [42].
Currently, heating is widely used as an effective and useful method for cancer therapy due to the burning of cancerous cells and tumors upon the enhancement of biological molecular temperature. Additionally, for non-superficial treatments, irradiation by laser at NIR was extensively investigated to minimize damage to non-specific surrounding tissues [42]. Among the promising photothermal materials, graphene-incorporated materials have attracted much attention because of their outstanding properties, especially the high absorption of NIR. Therefore, graphene/derivative graphene-based materials have been employed in diverse applications, such as antibacterial and anticancer therapies, drug delivery and tissue engineering. In this context, the photothermal conversion efficiency is the most fundamental factor, which requires only a low material concentration, irradiation power and shorter irradiation time. In particular, in the case of GO, the optical absorption is significantly increased due to stable colloidal suspension formation, eventually enhancing its photothermal conversion efficiency [43]. In comparison with others, for instance, Au nanoshells, nanorods, or Au-Ag alloys, graphene has attracted considerable attention because of its stable shape under high laser power, hence, altering their plasmonic resonances and conserving their photothermal conversion efficiency [44]. Furthermore, hydrogels are considered smart polymers because of their specific stimuli-absorbing capacity, including light and alternating magnetic fields, especially light from NIR laser, to generate and control local heating [45]. Nevertheless, hydrogels can easily alter their reversible structure upon variations in local conditions, such as temperature or pH [46]. To overcome these issues, immobilizing GO nanosheets is the most effective way to not only ameliorate the mechanical properties of the hydrogel, but also accomplish NIR light responsiveness; thus, it has been extensively investigated for biomedical applications [47][48][49]. Benefiting from the excellent photothermal properties of GO and the mechanical properties of hydrogel, GGels also exhibit high photothermal conversion efficiency that converting NIR light into heat and optimal mechanical properties for their promising potential in different photothermal-based biomedical applications.
3. Photothermal-Based Biomedical Applications of GGels
There are many exceptional advantages of photothermal-sensitive GGels in the field of biomedical applications, including NIR-mediated hyperthermic anticancer therapy, NIR-triggered drug release system, antimicrobial and wound healing, tissue engineering and bone regeneration, owing to their fascinating properties such as cost-effectiveness, straightforward functionalization and high photothermal conversion efficiency. GGels could act as innovative materials exhibiting significant potential in biomedical applications
References
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108.
- Nam, J.; Son, S.; Ochyl, L.J.; Kuai, R.; Schwendeman, A.; Moon, J.J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun. 2018, 9, 1–13.
- Yan, L.X.; Chen, L.J.; Zhao, X.; Yan, X.P. pH switchable nanoplatform for in vivo persistent luminescence imaging and precise photothermal therapy of bacterial infection. Adv. Funct. Mater. 2020, 30, 1909042.
- Zhang, W.; Gu, J.; Li, K.; Zhao, J.; Ma, H.; Wu, C.; Zhang, C.; Xie, Y.; Yang, F.; Zheng, X.; et al. A hydrogenated black TiO2 coating with excellent effects for photothermal therapy of bone tumor and bone regeneration. Mater. Sci. Eng. C 2019, 102, 458–470.
- Chen, Y.; Li, H.; Deng, Y.; Sun, H.; Ke, X.; Ci, T. Near-infrared light triggered drug delivery system for higher efficacy of combined chemo-photothermal treatment. Acta Biomater. 2017, 51, 374–392.
- Chen, Y.W.; Liu, T.Y.; Chen, P.J.; Chang, P.H.; Chen, S.Y. A High-Sensitivity and Low-Power Theranostic Nanosystem for Cell SERS Imaging and Selectively Photothermal Therapy Using Anti-EGFR-Conjugated Reduced Graphene Oxide/Mesoporous Silica/AuNPs Nanosheets. Small 2016, 12, 1458–1468.
- Ye, J.; Fu, G.; Yan, X.; Liu, J.; Wang, X.; Cheng, L.; Zhang, F.; Sun, P.Z.; Liu, G. Noninvasive magnetic resonance/photoacoustic imaging for photothermal therapy response monitoring. Nanoscale 2018, 10, 5864–5868.
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26.
- Xing, C.; Chen, S.; Qiu, M.; Liang, X.; Liu, Q.; Zou, Q.; Li, Z.; Xie, Z.; Wang, D.; Dong, B. Conceptually novel black phosphorus/cellulose hydrogels as promising photothermal agents for effective cancer therapy. Adv. Healthc. Mater. 2018, 7, 1701510.
- Li, S.; Deng, Q.; Zhang, Y.; Li, X.; Wen, G.; Cui, X.; Wan, Y.; Huang, Y.; Chen, J.; Liu, Z. Rational Design of Conjugated Small Molecules for Superior Photothermal Theranostics in the NIR-II Biowindow. Adv. Mater. 2020, 32, 2001146.
- Song, J.; Yang, X.; Jacobson, O.; Huang, P.; Sun, X.; Lin, L.; Yan, X.; Niu, G.; Ma, Q.; Chen, X. Ultrasmall gold nanorod vesicles with enhanced tumor accumulation and fast excretion from the body for cancer therapy. Adv. Mater. 2015, 27, 4910–4917.
- de Melo-Diogo, D.; Pais-Silva, C.; Dias, D.R.; Moreira, A.F.; Correia, I.J. Strategies to improve cancer photothermal therapy mediated by nanomaterials. Adv. Healthc. Mater. 2017, 6, 1700073.
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297.
- Li, C.; Zhang, W.; Liu, S.; Hu, X.; Xie, Z. Mitochondria-targeting organic nanoparticles for enhanced photodynamic/photothermal therapy. ACS Appl. Mater. Interfaces 2020, 12, 30077–30084.
- Wang, J.; Wu, X.; Shen, P.; Wang, J.; Shen, Y.; Shen, Y.; Webster, T.J.; Deng, J.J.I.J.O.N. Applications of Inorganic Nanomaterials in Photothermal Therapy Based on Combinational Cancer Treatment. Int. J. Nanomed. 2020, 15, 1903.
- Khafaji, M.; Zamani, M.; Golizadeh, M.; Bavi, O. Inorganic nanomaterials for chemo/photothermal therapy: A promising horizon on effective cancer treatment. Biophys. Rev. 2019, 1–18.
- Luo, Y.; Wei, X.; Wan, Y.; Lin, X.; Wang, Z.; Huang, P. 3D printing of hydrogel scaffolds for future application in photothermal therapy of breast cancer and tissue repair. Acta Biomater. 2019, 92, 37–47.
- Ha, J.H.; Shin, H.H.; Choi, H.W.; Lim, J.H.; Mo, S.J.; Ahrberg, C.D.; Lee, J.M.; Chung, B. Electro-responsive hydrogel-based microfluidic actuator platform for photothermal therapy. Lab Chip 2020, 20, 3354–3364.
- Nele, V.; Wojciechowski, J.P.; Armstrong, J.P.; Stevens, M. Tailoring Gelation Mechanisms for Advanced Hydrogel Applications. Adv. Funct. Mater. 2020, 30, 2002759.
- Guo, Y.; Zhao, F.; Zhou, X.; Chen, Z.; Yu, G. Tailoring nanoscale surface topography of hydrogel for efficient solar vapor generation. Nano Lett. 2019, 19, 2530–2536.
- Zong, H.; Wang, B.; Li, G.; Yan, S.; Zhang, K.; Shou, Y.; Yin, J. Biodegradable High-Strength Hydrogels with Injectable Performance Based on Poly (l-Glutamic Acid) and Gellan Gum. ACS Biomater. Sci. Eng. 2020, 6, 4702–4713.
- Giobbe, G.G.; Crowley, C.; Luni, C.; Campinoti, S.; Khedr, M.; Kretzschmar, K.; De Santis, M.M.; Zambaiti, E.; Michielin, F.; Meran, L. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 2019, 10, 1–14.
- Buitrago, J.O.; Patel, K.D.; El-Fiqi, A.; Lee, J.-H.; Kundu, B.; Lee, H.-H.; Kim, H.-W. Silk fibroin/collagen protein hybrid cell-encapsulating hydrogels with tunable gelation and improved physical and biological properties. Acta Biomater. 2018, 69, 218–233.
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003.
- Chen, Y.; Gao, Y.; da Silva, L.P.; Pirraco, R.P.; Ma, M.; Yang, L.; Reis, R.L.; Chen, J. A thermo-/pH-responsive hydrogel (PNIPAM-PDMA-PAA) with diverse nanostructures and gel behaviors as a general drug carrier for drug release. Polym. Chem. 2018, 9, 4063–4072.
- Yata, T.; Takahashi, Y.; Tan, M.; Nakatsuji, H.; Ohtsuki, S.; Murakami, T.; Imahori, H.; Umeki, Y.; Shiomi, T.; Takakura, Y. DNA nanotechnology-based composite-type gold nanoparticle-immunostimulatory DNA hydrogel for tumor photothermal immunotherapy. Biomaterials 2017, 146, 136–145.
- Sang, Y.; Li, W.; Liu, H.; Zhang, L.; Wang, H.; Liu, Z.; Ren, J.; Qu, X. Construction of Nanozyme-Hydrogel for Enhanced Capture and Elimination of Bacteria. Adv. Funct. Mater. 2019, 29, 1900518.
- Sun, L.; Zhong, Y.; Gui, J.; Wang, X.; Zhuang, X.; Weng, J. A hydrogel biosensor for high selective and sensitive detection of amyloid-beta oligomers. Int. J. Nanomed. 2018, 13, 843.
- Jung, I.Y.; Kim, J.S.; Choi, B.R.; Lee, K.; Lee, H. Hydrogel based biosensors for in vitro diagnostics of biochemicals, proteins, and genes. Adv. Healthc. Mater. 2017, 6, 1601475.
- Shao, J.; Ruan, C.; Xie, H.; Li, Z.; Wang, H.; Chu, P.K.; Yu, X.F. Black-Phosphorus-Incorporated Hydrogel as a Sprayable and Biodegradable Photothermal Platform for Postsurgical Treatment of Cancer. Adv. Sci. 2018, 5, 1700848.
- Liu, Y.; Li, F.; Guo, Z.; Xiao, Y.; Zhang, Y.; Sun, X.; Zhe, T.; Cao, Y.; Wang, L.; Lu, Q. Silver nanoparticle-embedded hydrogel as a photothermal platform for combating bacterial infections. Chem. Eng. J. 2020, 382, 122990.
- He, J.; Shi, M.; Liang, Y.; Guo, B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020, 124888.
- Yang, Y.; Tan, Y.; Wang, X.; An, W.; Xu, S.; Liao, W.; Wang, Y. Photothermal nanocomposite hydrogel actuator with electric-field-induced gradient and oriented structure. ACS Appl. Mater. Interfaces 2018, 10, 7688–7692.
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127.
- Suvarnaphaet, P.; Pechprasarn, S.J.S. Graphene-based materials for biosensors: A review. Sensors 2017, 17, 2161.
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317.
- Zhang, D.; Wang, S.; Ma, Y.; Yang, S. Two-dimensional nanosheets as building blocks to construct three-dimensional structures for lithium storage. J. Energy Chem. 2018, 27, 128–145.
- Li, C.; Qiu, J.; Ou, J.-Y.; Liu, Q.H.; Zhu, J. High-sensitivity refractive index sensors using coherent perfect absorption on graphene in the vis-nir region. ACS Appl. Nano Mater. 2019, 2, 3231–3237.
- Liu, W.; Zhang, X.; Zhou, L.; Shang, L.; Su, Z. Reduced graphene oxide (rGO) hybridized hydrogel as a near-infrared (NIR)/pH dual-responsive platform for combined chemo-photothermal therapy. J. Colloid Interface Sci. 2019, 536, 160–170.
- Lima-Sousa, R.; de Melo-Diogo, D.; Alves, C.G.; Cabral, C.S.; Miguel, S.P.; Mendonça, A.G.; Correia, I.J. Injectable in situ forming thermo-responsive graphene based hydrogels for cancer chemo-photothermal therapy and NIR light-enhanced antibacterial applications. Mater. Sci. Eng. C 2020, 117, 111294.
- Chen, Y.-W.; Su, Y.-L.; Hu, S.-H.; Chen, S.-Y. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv. Drug Del. Rev. 2016, 105, 190–204.
- Savchuk, O.A.; Carvajal, J.; Massons, J.; Aguiló, M.; Díaz, F. Determination of photothermal conversion efficiency of graphene and graphene oxide through an integrating sphere method. Carbon 2016, 103, 134–141.
- Yang, K.; Wan, J.; Zhang, S.; Tian, B.; Zhang, Y.; Liu, Z. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials 2012, 33, 2206–2214.
- Link, S.; El-Sayed, M.A. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem. 2000, 19, 409–453.
- Zhu, C.H.; Lu, Y.; Peng, J.; Chen, J.F.; Yu, S.H. Photothermally sensitive poly (N-isopropylacrylamide)/graphene oxide nanocomposite hydrogels as remote light-controlled liquid microvalves. Adv. Funct. Mater. 2012, 22, 4017–4022.
- Schmaljohann, D. Thermo-and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670.
- Liu, J.-H.; Yang, S.-T.; Wang, H.; Chang, Y.; Cao, A.; Liu, Y. Effect of size and dose on the biodistribution of graphene oxide in mice. Nanomedicine 2012, 7, 1801–1812.
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2011, 6, 8.
- Fan, H.; Wang, L.; Zhao, K.; Li, N.; Shi, Z.; Ge, Z.; Jin, Z. Fabrication, mechanical properties, and biocompatibility of graphene-reinforced chitosan composites. Biomacromolecules 2010, 11, 2345–2351.





