
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Arne Baudach | + 2931 word(s) | 2931 | 2021-04-15 09:48:54 | | | |
| 2 | Rita Xu | Meta information modification | 2931 | 2021-04-16 06:02:15 | | |
Video Upload Options
The European map butterfly (Araschnia levana) looks different in spring and summer due to day length and temperature. If the butterfly’s caterpillars receive at least 16 h of light per day, the resulting butterfly hatches a few weeks later in summer with blackish wings (f. prorsa). However, if caterpillars receive less than 15.5 h of daylight, many overwinter as pupae. The imagos emerging in the following spring, have predominantly organge wings with black spots (f. levana). This whole process is guided by hormones and epigenetics.
1. Introduction
The phenotype of an organism is dependent on the genome and its epigenetic regulation, based on a combination of cellular memory and interactions with the environment [1]. The term phenotypic plasticity thus refers to the ability of an organism to generate different phenotypes from the same genotype under different environmental conditions [2]. Phenotypic plasticity often facilitates adaptive changes by increasing phenotypic diversity in response to environmental challenges. Polyphenism is a special case of phenotypic plasticity in which the outputs are discrete and discontinuous, resulting in multiple distinct phenotypes from the same genetic background [3]. Some of the most striking examples of polyphenism in animals include sex determination in reptiles and fish regulated by temperature and social factors [4][5], the defense polyphenism in cladocerans [6], the sexual and wing polyphenism in aphids [7], and seasonal polyphenism in butterflies [8].
In 1758, Carl Linnaeus described two apparently distinct butterfly species, which he named Papilio levana and P. prorsa, but subsequent field observations and breeding experiments revealed them to be seasonal variants of the same bivoltine species: The European map butterfly Araschnia levana [9][10]. The dorsal wing of the spring generation (A. levana f. levana) is orange to reddish-brown (basic coloration) with black spots, some white dots, and a bluish dotted rim on the posterior of the hindwing (Figure 1). Conversely, the dorsal wing of the summer generation (A. levana f. prorsa) is brownish to bluish-black with a prominent white band (featuring varying degrees of melanization) located basally with respect to 1–3 apical orange bands (Figure 1). Many variations between these two phenotypes have been reported, and they are best described as a spectrum. Some of these occur naturally as A. levana f. porima, with patterning and coloration appearing intermediate between levana and prorsa. However, most are the result of experimental manipulation [10][11]. The elements of the wing underside are consistent between morphs and gave rise to the genus name Araschnia. The basic coloration is a darkish brown with another prominent whitish band separating the apical and basal parts of the wings. The veins have whitish scales and, with their fine-crossed connections, form a grid that is reminiscent of a spider web or map.
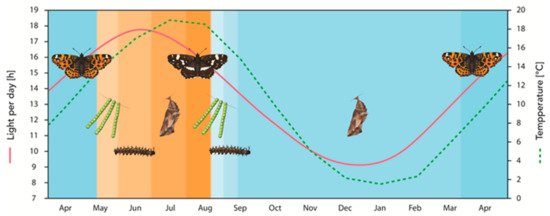
Figure 1. Annual life cycle and phenotype succession of A. levana depending on photoperiod and temperature. Values for day length and temperature correspond to the administrative district of Giessen, Hesse, Germany. Long day lengths (>15.5 h) and high temperatures (larval development from spring to midsummer) result in pupal subitaneous development and the expression of the adult prorsa form, emerging in summer. Conversely, short day lengths (<15.5 h) and low temperatures (larval development from late summer to early autumn) result in pupal diapause and the development of the adult levana form, emerging in spring. The brown color gradient represents prorsa development, blue color gradient represents levana development. The dotted line corresponds to the threshold day length of >15.5 h per day, below which levana development becomes increasingly likely. The developmental trajectory is not affected by environmental cues during embryonic development (green egg towers). For details, see text.
The remarkable change from vernal to estival phenotypes has attracted much interest from scientists. Naturalists initially tested the effect of temperature to determine the biophysical basis of the phenomenon [12][13][14], but their findings were also contested by contemporaries [15]. Alternatively, strict cyclic heritability was proposed to explain the rhythmic alternation of spring and summer phenotypes [16]. The first studies on seasonal changes in insects were conducted by Marcovitch in the 1920s, who revealed a connection between the appearance of sexual forms in relation to day length in aphids [17]. However, it was not until the 1950s that the true ecological parameters responsible for the polyphenic shift in A. levana were revealed. Müller (1955) demonstrated that larvae of either (parental) generation developed directly to become subitaneous pupae and then displayed the adult prorsa (long-day or summer) phenotype if they were exposed to light for more than 16 h per day, whereas all larvae became diapause pupae and thus displayed the adult levana (short-day or spring) phenotype if they were exposed to light for less than 8 h per day [18]. Following this breakthrough discovery, the physiological basis of polyphenism was the next problem to be addressed [19]. Another species, the Satyrid Bicyclus anynana, seasonally exhibits striking differences in the ventral wing pattern too [20]. In this model species, the plasticity of the eyespot size is mostly regulated by temperature, which—in the wandering stage—leads to changing titers of the hormone 20-hydroxyecdysone (20E) [21]. If the 20E signaling is manipulated at that specific time in development, eyespot size can easily be modified. However, for A. levana, it remained unclear for over 60 years how the physiological switches were likely regulated at the molecular level to orchestrate the manifestation of two discrete phenotypes [22][23].
2. Environment and Phenotype
2.1. Photoperiodism and Temperature
The longstanding assumption that temperature was the abiotic factor responsible for the phenotypic switch in A. levana was elegantly refuted by Müller (1955). Before presenting his findings, he remarked on the matter of temperature: “In the field, it cannot be the cause, as it is on average about the same during the crucial developmental phases of the two generations” [18]. In his experiment, he assigned offspring from both generations into four treatment groups. He then reared one group from each parental generation under two separate light regimes but otherwise identical conditions, in particular, at the same temperature. The two groups exposed to more than 16 h of light per day developed exclusively into subitaneous pupae and thus into the prorsa form, whereas the two groups reared under short-day conditions (8 h of light per day) invariably developed into diapause pupae and thus into the levana form, regardless of the parental generation [18].
Because the potentially modifying influence of temperature was still unclear at this point, Müller subsequently investigated the effects of the distinct light regimes at two different temperatures: 20 and 30 °C [24]. At 20 °C, all larvae developed into the levana form if exposed to fixed light regimes of 4–15 h per day, whereas there was an inverse relationship between light duration and the proportion of prorsa individuals in the same photoperiodic range when larvae were reared at 30 °C. Specifically, longer photoperiods led to a steady decline in the proportion of prorsa individuals. When the day length was 6 h, the proportions of prorsa and levana adults were approximately equal, but when the day length was 12 h the ratio was 3% prorsa to 97% levana. However, at both temperatures, a switch occurred between day lengths of 15.5 and 16.5 h. More than 16.5 h resulted in the complete inhibition of levana development, yielding 100% prorsa adults. When the photoperiod falls below 15–16 h (daylight lasts for 15.5 h between the middle of May and late July, in this study at app. 51°8′ N 11°1′ E), the temperature is, therefore, used as an additional cue to determine whether direct development or diapause is preferred.
Later work showed that the critical photoperiod is longer at lower temperatures, with a temperature regime of 15 °C shifting the photoperiod needed for direct development towards longer days [25]. Exposure to 16 h of daylight at this temperature still committed little more than half of all larvae to direct development. These findings indicate that longer photoperiods are required to induce direct development at lower temperatures, whereas shorter photoperiods are sufficient at higher temperatures, although the latter only applies to photoperiods of less than 12 h. In an ecological context, this means that warmer temperatures tip the risk–benefit ratio in favor of direct development (betting on continued beneficial conditions), whereas cooler temperatures have the opposite effect (betting on an overwintering strategy). The increase in critical day length is more likely to prevent direct development and consequently the formation of a potential third butterfly generation. In Central Europe, this reflects conditions in the wild, where prorsa larvae develop from mid-May to mid-July at day lengths of at least 16.5 h and mean temperatures of 15–18 °C [18][24][25] (Figure 1). In contrast, levana larvae develop in August and September, when the mean temperature is initially ~19 °C but quickly declines to ~11 °C by the beginning of October [18]. Day length during the same period declines from 15.5 to 11.5 h (Figure 1). This suggests that the photoperiod takes precedence as the key climate predictor with the ultimate decision-making role, but it can be modified and fine-tuned by prevailing temperatures.
The development of subitaneous or diapause pupae depends on the day length in the mid (but not early or late) larval stages, with a critical photoperiod of ~15.5 h [24][26]. These findings have been modified by a more recent study [27], although direct comparisons are not possible because the data provided in the original studies are not precise. Larvae were reared at 23 °C under short-day conditions (12 h photoperiod) or long-day conditions (20 h photoperiod), and subsets were transferred between conditions in both directions in each of the five instars. Transfer from long-day to short-day conditions during the first three larval stages invariably led to diapause development, whereas the transferred fourth-instar larvae yielded ~40% prorsa adults and transferred final-instar larvae yielded 100% prorsa adults [27]. A fixed number of long days (18 h photoperiod) is necessary for direct development, representing up to half of the entire larval development period (~23 days) at 20 °C [25]. This indicates that there is a point of commitment during the fourth-instar stage beyond which diapause development is no longer possible, an advantageous strategy given the additional preparations needed to survive winter, such as general physiological changes, the formation of denser tissues, and the thickening of the cuticle [27]. On the other hand, it also implies that natural selection favors a decision made late in larval development, when larvae have the most current information about their position in the season. Friberg and colleagues also showed that transfer from short-day to long-day conditions during the first four larval stages led reproducibly to 100% prorsa adults, and even when switched during the final larval stage, there was still a 50% likelihood of prorsa development. In nature, longer day lengths correspond to spring and early summer (Figure 1). In years with early-season high temperatures, imagoes may emerge ahead of time, as reported in 1990 in the south-west of Germany [28]. After mating and oviposition, larval development may, therefore, start when day lengths are below the critical photoperiod for prorsa development of >15.5 h. This threshold is likely to be even higher, given that the mean early-season temperatures are still comparatively low even in unusually warm years and low temperatures require longer day lengths in order to achieve direct development (contrast with the modification of critical day length by low temperatures, as discussed above). In such cases, it would, therefore, be beneficial if larvae were able to identify and respond to a “switch from short days to long days” and accordingly favor the subitaneous pathway over diapause throughout larval development.
Temperature can also modify photoperiod effects at later stages of development. As with many nymphalids and other butterflies, the influence of higher or lower temperatures during early pupal development can lead to a brightening or darkening of wing color patterns in both Araschnia generations. However, a complete change from levana to prorsa or vice versa is not possible [29]. For subsequent development, only the temperature is relevant because both pupae and imagoes are profoundly insensitive to day length. During diapause, pupae must undergo a cool period (0–10 °C) lasting at least 3 months before eclosion can be induced by spring temperatures of 12–24 °C [26].
2.2. Food Quality
Not only day length and temperature change seasonally but also availability, composition, and quality of food sources. These may, therefore, have an impact on the phenotype in their own right. In both generations, A. levana larvae are strictly monophagous and feed exclusively on leaves of the stinging nettle Urtica dioica. However, the nutritional quality of this plant deteriorates as the nettle matures and thus also varies seasonally [30][31][32]. Seasonal variations in adult food sources are also conceivable, suggesting that food quality may influence the fitness of A. levana, which may contribute to polyphenism. Access to carbohydrates, nitrogen, and amino acids across juvenile and adult stages is known to influence the adult size, longevity, and fecundity in many lepidopteran species [30][33]. Adult female A. levana f. prorsa reared on a low-quality larval diet preferred a high-quality nectar mimic containing both essential and non-essential amino acids [33]. The authors proposed that such a preference implies that adult resources are more important when larval reserves are poor and that butterflies may compensate for adverse larval conditions by selective adult feeding. They further demonstrated a negative relationship between emergence mass and amino acid preference regardless of the larval diet, with amino acid preference diminishing as female mass increased. Interestingly, male larvae did not display any food preference regardless of the larval feeding state or emergence mass, suggesting this trait is linked to female fecundity or sex determination.
A follow-up study tested whether the use of nectar amino acids by adult female A. levana f. prorsa increased fecundity [30]. The authors evaluated the effect of low-quality and high-quality larval diets (based on the U. dioica leaf nitrogen content) combined with adult high-quality or low-quality nectar mimics (with or without amino acids). They compared the effects of these four diets on multiple fecundity parameters, including the number of eggs laid, egg mass, longevity, and hatching rate. Female emergence mass (reflecting larval food quality), adult nectar diet, and the amount of nectar consumed all had significant effects on the number of eggs laid. Individuals produced fewer eggs only if they were reared on a low-quality larval diet and, as adults, nectar lacking amino acids. The egg number was on par in the three other groups, including individuals reared on a low-quality larval diet but switched to the amino acid-rich high-quality nectar as adults. However, the egg mass, carbon to nitrogen (C/N) ratio, and hatching rate stayed the same even under adverse conditions, indicating that the fitness cost is purely quantitative. Furthermore, there was no significant effect on longevity in any treatment group, and the C/N ratio of abdomens from female specimens did not vary. The authors reported a positive correlation between butterfly emergence mass and the total number of eggs laid. Emergence mass depends on larval nutrition, and poor-quality foliage is typical for wild A. levana larvae feeding later in the year. This supports the presence of polyphenism in addition to compensatory adult feeding.
Differences have been reported in the body composition of pupae and imagoes depending on sex and/or light regime applied during the rearing of larvae [32]. Groups were reared with a 16 h photoperiod representing the spring and early summer conditions of the subitaneous prorsa generation and a 12 h photoperiod representing the late summer and early autumn conditions of the diapausing levana generation [32]. The water content of male and female pupae was lower in levana than prorsa individuals, and the male levana pupae also featured lower concentrations of lipids, but there were no significant differences in sugar or protein content and dry weight. Interestingly, the differences were not apparent in the adults. Indeed, the water content was higher in levana than prorsa imagoes at eclosion, and there was no seasonal difference in lipid content. These findings were put forward as evidence for phenotypic traits associated with pupal diapause and overwintering [32]. For example, diapausing levana pupae may be adapted specifically to prevent further dehydration, allowing them to enter pupation with a lower water content, protecting them against freezing. The authors did not report an increase in sugar content, but suggested that more subtle changes in sugars related to freeze tolerance in levana pupae may have been overlooked. This is because their methods were insensitive to variations in sugars associated with regular metabolism (such as glucose) and sugars that act as cryoprotectants (such as trehalose) but also polyols such as glycerol with a similar role.
The levana adults weighed less than their prorsa counterparts, which may reflect the metabolic cost of diapause and overwintering [32]. Notably, the lower body mass primarily affected the adult head, thorax, and wings. Because levana adults also emerged with lower protein concentrations, the flight musculature (and, by extension, flight capacity) is likely to be affected, consistent with findings in field-caught butterflies [32][34][35]. Both morphs in the study were fed on the same larval diet of freshly-picked U. dioica collected between July and August, representing the mediocre to poor food quality typically encountered by levana larvae. The study design is, therefore, likely to have masked any polyphenic effects related to differences in food quality.
Recently, a study by Esperk and Tammaru (2021) reported comparisons of various parameters of larval growth schedules in a 2 × 2 × 2 crossed design with photoperiod, temperature, and host plant quality as the varied factors [36]. Specifically (among other findings), they showed that levana larvae spent more time in both final and penultimate larval instars. Lower (but not higher) temperatures, also promoted lower levana pupal masses. In contrast, prorsa larvae displayed higher growth rates—a pattern that was consistent across different rearing conditions, sexes, and larval instars. These authors concluded that their findings demonstrated that the between-generation differences in development have a significant element of anticipatory plasticity and thus should be considered adaptive.
The latter study elegantly demonstrated how the approaches of the other studies described above could be combined to disentangle further the responses to food regimes as well as polyphenic adaptations to predictable seasonal variations in nutrition.
References
- Panzeri, I.; Pospisilik, J.A. Epigenetic control of variation and stochasticity in metabolic disease. Mol. Metab. 2018, 14, 26–38.
- Geng, Y.; Gao, L.; Yang, J. Epigenetic Flexibility Underlying Phenotypic Plasticity. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2013; pp. 153–163.
- Yang, C.H.; Pospisilik, J.A. Polyphenism—A window into gene-environment interactions and phenotypic plasticity. Front. Genet. 2019, 10, 132.
- Janzen, F.J.; Phillips, P.C. Exploring the evolution of environmental sex determination, especially in reptiles. J. Evol. Biol. 2006, 19, 1775–1784.
- Liu, H.; Todd, E.V.; Lokman, P.M.; Lamm, M.S.; Godwin, J.R.; Gemmell, N.J. Sexual plasticity: A fishy tale. Mol. Reprod. Dev. 2017, 84, 171–194.
- Miyakawa, H.; Gotoh, H.; Sugimoto, N.; Miura, T. Effect of juvenoids on predator-induced polyphenism in the water flea, Daphnia pulex. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2013, 319, 440–450.
- Mukherjee, K.; Baudach, A. Epigenetic Control of Polyphenism in Aphids. In Biology and Ecology of Aphids; Vilcinskas, A., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 89–99.
- Brakefield, P.M.; Frankino, W.A. Polyphenisms in Lepidoptera: Multidisciplinary approaches to studies of evolution. In Phenotypic Plasticity in Insects. Mechanisms and Consequences; Whitman, D.W., Ananthakrishnan, N.T., Eds.; Science Publishers: Plymouth, UK, 2007; pp. 121–151.
- Von Linnaeus, C. Systema Naturae; Laurentii Salvii: Stockholm, Sweden, 1758.
- Reinhardt, R. Der Landkärtchenfalter Araschnia levana—Einfluß der Umwelt auf den Gestaltswechsel, 2nd ed.; Die Neue Brehm-Bücherei: Karl-Marx-Stadt, Germany, 1984.
- Fric, Z.; Konvicka, M. Adult population structure and behaviour of two seasonal generations of the European Map Butterfly, Araschnia levana, species with seasonal polyphenism (Nymphalidae). Nota Lepidopterol. 2000, 23, 2–25.
- Dorfmeister, G. Über die Einwirkung verschiedener, während der Entwicklungsperioden angewendeter Wärmegrade auf die Färbung und Zeichnung der Schmetterlinge. Mitt. Naturw. Ver. Steiermark 1864, 99–108.
- Dorfmeister, G. Über den Einfluß der Temperatur bei der Erzeugung von Schmetterlingsvaritäten. Mitt. Naturw. Ver. Steiermark 1879, 1–8.
- Standfuß, M. Experimentelle zoologische Studien mit Lepidopteren. A Temperaturexperimente. N. Denkschr. Schweiz. Ges. 1891, 36, 1–82.
- Fischer, E. Zur Physiologie der Aberrationen- und Varietätenbildung der Schmetterlinge. Arch. Rassen Ges. 1907, 761–792.
- Weismann, A. Studien zur Descendenz-Theorie. I. Über den Saison-Dimorphismus der Schmetterlinge; Verlag von Wilhelm Engelmann: Leipzig, Germany, 1875; ISBN 9788578110796.
- Marcovitch, S. The migration of the aphididae and the appearance of the sexual forms as affected by the relative length of daily light exposure. J. Agric. Res. 1924, XXVII, 513–522.
- Müller, H.J. Die Saisonformenbildung von Arachnia levana, ein photoperiodisch gesteuerter Diapauseeffekt. Naturwissenschaften 1955, 42, 134–135.
- Koch, P.B.; Bückmann, D. Hormonal control of seasonal morphs by the timing of ecdysteroid release in Araschnia levana L. (Nymphalidae: Lepidoptera). J. Insect Physiol. 1987, 33, 823–829.
- Brakefield, P.M.; Reitsma, N. Phenotypic plasticity, seasonal climate and the population biology of Bicyclus butterflies (Satyridae) in Malawi. Ecol. Entomol. 1991, 16, 291–303.
- Monteiro, A.; Tong, X.; Bear, A.; Liew, S.F.; Bhardwaj, S.; Wasik, B.R.; Dinwiddie, A.; Bastianelli, C.; Cheong, W.F.; Wenk, M.R.; et al. Differential Expression of Ecdysone Receptor Leads to Variation in Phenotypic Plasticity across Serial Homologs. PLoS Genet. 2015, 11, 1–20.
- Vilcinskas, A.; Vogel, H. Seasonal phenotype-specific transcriptional reprogramming during metamorphosis in the European map butterfly Araschnia levana. Ecol. Evol. 2016, 6, 3476–3485.
- Mukherjee, K.; Baudach, A.; Vogel, H.; Vilcinskas, A. Seasonal phenotype-specific expression of microRNAs during metamorphosis in the European map butterfly Araschnia levana. Arch. Insect Biochem. Physiol. 2020, 104, e21657.
- Müller, H.J. Die Wirkung verschiedener diurnaler Licht-Dunkel-Relationen auf die Saisonformenbildung von Araschnia levana. Naturwissenschaften 1956, 21, 503–504.
- Kratochwil, A. Die Anpassung der Generationenfolge von Araschnia levana L. (Lepidoptera, Nymphalidae) an den jahreszeitlichen Witterungsverlauf. Verh. Ges. Okol. 1980, Vlll, 395–401.
- Müller, H.J.; Reinhardt, R. Die Bedeutung von Temperatur und Tageslänge für die Entwicklung der Saisonformen von Araschnia levana L. (Lep. Nymphalidae). Entomol. Berichte 1969, 93–100.
- Friberg, M.; Haugen, I.M.A.; Dahlerus, J.; Gotthard, K.; Wiklund, C. Asymmetric life-history decision-making in butterfly larvae. Oecologia 2011, 165, 301–310.
- Steiner, A. Extreme Flugzeiten von Schmetterlingen in den Jahren 1989 und 1990—Auswirkungen der weltweiten Klimaveränderung? Atalanta 1991, 22, 237–244.
- Müller, H.J. Die Bedeutung der Photoperiode im Lebenslauf der Insekten. Z. Entomol. 1960, 47, 7–24.
- Mevi-Schütz, J.; Erhardt, A. Amino acids in nectar enhance butterfly fecundity: A long-awaited link. Am. Nat. 2005, 165, 411–419.
- Pullin, A.S. Changes in Leaf Quality Following Clipping and Regrowth of Urtica dioica, and Consequences for a Specialist Insect Herbivore, Aglais urticae. Oikos 1987, 49, 39.
- Morehouse, N.I.; Mandon, N.; Christides, J.P.; Body, M.; Bimbard, G.; Casas, J. Seasonal selection and resource dynamics in a seasonally polyphenic butterfly. J. Evol. Biol. 2013, 26, 175–185.
- Mevi-Schütz, J.; Erhardt, A. Larval nutrition affects female nectar amino acid preference in the map butterfly (Araschnia levana). Ecology 2003, 84, 2788–2794.
- Fric, Z.; Klimova, M.; Konvicka, M. Mechanical design indicates differences in mobility among butterfly generations. Evol. Ecol. Res. 2006, 8, 1511–1522.
- Fric, Z.; Konvička, M. Generations of the polyphenic butterfly Araschnia levana differ in body design. Evol. Ecol. Res. 2002, 4, 1017–1032.
- Esperk, T.; Tammaru, T. Ontogenetic Basis of Among-Generation Differences in Size-Related Traits in a Polyphenic Butterfly. Front. Ecol. Evol. 2021, 9, 1–11.




