
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gauri Gangapurwala | + 1489 word(s) | 1489 | 2021-01-13 08:00:15 | | | |
| 2 | Camila Xu | + 1 word(s) | 1490 | 2021-04-15 10:17:35 | | | | |
| 3 | Camila Xu | Meta information modification | 1490 | 2021-04-27 04:44:54 | | |
Video Upload Options
Supercritical carbon dioxide (SC-CO2) based techniques can be exploited for the formulation of polymeric nanocarriers, limiting the use of toxic organic solvent. The currently approved FDA pharmaceutical polymers like PLA and PLGA particles can be obtained in the micro-and nanometer range by techniques that involve SC-CO2 as solvent (RESS, RESOLV), anti-solvent (SAS, SEDS, SAILA) or extractant (SFEE), depending on the SC-CO2 compatibility with the system materials and the final product.
1. Introduction
The targeted treatment of diseases with drug-loaded carriers is becoming a substantial reality in clinical practice [1]. Diverse nano- and micrometer-sized systems based on liposomes, polymeric nanoparticles (NPs), dendrimers, and micelles are on their way to change modern medicine. The sustainability of the production of these systems, however, is still a challenge.
Conventional production strategies for the preparation of drug-loaded liposomes [2][3] or particles [4][5] usually involve organic solvents, such as tetrahydrofuran or ethyl acetate, which are often considered a health risk and contribute to environmental risks [6]. Thus, they have to be removed from the final product, together with the excess of stabilizers, by additional purification or cleaning procedures [7] which is usually followed by freeze-drying or lyophilization that leads to dry powdered product [8]. Consequently, there is an increasing demand for efficient alternative strategies that rely on sustainable chemistry and can replace (potentially) toxic organic solvents, and which additionally, offer advantages in biodegradation, waste reduction, time and energy efficiency, and in the use of renewable raw materials [9][10]. One method that is attracting significant attention in recent years is the supercritical fluid (SCF) technology, in particular the liposome and particle formulation with supercritical carbon dioxide (SC-CO2) [11]. SC-CO2 is the most economical, chemically stable, and benign compound in its supercritical state, and of high interest to replace common organic solvents.
2. Carbon Dioxide as Supercritical Fluid
SC-CO2 is presently very popular as a green alternative for several applications including extraction, production of polymers, removal of residual solvents or monomers and formulation of polymeric particles, foams or scaffolds [12]. The word “supercritical” defines the state of the solvent, which is a prerequisite for using it in the areas mentioned above. This physical state was already described in 1822 by Cagnaird de la Tour, who observed the disappearance of the boundaries between the liquid and gaseous phases when increasing the temperature far above the boiling point of water [13]. The author described the critical pressure and critical temperature concepts, including the critical point (Figure 1). SCFs impart valuable physicochemical properties such as low viscosity, high diffusivity, and high compressibility that are a combination of the liquid and gaseous states of the compound and are largely influenced by a slight change in pressure and temperature. The generation of SCFs is a reversible process; i.e., SCF goes back to liquid or gaseous phase depending on the pressure and temperature of the system. Examples of commonly known SCFs are CO2, acetone, chlorodifluoromethane, ethanol, hexane, propane, pentane, toluene as well as water. The supercritical conditions for acetone, ethanol, propane, toluene, and water are hard to achieve due to the high pressure and temperature required. Moreover, water in its supercritical state is highly corrosive and harsh [14]. On the contrary, CO2 can achieve its supercritical state at “mild” conditions, T = 31 °C and P = 73 bar. Additionally, it is non-toxic, chemically stable, non-flammable, presents low surface tension and is cost-effective.
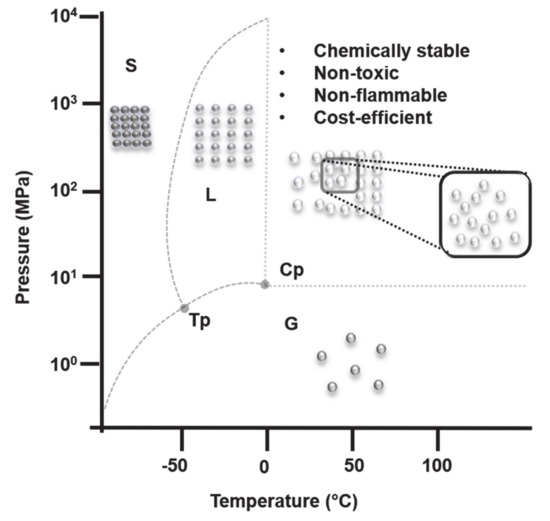
Figure 1. Phase diagram of carbon dioxide (CO2) (not to scale). S, L, and G denote the solid, liquid and gaseous phases respectively. Cp denotes the critical pressure point (31 °C, 7.3 Mpa) and Tp denotes the triple point (−56 °C, 0.5 MPa) for CO2.
3. Solubility of Polyesters in SC-CO2
In the light of prior research and studies, SC-CO2 is an established solvent for small molecules but not for organic polymers. Also, SC-CO2 is nonpolar and aprotic and hence exhibits certain selective solubility for ionic and polar compounds as well as for higher molar mass polymers [15]. The solubility of a polymer in SC-CO2 depends on its chemical structure as well as on its physicochemical and mechanical properties, e.g., molar mass, end groups (e.g., fluorine, amine), the architecture (branched or linear), Tg and crystallinity. However, it also depends on the experimental conditions, such as pressure, temperature, and the use or not of a co-solvent in the system. The most important parameters influencing the solubility of a polymer in SC-CO2 are presented in Figure 2. Moreover, the main parameters for the solubility of polyesters are briefly discussed in the following.
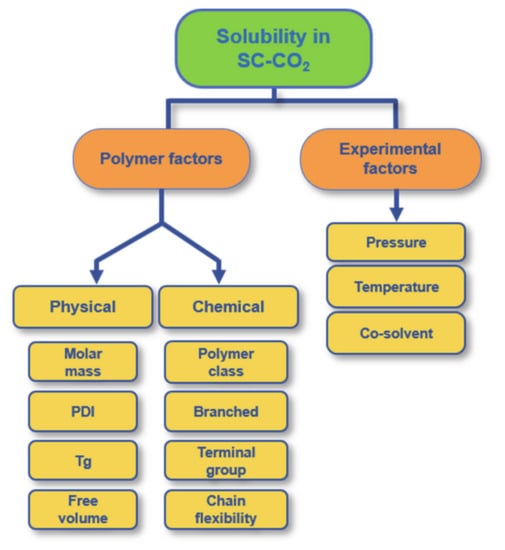
Figure 2. Parameters that influence the solubility of a polymer in SC-CO2 divided into the two main categories “polymer factors” and “experimental factors” with their illustrated subgroups.
Compared to other polymer classes such as fluoropolymers, polyesters such as PLA generally reveal a limited solubility in SC-CO2, which was explained among other factors by their lower chain flexibility [15]. Nevertheless, the solubility of the polyesters can be positively influenced, e.g., by decreasing the molar mass and dispersity of the polymer. It is also worth mentioning that the introduction of terminal groups, such as carboxyl, hydroxyl, acetate, or fluorine groups that modify the interactions between the polymer and the CO2 molecule, plays an important role in the solubility properties of the polymer. Early study from J. Gregorowicz et al. confirmed the influence of molar masses and terminal groups on the solubility by illustrating the difference of solubility between two different molar mass PLLA polymers with two different terminal groups. The study suggests that for low molar mass systems the solubility is controlled by the end groups rather than by the molar mass [16]. The architecture of the polymer and subsequently its free volume plays a vital role in the solubility in SC-CO2. The free volume refers hereby to the free spaces left in the solid-state of the polymeric chain at the molecular level [17]. The free volume of the polymer chain is directly proportional to the polymer chain flexibility and leads to higher solvent diffusion and enhanced solubility in the SC-solvent. Influenced by the architecture, the free volume of a highly branched polymer is higher and, thus, it is more likely to be soluble compared to a linear polymer [18][19]. Moreover, the solubility can be positively influenced by hyperbranching, a shorter chain length including the addition of several acetate or fluorine moieties as terminal groups [20][21]. Finally, the solubility of a polymer is determined by the density of SC-CO2, which is dependent on the process parameters and tends to elevate with pressure. With increasing pressure, the polymer solubility is likely to be enhanced.
However, if the polymer remains insoluble in SC-CO2, even under high-pressure conditions, polar co-solvents such as ethanol or acetone are used. The addition of these solvents contributes to decreasing the difference in the free volume between the polymer and the SCF solvent and, hence, increasing the solubility of the polymer in the system [18].
4. SC-CO2-based formulation of PLA/PLGA particles
The previous section provided a basic understanding of SC-CO2-based principles. This section intends to serve as an overview of completed studies related to the production of drug delivery systems consisting of PLA/PLGA-based polymers until recently. J. W.Tom and P. G. Debenedetti in 1991 introduced the use of SC-CO2 for the formulation of polyester particles. A recent study used a mixture of dimethyl sulfoxide (DMSO) and CH2Cl2 (ratio 1:1 v/v %) within the SAS process for the production of PLLA-coated 5-Fu (5-Fluorouracil). The obtained size for the coated particles varied from 0.6 to 1.2 µm (at 35 and 50 ◦C respectively) and the drug load ranged from 32 to 42%. The dissolution profile clearly showed a slower release rate from the PLLA-coated 5-Fu particles compared to the micronized particles.
The most applied technique is Supercritical Fluid emulsion extraction was published in 2019 by C. Gimenez-Rota et al. β-carotene was encapsulated together with the anti-oxidants α-tocopherol and rosmarinic acid into PLLA and PLGA particles. Experiments with several different double emulsions and various external phases were shown and the SFEE process was carried out at 80 bar and 37 ◦C throughout all the experiments with a constant liquid-gas ratio of 0.1. The final sizes for the loaded PLLA particles ranged from 0.5 to 1.4 µm with the EE was calculated to be 72% and 62%, respectively. For PLGA, the same droplet sizes (2.1 and 1.2 µm) were tested and particles of 2 and 0.3 µm with an EE of 52% and 62%, respectively, were reported.
The supercritical fluid-based technologies are environmental friendly approaches for the formulation of drug delivery systems. Although the process seems convenient, previous work demonstrates the need for optimization for each polymer system and cannot be universally applicable. SC-CO2 is the best choice as SCF for the pharmaceutical industry, but the SC-CO2-based techniques are not yet answered for scale-up processes, e.g., the GMP cost estimation, calculation for estimation of parts of GMP equipment, together with the above-mentioned lack of standard processing conditions. Nevertheless, it is obvious that although the advantages of the SC-CO2-based processes are numerous, many gaps in knowledge still need to be bridged. Thus, rigorous evaluation and detailed investigation are necessary for a broad and successful application of this technology in the near future.
References
- Farjadian, F.; Ghasemi, A.; Gohar, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine (London) 2019, 14, 93–126.
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799.
- Chang, H.-I.; Yeh, M.-K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60.
- Schubert, S.; Delaney, J.T.; Schubert, U.S. Nanoprecipitation and nanoformulation of polymers: From history to powerful possibilities beyond poly(lactic acid). Soft Matter 2011, 7, 1581–1588.
- Rosca, I.D.; Watari, F.; Uo, M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J. Control. Release 2004, 99, 271–280.
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7.
- Shkodra-Pula, B.; Grune, C.; Traeger, A.; Vollrath, A.; Schubert, S.; Fischer, D.; Schubert, U.S. Effect of surfactant on the size and stability of PLGA nanoparticles encapsulating a protein kinase C inhibitor. Int. J. Pharm. 2019, 566, 756–764.
- Konan, Y.N.; Gurny, R.; Allemann, E. Preparation and characterization of sterile and freeze-dried sub-200 nm nanoparticles. Int. J. Pharm. 2002, 233, 239–252.
- Raston, C. Renewables and Green Chemistry. Green Chem. 2005, 7, 57.
- Butler, M.; Olga, K. Introduction. In Green Chemistry Guide; Butler, M., Rivin, J.M., Eds.; National Pollution Prevention Roundtable: Los Angeles, CA, USA, 2015; pp. 1–5. Available online: (accessed on 19 November 2020).
- Ciaglia, E.; Montella, F.; Trucillo, P.; Ciardulli, M.C.; Di Pietro, P.; Amodio, G.; Remondelli, P.; Vecchione, C.; Reverchon, E.; Maffulli, N.; et al. A bioavailability study on microbeads and nanoliposomes fabricated by dense carbon dioxide technologies using human-primary monocytes and flow cytometry assay. Int. J. Pharm. 2019, 570, 118686.
- DeSimone, J.M.; Tumas, W. Green Chemistry Using Liquid and Supercritical Carbon Dioxide; Oxford University Press: New York, NY, USA, 2003.
- Berche, B.; Henkel, M.; Kenna, R. Fenômenos críticos: 150 anos desde Cagniard de la Tour. Rev. Bras. de Ensino de Fis. 2009, 31, 2602-1–2602-4.
- Williams, J.R.; Clifford, A.A. Introduction to Supercritical Fluids and Their Applications; Humana Press: Totowa, NJ, USA, 2003; pp. 1–16.
- Girard, E.; Tassaing, T.; Marty, J.-D.; Destarac, M. Structure-Property Relationships in CO2-philic (Co)polymers: Phase Behavior, Self-Assembly, and Stabilization of Water/CO2 Emulsions. Chem. Rev. 2016, 116, 4125–4169.
- Gregorowicz, J.; Bernatowicz, P. Phase behaviour of L-lactic acid based polymers of low molecular weight in supercritical carbon dioxide at high pressures. J. Supercrit. Fluids 2009, 51, 270–277.
- Wilson, R.; George, S.C.; Kumar, S.A.; Thomas, S. Liquid Transport Characteristics in Polymeric Systems. In Transport Properties of Polymeric Membranes; Elsevier B.V.: Amsterdam, The Netherlands, 2018; pp. 3–13.
- Kirby, C.F.; McHugh, M.A. Phase Behavior of Polymers in Supercritical Fluid Solvents. Chem. Rev. 1999, 99, 565–602.
- Rindfleisch, F.; DiNoia, T.P.; McHugh, M.A. Solubility of Polymers and Copolymers in Supercritical CO2. J. Phys. Chem. 1996, 100, 15581–15587.
- Suttiruengwong, S.; Rolker, J.; Smirnova, I.; Arlt, W.; Seiler, M.; Luderitz, L.; Perez de Diego, Y.; Jansens, P.J. Hyperbranched polymers as drug carriers: Microencapsulation and release kinetics. Pharm. Dev. Technol. 2006, 11, 55–70.
- Gregorowicz, J.; Fras, Z.; Parzuchowski, P.; Rokicki, G.; Kusznerczuk, M.; Dziewulski, S. Phase behaviour of hyperbranched polyesters and polyethers with modified terminal OH groups in supercritical solvents. J. Supercrit. Fluids 2010, 55, 786–796.




