
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | XUEJING ZHANG | + 1875 word(s) | 1875 | 2021-04-08 05:29:53 | | | |
| 2 | Dean Liu | -10 word(s) | 1865 | 2021-04-15 09:41:45 | | |
Video Upload Options
Protein kinase D (PKD) is a family of serine/threonine protein kinases operating in the signaling network of the second messenger diacylglycerol. PKD belongs to the Calcium/calmodulin-dependent protein kinases superfamily and consists of three isoforms in mammals, notably, PKD1, PKD2 and PKD3. Activated PKD resides in diverse subcellular locations such as cytosol, Golgi apparatus, nucleus, mitochondria to regulate a number of cellular functions.
1. Introduction
Protein kinase D (PKD) was discovered near the turn of the second millennium, with PKD1 first reported in 1994 [1][2], followed by PKD3 in 1999 [3] and PKD2 in 2001 [4]. Due to the presence of a diacylglycerol (DAG)-binding C1 domain in its structure, PKD was initially classified as an atypical protein kinase C (PKC) and given the name PKCµ for human PKD1 and PKCν for PKD3. Later, recognizing the similarity of its catalytic domain to Ca2+/calmodulin-dependent protein kinases (CAMKs), PKD was reclassified into the CAMK group in the human kinome. Interestingly, PKD not only binds DAG but also is activated by PKC through direct phosphorylation, a unique feature allowing PKD to integrate signal inputs from both DAG and PKC.
In the past two decades, extensive progress has been made towards the understanding of PKD structure, regulation, function, and signaling mechanisms. PKD has now emerged as a key signaling node in the DAG network, activated by a variety of cellular stimuli including growth factors, G protein-coupled receptor (GPCR) agonists, hormones, bioactive peptides, cellular stresses, and cytokines/chemokines, and coordinately regulates various downstream cellular processes, such as proliferation, survival, motility, secretion, and gene expression. PKD resides or can be mobilized to different subcellular locations, including the plasma membrane, Golgi, mitochondria, and nucleus, to carry out unique functions. Coinciding with its important roles in normal biology, dysregulation of PKD has detrimental impacts and associates with a variety of pathological conditions and diseases such as cancer, cardiac diseases, metabolic disorders, inflammatory diseases, neuronal dysfunctions, and immune dysregulation.
2. PKD Structure and Regulation
2.1. Structure, Isoforms, and Expression/Tissue Distribution
The family of PKD is evolutionarily highly conserved with three isoforms (PKD1, PKD2, and PKD3) identified in mammals. The three PKDs are similar in sizes (912, 878, and 890 amino acids (aa) for human PKD1, -2, and -3, respectively). PKD1 and PKD2 are most homologous and may originate from a common ancestor [5]. PKDs are widely distributed in the body, although their relative expression varies with different organs, with PKD1 and PKD2 being more prevalent in the lung, brain, kidney, heart, smooth muscle, pancreas, and prostate, and with PKD3 being more ubiquitous [1][2][3][4][6]. The conserved structure of PKD contains a N-terminal regulatory region that mainly comprises a C1 domain and a pleckstrin homology (PH) domain, followed by a C-terminal catalytic domain. The C1 domain constitutes two cysteine-rich Zn-finger-like motifs, Cla and Clb, that bind DAG and phorbol esters, the pharmacological analogs of DAG, with high affinity and regulate PKD localization to the nucleus, Golgi, and plasma membrane [7][8]. The PH domain exerts an autoinhibitory function on the catalytic domain to keep the kinase inactive at basal state [9][10]. Additionally, the regulatory domain contains an alanine–proline rich (AP) region at the N-terminus for PKD1 and a proline-rich (P) region for PKD2, and an acidic amino-acid-rich region (AD) between Clb and PH domains, and the functions of these domains remain obscure [11]. Recently, a ubiquitin-like domain (ULD) shared by all three PKD isoforms was identified at the N-terminus following the AP or P region. Based on the X-ray crystal structure of the ULD-C1a domain in the C. elegans PKD homolog DKF-1, ULD may act to initiate PKD dimerization at the membrane for trans-autophosphorylation at the activation loop in response to increased DAG concentration, leading to PKD activation possibly independent of PKC [5][12]. This domain appears to be conserved in all three human PKD isoforms [5][12]. The structure of PKD1 and PKD2 also contains a C-terminal PDZ domain that is thought to facilitate protein substrate recognition [7]. Within the PDZ domain, there is an autophosphorylation site (S910 for PKD1, S876 for PKD2), which is commonly used as a measure for PKD activation status, although its phosphorylation likely also plays a functional role [7][13][14] (see Figure 1 for a schematic diagram of human PKD1, -2, and -3).
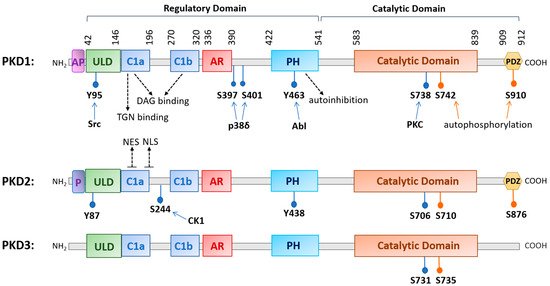
Figure 1. A diagram illustrating the conserved structural domains and major phosphorylation sites in human protein kinase D (PKD) isoforms. The structure of PKD contains a newly identified ubiquitin-like domain (ULD) for dimerization, a C1 domain (Cla and Clb) that binds diacylglycerol, a pleckstrin homology (PH) domain for autoinhibition, a catalytic domain for substrate phosphorylation, and a PDZ domain in PKD1 and PKD2 for protein interactions. Other domains with less known functions are the acidic amino-acid-rich region (AR) and an alanine–proline-rich region (AP) for PKD1 and a proline-rich region (P) for PKD2. Major phosphorylation sites and the upstream kinases that confer the phosphorylation are indicated as well as the nuclear export signal (NES) and nuclear localization signal (NLS) for PKD2. Abbreviations: trans-Golgi network (TGN), Abelson murine leukemia viral oncogene homolog 1 (Abl), casein kinase 1 (CK1).
2.2. Mechanisms of Regulation
PKD can be activated downstream of GPCRs or receptor tyrosine kinases (RTKs) by a variety of stimuli such as hormones, growth factors, neuropeptides, lipids, and cellular stresses [7]. In a canonical activation pathway, following receptor stimulation, phospholipase Cs (PLCs) are activated to hydrolyze phosphatidylinositol 4,5-biphosphate (PIP2) to generate inositol 1,4,5-trisphosphate (IP3) and DAG. IP3 mobilizes internal calcium and DAG along with calcium (for cPKC) binds and anchors classic or novel protein kinases C (c/nPKC) to the plasma membrane and triggers their activation. DAG also recruits cytosolic PKD to the plasma membrane by binding to its C1 domain; this process may induce a conformational change that allows PKC to colocalize with PKD at the plasma membrane to transphosphorylate a conserved serine residue (Ser738 for PKD1, Ser706 for PKD2, Ser731 for PKD3) in the activation loop of PKD, leading to the autophosphorylation of an adjacent serine residue (Ser742 for PKD1, Ser710 for PKD2, and Ser735 for PKD3) and relief of autoinhibition by the PH domain for full activation of the kinase [15][16][17]. Given the lack of crystal structure of PKD, there remain many questions regarding the exact sequence of events occurring during the activation of PKD by DAG and PKC, particularly in light of the discovery of a N-terminal ULD domain (see detailed discussion in ref. [5]).
PKD can be activated at multiple subcellular locations and can also be mobilized to different cellular compartments to carry out unique functions at each site (Figure 2). PKD was first identified as a trans-Golgi network (TGN)-resident enzyme [18]; a series of landmark studies by the Malhotra group shows that PKD plays a critical role in regulating the fission of transport carriers from TGN to the cell surface [19][20][21]. As with its activation at the plasma membrane, PKD is recruited by DAG to the TGN [22]. An intact C1a domain and the catalytic activity of PKD are both required for its binding and regulation of vesicle trafficking at the TGN [20][23]. The regulation of the TGN by PKD is essential for many important secretory processes, and the best known is the regulation of insulin secretion from pancreatic β cells [24] A well characterized substrate of PKD at the TGN is phosphatidylinositol-4 kinase IIIβ (PI4KIIIβ). Phosphorylation and activation of PI4KIIIβ is critical for protein transport at the TGN [25].
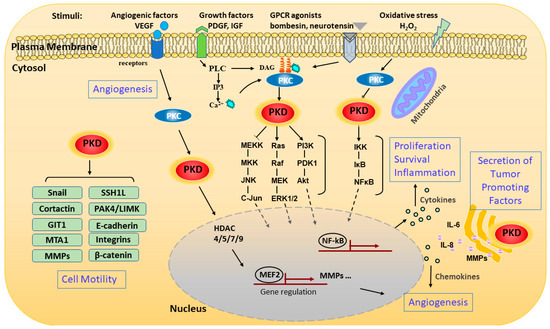
Figure 2. Schematic representation of signaling pathways and pathological processes regulated by PKD. The schematic representation shows the pathways that activate PKD and the various downstream signaling events and functions modulated by the kinase. PKD can be activated through stimulating various membrane receptors such as G-protein-coupled receptors (GPCRs) and growth factor receptors. The extracellular stimuli activate phospholipase C (PLC), which catalyzes the formation of diacylglycerol (DAG). DAG modulates PKD activation by binding and recruiting it to the cell membrane for activation by protein kinase C (PKC). PKD can also be activated on the outer mitochondrial membrane by oxidative stress through binding to DAG and PKC. Activated PKD is rapidly translocated from the plasma membrane to the cytosol and then to the nucleus, where it regulates a set of transcription factors in the nucleus. Activated PKD regulates a battery of pathological processes including cell proliferation, survival, migration, invasion, gene transcription, inflammation, angiogenesis, and secretion of tumor-associated factors through several major signaling pathways. Abbreviations: matrix metalloproteinase (MMP), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), metastasis-associated 1 (MTA1), slingshot-1L (SSH1L), GPCR kinase-interacting protein 1 (GIT1).
Following its activation at the plasma membrane, activated PKD can shuttle in and out of the nucleus to regulate the activity and availability of several transcription factors. Nuclear localization of PKD is regulated by more than one region of the domain structure, and the precise export and import mechanisms for each isoform may vary with the specific isoform and cellular context. In unstimulated cells, PKD1 and PKD2 exist primarily in the cytoplasm, while PKD3 is present in both the nucleus and cytoplasm as a result of continuous shuttling [26]. In earlier studies, it was shown that the Clb motif controls the nuclear import of PKD1, with evidence supporting the use of a nonclassical nuclear localization signal (NLS). The nuclear export of PKD1 is regulated by the PH domain in a Crm-dependent manner [27]. A recent study identified a complex formed between the catalytic domain of PKD1 and Hsp20, which is essential for PKD1 nuclear translocation and downstream signaling events leading to cardiac hypertrophy [28]. This supports a potential complex and cell context-dependent regulation of PKD1 nuclear localization. For PKD2, an isoform that has been more extensively studied in this aspect, the C-terminus of C1a and the linker region between Cla and Clb contains a putative bipartite NLS (key residues 192RKRR195), which is critical for the nuclear import of PKD2, while Cla contains a functional nuclear export signal (NES) that is required for the nuclear export of PKD2 [29] (Figure 1). Interestingly, the nuclear location of PKD2 can be regulated by a casein kinase 1-mediated S244 phosphorylation in the C1a/C1b linker region of PKD2 [30]. For the nuclear import of PKD3, it appears that the catalytic activity of the kinase is essential, implying the involvement of other import partners, and the export is similarly dependent on Crm1 [26][31].
PKD can also be activated in response to an increase in mitochondrial or cellular reactive oxygen species (ROS). Work by the Toker and Storz groups shows that increased ROS induces several tyrosine phosphorylations in PKD1 (Tyr95, Tyr432, Tyr463, and Tyr502) [32][33][34]. Furthermore, Abelson murine leukemia viral oncogene homolog 1 (Abl) directly phosphorylates PKD at Tyr463 in the PH domain to initiate a conformational change which allows PKD1 to translocate to the mitochondrial membrane through binding to DAG, which is generated by activated phospholipase D1 downstream of mitochondrial ROS [35]. Phosphorylation of PKD1 at Tyr95 by proto-oncogene tyrosine kinase Src then allows PKCδ to bind and fully activate PKD1 via activation loop phosphorylation. PKD1 then induces the activation of the Nuclear Factor-κB (NF-κB) signaling pathway to promote cell survival and detoxification of mitochondrial ROS via induction of manganese-dependent superoxide dismutase (MnSOD) [33][34][36]. In recent years, isoform- and cell context-specific regulation of ROS-induced PKD activation has been observed along with downstream signaling events. Detailed discussions on this topic can be found in three reviews (see references [13][37][38]).
References
- Johannes, F.J.; Prestle, J.; Eis, S.; Oberhagemann, P.; Pfizenmaier, K. PKCu is a novel, atypical member of the protein kinase C family. J. Biol. Chem. 1994, 269, 6140–6148.
- Valverde, A.M.; Sinnett-Smith, J.; Van Lint, J.; Rozengurt, E. Molecular cloning and characterization of protein kinase D: A target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc. Natl. Acad. Sci. USA 1994, 91, 8572–8576.
- Hayashi, A.; Seki, N.; Hattori, A.; Kozuma, S.; Saito, T. PKCnu, a new member of the protein kinase C family, composes a fourth subfamily with PKCmu. Biochim. Biophys. Acta 1999, 1450, 99–106.
- Sturany, S.; Van Lint, J.; Muller, F.; Wilda, M.; Hameister, H.; Hocker, M.; Brey, A.; Gern, U.; Vandenheede, J.; Gress, T.; et al. Molecular cloning and characterization of the human protein kinase D2. A novel member of the protein kinase D family of serine threonine kinases. J. Biol. Chem. 2001, 276, 3310–3318.
- Reinhardt, R.; Truebestein, L.; Schmidt, H.A.; Leonard, T.A. It Takes Two to Tango: Activation of Protein Kinase D by Dimerization. Bioessays 2020, 42, e1900222.
- Azoitei, N.; Cobbaut, M.; Becher, A.; Van Lint, J.; Seufferlein, T. Protein kinase D2: A versatile player in cancer biology. Oncogene 2018, 37, 1263–1278.
- Roy, A.; Ye, J.; Deng, F.; Wang, Q.J. Protein kinase D signaling in cancer: A friend or foe? Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 283–294.
- Chen, J.; Giridhar, K.V.; Zhang, L.; Xu, S.; Wang, Q.J. A protein kinase C/protein kinase D pathway protects LNCaP prostate cancer cells from phorbol ester-induced apoptosis by promoting ERK1/2 and NF-B activities. Carcinogenesis 2011, 32, 1198–1206.
- LaValle, C.R.; George, K.M.; Sharlow, E.R.; Lazo, J.S.; Wipf, P.; Wang, Q.J. Protein kinase D as a potential new target for cancer therapy. Biochim. Biophys. Acta 2010, 1806, 183–192.
- Waldron, R.T.; Rozengurt, E. Protein kinase C phosphorylates protein kinase D activation loop Ser744 and Ser748 and releases autoinhibition by the pleckstrin homology domain. J. Biol. Chem. 2003, 278, 154–163.
- Sundram, V.; Chauhan, S.C.; Jaggi, M. Emerging roles of protein kinase D1 in cancer. Mol. Cancer Res. 2011, 9, 985–996.
- Elsner, D.J.; Siess, K.M.; Gossenreiter, T.; Hartl, M.; Leonard, T.A. A ubiquitin-like domain controls protein kinase D dimerization and activation by trans-autophosphorylation. J. Biol. Chem. 2019, 294, 14422–14441.
- Wood, B.M.; Bossuyt, J. Emergency Spatiotemporal Shift: The Response of Protein Kinase D to Stress Signals in the Cardiovascular System. Front. Pharmacol. 2017, 8, 9.
- Durand, N.; Borges, S.; Storz, P. Protein Kinase D Enzymes as Regulators of EMT and Cancer Cell Invasion. J. Clin. Med. 2016, 5, 20.
- Rozengurt, E.; Rey, O.; Waldron, R.T. Protein kinase D signaling. J. Biol. Chem. 2005, 280, 13205–13208.
- Simsek Papur, O.; Sun, A.; Glatz, J.F.C.; Luiken, J.; Nabben, M. Acute and Chronic Effects of Protein Kinase-D Signaling on Cardiac Energy Metabolism. Front. Cardiovasc Med. 2018, 5, 65.
- Rozengurt, E. Protein kinase D signaling: Multiple biological functions in health and disease. Physiology (Bethesda) 2011, 26, 23–33.
- Prestle, J.; Pfizenmaier, K.; Brenner, J.; Johannes, F.J. Protein kinase C mu is located at the Golgi compartment. J. Cell Biol. 1996, 134, 1401–1410.
- Liljedahl, M.; Maeda, Y.; Colanzi, A.; Ayala, I.; Van Lint, J.; Malhotra, V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell 2001, 104, 409–420.
- Bossard, C.; Bresson, D.; Polishchuk, R.S.; Malhotra, V. Dimeric PKD regulates membrane fission to form transport carriers at the TGN. J. Cell Biol. 2007, 179, 1123–1131.
- Jamora, C.; Yamanouye, N.; Van Lint, J.; Laudenslager, J.; Vandenheede, J.R.; Faulkner, D.J.; Malhotra, V. Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell 1999, 98, 59–68.
- Baron, C.L.; Malhotra, V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 2002, 295, 325–328.
- Maeda, Y.; Beznoussenko, G.V.; Van Lint, J.; Mironov, A.A.; Malhotra, V. Recruitment of protein kinase D to the trans-Golgi network via the first cysteine-rich domain. Embo J. 2001, 20, 5982–5990.
- Sumara, G.; Formentini, I.; Collins, S.; Sumara, I.; Windak, R.; Bodenmiller, B.; Ramracheya, R.; Caille, D.; Jiang, H.; Platt, K.A.; et al. Regulation of PKD by the MAPK p38delta in insulin secretion and glucose homeostasis. Cell 2009, 136, 235–248.
- Hausser, A.; Storz, P.; Martens, S.; Link, G.; Toker, A.; Pfizenmaier, K. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex. Nat. Cell Biol. 2005, 7, 880–886.
- Rey, O.; Yuan, J.; Young, S.H.; Rozengurt, E. Protein kinase C nu/protein kinase D3 nuclear localization, catalytic activation, and intracellular redistribution in response to G protein-coupled receptor agonists. J. Biol. Chem. 2003, 278, 23773–23785.
- Rey, O.; Sinnett-Smith, J.; Zhukova, E.; Rozengurt, E. Regulated nucleocytoplasmic transport of protein kinase D in response to G protein-coupled receptor activation. J. Biol. Chem. 2001, 276, 49228–49235.
- Sin, Y.Y.; Martin, T.P.; Wills, L.; Currie, S.; Baillie, G.S. Small heat shock protein 20 (Hsp20) facilitates nuclear import of protein kinase D 1 (PKD1) during cardiac hypertrophy. Cell Commun. Signal. 2015, 13, 16.
- Auer, A.; von Blume, J.; Sturany, S.; von Wichert, G.; Van Lint, J.; Vandenheede, J.; Adler, G.; Seufferlein, T. Role of the regulatory domain of protein kinase D2 in phorbol ester binding, catalytic activity, and nucleocytoplasmic shuttling. Mol. Biol Cell 2005, 16, 4375–4385.
- von Blume, J.; Knippschild, U.; Dequiedt, F.; Giamas, G.; Beck, A.; Auer, A.; Van Lint, J.; Adler, G.; Seufferlein, T. Phosphorylation at Ser244 by CK1 determines nuclear localization and substrate targeting of PKD2. Embo J. 2007, 26, 4619–4633.
- Rey, O.; Papazyan, R.; Waldron, R.T.; Young, S.H.; Lippincott-Schwartz, J.; Jacamo, R.; Rozengurt, E. The nuclear import of protein kinase D3 requires its catalytic activity. J. Biol. Chem. 2006, 281, 5149–5157.
- Storz, P.; Doppler, H.; Johannes, F.J.; Toker, A. Tyrosine phosphorylation of protein kinase D in the pleckstrin homology domain leads to activation. J. Biol. Chem. 2003, 278, 17969–17976.
- Doppler, H.; Storz, P. A novel tyrosine phosphorylation site in protein kinase D contributes to oxidative stress-mediated activation. J. Biol. Chem. 2007, 282, 31873–31881.
- Storz, P.; Toker, A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J. 2003, 22, 109–120.
- Cowell, C.F.; Doppler, H.; Yan, I.K.; Hausser, A.; Umezawa, Y.; Storz, P. Mitochondrial diacylglycerol initiates protein-kinase D1-mediated ROS signaling. J. Cell Sci 2009, 122, 919–928.
- Storz, P.; Doppler, H.; Toker, A. Protein kinase D mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol. Cell Biol. 2005, 25, 8520–8530.
- Cobbaut, M.; Van Lint, J. Function and Regulation of Protein Kinase D in Oxidative Stress: A Tale of Isoforms. Oxid. Med. Cell Longev. 2018, 2018, 2138502.
- Doppler, H.; Storz, P. Mitochondrial and Oxidative Stress-Mediated Activation of Protein Kinase D1 and Its Importance in Pancreatic Cancer. Front. Oncol. 2017, 7, 41.





