
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Surinder Singh | + 2227 word(s) | 2227 | 2021-04-07 07:55:44 | | | |
| 2 | Rita Xu | Meta information modification | 2227 | 2021-04-14 07:52:32 | | |
Video Upload Options
Psidium guajava (L.) belongs to the Myrtaceae family and it is an important fruit in tropical areas like India, Indonesia, Pakistan, Bangladesh, and South America. The leaves of the guava plant have been studied for their health benefits which are attributed to their plethora of phytochemicals, such as quercetin, avicularin, apigenin, guaijaverin, kaempferol, hyperin, myricetin, gallic acid, catechin, epicatechin, chlorogenic acid, epigallocatechin gallate, and caffeic acid. Extracts from guava leaves (GLs) have been studied for their biological activities, including anticancer, antidiabetic, antioxidant, antidiarrheal, antimicrobial, lipid-lowering, and hepatoprotection activities.
1. Introduction
Plants are a predominant natural source of numerous bioactive compounds [1][2]. Several diseases have been cured using a variety of plant preparations in folk medicine since ancient times [3] and, presently, cosmetic, pharmaceutical, and nutraceutical industries are paying more attention to plant preparations and pure phytochemicals. The projected growth of the plant preparation market is around USD 86.74 billion by 2022, with the largest market share belonging to the pharmaceutical sector, followed by the nutraceutical industry. Interestingly, the utilization of plant preparations for cosmetics, beverages, food, and medicine is mainly dependent on plant leaves. Among all plant organs, leaves are the largest accumulators of bioactive compounds, such as secondary metabolites. Several recent studies reported phytochemical profiles and biological activities of leaf extracts of various cultivated plants [2][4][5][6]. Hence, although plant leaves are considered as agricultural waste, they are a rich source of high-value nutra-pharmaceutical compounds.
The guava (Psidium guajava L.) tree (Figure 1), belonging to the Myrtaceae family, is a very unique and traditional plant which is grown due to its diverse medicinal and nutritive properties. Guava has been grown and utilized as an important fruit in tropical areas like India, Indonesia, Pakistan, Bangladesh, and South America. Different parts of the guava tree, i.e., roots, leaves, bark, stem, and fruits, have been employed for treating stomachache, diabetes, diarrhea, and other health ailments in many countries. Guava leaves (Psidii guajavae folium; GL) are dark green, elliptical, oval, and characterized by their obtuse-type apex. Guava leaves, along with the pulp and seeds, are used to treat certain respiratory and gastrointestinal disorders, and to increase platelets in patients suffering from dengue fever [7]. GLs are also widely used for their antispasmodic, cough sedative, anti-inflammatory, antidiarrheic, antihypertension, antiobesity, and antidiabetic properties [8]. Studies on animal models have also established the role of GL isolates as potent antitumor, anticancer, and cytotoxic agents [9][10].

Figure 1. (A) Guava fruit and leaves, (B) bunch of guava leaves with dorsal view on the left and ventral view on the right, (C) guava leaf with dorsal view on the left and ventral view on the right.
GLs are widely employed for treating diarrhea and digestive ailments, while the fruit pulp is utilized to enhance the platelet count for treating dengue fever. The potential of guava leaf extracts for diarrhea treatment was also studied [11][12]. The flavonoids present in guava leaf extract chiefly determine their antibacterial activity, while quercetin, which is the most predominant flavonoid of guava leaves, exhibits strong antidiarrheal activities. The antidiarrheal activity of quercetin is ascribed to its relaxing effect on the intestinal muscle lining which prevents bowel contractions. Guava leaf polysaccharides (GLPs) can be utilized as an antioxidant additive in food and for diabetes treatment.
The presence of a unique variety of bioactive polyphenolic compounds, like quercetin and other flavonoids, and ferulic, caffeic, and gallic acids, present in guava leaves primarily determine their bioactive and therapeutic properties [8][13]. These phenolic compounds are known as secondary metabolites which exhibit strong antioxidant and immunostimulant activities.
2. Chemical Composition
2.1. Proximate Composition
Guava leaves (GLs) are a rich source of various health-promoting micro- and macronutrients as well as bioactive compounds. They contain 82.47% moisture, 3.64% ash, 0.62% fat, 18.53% protein, 12.74% carbohydrates, 103 mg ascorbic acid, and 1717 mg gallic acid equivalents (GAE)/g total phenolic compounds [14]. The overall proximate profile of GLs is presented in Table 1.
2.1.1. Polysaccharides
Polysaccharides are macromolecules that are ubiquitously present in nature. They are made of long polymeric chains, which are composed of monosaccharide units. These polysaccharides demonstrate various physicochemical, biological, and pharmacological properties, such as antioxidant, anti-inflammatory, antidiabetic, immunomodulatory, and antitumor activities [15]. Guava leaf polysaccharides (GLPs) can be isolated using ultrasound-assisted extraction (UAE) (time: 20 min, power: 404 W, temperature: 62 °C). These GLPs contain about 9.13% uronic acid and 64.42% total sugars, out of which 2.24% are reducing sugars. GLPs are soluble in water, while insoluble in organic solvents like ethanol, diethyl ether, ethyl acetate, acetone, and chloroform. Extracted GLP with a concentration of 100 μg/mL exhibits good antioxidant capacity with 56.38% and 51.73% 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical- and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical cation-scavenging capacity, respectively [16]. Similar results were also reported by Kong et al. [17]. They obtained up to 0.51% GLP using UAE that exhibited good DPPH•- and •OH-scavenging activity (72–86% and 42.94–58.33%). GLPs can be categorized into two groups: unsulfated and sulfated GLPs. Sulfated GLP contains about 18.58% sulfate content. Sulfated GLP exhibited good antioxidant activity in terms of DPPH, hydroxyl, and alkyl radical-scavenging activity (0.10, 0.02, and 0.17 IC50, mg/mL, respectively). Studies showed that guava leaves extracts (GLE) effectively reduced the oxidative stress and toxicity caused by hydrogen peroxide in mammalian cell lines (Vero cells) [18]. GLPs are also found to be beneficial in treating diabetes mellitus symptoms. Acarbose (an antidiabetic drug) is commonly used for the treatment of type 2 diabetes [16]. It acts as an inhibitor of glycoside hydrolases like α-glucosidase and α-amylase and thus prevents rapid glucose release from complex carbohydrates [19]. This activity causes some of the incompletely digested complex carbohydrates to remain in the intestine and be transported to the colon. The intestinal microflora digests these complex carbohydrate fractions, causing gastrointestinal problems like diarrhea and flatulence. A study reported that GLP inhibited α-glucosidase more efficiently than acarbose without significantly blocking the α-amylase activity [19]. Moreover, it also caused a substantial drop in fasting blood sugar, total cholesterol, total triglycerides, glycated serum protein, creatinine, and malonaldehyde in diabetic mice without causing any major side effect [15]. Therefore, GLP can be used as a replacement of acarbose for managing diabetes mellitus and also as an antioxidant additive in foods.
Table 1. Nutritional profile of guava leaves.
| Compounds | Content/Composition | References |
|---|---|---|
| Elements and ascorbic acid | [20] | |
| Potassium | 1.11% | |
| Phosphorus | 0.23% | |
| Nitrogen | 1.02% | |
| Ascorbic acid | 142.55 mg/100 g | |
| Carbohydrates/phenols/sulfates | [18] | |
| Fucose | 1.44% | |
| Rhamnose | 3.88% | |
| Arabinose | 22.6% | |
| Galactose | 29.41% | |
| Glucose | 33.79% | |
| Mannose | 0.59% | |
| Xylose | 7.71% | |
| Phenol | 15.28% | |
| Sulfate | 18.58% | |
| Carbohydrate | 48.13% | |
| Sulfate polysaccharide | 66.71% | |
| Protein | ||
| Association of Official Analytical Chemists (AOAC) method | 22.98 ± 0.036% [dry weight (DW) basis] | [21] |
| AOAC method | 9.73% | [22] |
| Lowry’s method | 16.8 mg/100 g | [23] |
| Ninhydrin method | 8.0 mg/100 g |
2.1.2. Proteins
Guava leaves contains 9.73% protein on a dry weight basis [22]. Proteins are large biomolecules composed of amino acids and act as building blocks of cells. Proteins play a major role in growth and maintenance, enzyme regulation, and cell signaling, and also as biocatalysts [24]. Recently, plant-based nutrients have gained potential because of the high demand for nutritionally rich food, particularly protein. A great effort is now being made to find highly sustainable nutritionally rich food sources [25]. Thomas et al. [23] reported 16.8 mg protein/100g and 8 mg amino acids/100g in guava leaves as estimated according to Lowry’s and ninhydrin methods, respectively. Jassal et al. [21] reported that guava leaves can be utilized as a novel and sustainable dietary source as they are a rich source of proteins, carbohydrates, and dietary fibers.
2.1.3. Minerals and Vitamins
Guava leaves are the rich source of minerals, such as calcium, potassium, sulfur, sodium, iron, boron, magnesium, manganese, and vitamins C and B. The higher concentrations of Mg, Na, S, Mn, and B in GLs makes them a highly suitable choice for human nutrition and also as an animal feed to prevent micronutrient deficiency [26]. Thomas et al. [23] reported the concentration of minerals such as Ca, P, K, Fe, and Mg as 1660, 360, 1602, 13.50, and 440 mg per 100g of guava leaf dry weight (DW), respectively. The concentration of vitamins C and B was 103.0 and 14.80 mg per 100g DW, respectively. Consumption of Ca- and P-rich GLs reduces the risk of deficiency-related diseases like hypocalcemia, hypophosphatemia, and osteoporosis. The study also reported that the concentration of Ca, P, Mg, Fe, and vitamin B in GLs was higher than that in guava fruit. The higher vitamin C content in GLs may help in improving the immune system and maintain the health of blood vessels, whereas vitamin B plays an important role in improving blood circulation, nerve relaxation, and cognitive function stimulation.
2.2. Phytochemical Profile
2.2.1. Essential Oil Profile
GLs are a rich source of essential oils (Table 2). The major constituent of GL essential oil includes 1,8-cineole and trans-caryophyllene [27]. Chen et al. [8] identified 50 compounds in GL essential oil using gas chromatography (GC) and gas chromatography/mass spectrometry (GC–MS), where they found β-caryophyllene, α-pinene, and 1,8-cineole to be the major ones. GL essential oil from the Philippines was found to contain a different profile, with limonene, α-pinene, β-caryophyllene, and longicyclene as major compounds [28]. Ecuadorian GL essential oil contained a higher content of monoterpenes (limonene and α-pinene) whereas Tunisian guava leaf oil displayed a higher content of veridiflorol and trans-caryophyllene [29][30]. Soliman et al. [31] reported a larger amount of monoterpenes, contrary to the other studies, where sesquiterpenes constituted the major compound in GL essential oil. El-Ahmady et al. [32] reported 4 α-selin-7(11)-enol, α-selinene, β-caryophyllene, and β-caryophyllene oxide as the major constituents of GL essential oil. In another study, sixty-four different compounds were determined in essential oil extracted from GLs by gas chromatography–mass spectrometry (GC–MS). Among them, caryophyllene (24.97%) was found to be predominantly present, which acts as an antioxidant, anticancer, anti-inflammatory, and antimicrobial agent [21]. This study reported the concentration of non-oxygenated sesquiterpenes, oxygenated sesquiterpenes, and monoterpenes as 73.67, 12.94, and 8.55%, respectively.
Table 2. Essential oil components of guava leaves.
| Compounds | Content/Composition | References |
|---|---|---|
| Essential oil components | [31] | |
| α-Pinene | 1.53% | |
| Benzaldehyde | 0.83% | |
| p-cymene | 0.52% | |
| Limonene | 54.7% | |
| 1,8-Cineole | 32.14% | |
| β-cis-Ocimene | 0.28% | |
| γ-Terpinene | 0.38% | |
| α-Terpineol | 1.79% | |
| β-Caryophyllene | 2.91% | |
| α-Humulene | 0.77% | |
| Total identified constituents | 95.85% | |
| Caryophyllene, copaene, nerolidol, caryophyllene oxide, humulene, limonene, eucalyptol, beta-bisabolene, cadin-4-en-10-ol, trans-cadina-1,4-diene, sesquiterpenes, eugenol, isoeugenol, cevadine, emetine (extracted from guava leaves, Ludhiana, India using hydro-distillation by Clevenger-type apparatus) | - | [21] |
2.2.2. Phenolic Compounds
GLs are widely popular as a traditional source of medicine in Asian countries due to their antihyperglycemic effect. As mentioned in the previous sections, they contain superior quality bioactive polysaccharides, proteins, lipids, essential oils, vitamins, and minerals. The various secondary metabolites present in GLs include phenolic acids, flavonoids, triterpenoids, sesquiterpenes, glycosides, alkaloids, and saponins. Phenolic compounds (PCs) serve as key bioactive compounds which provide antioxidant and hypoglycemic properties to GLs. Generally, these PCs play a major role in managing various metabolic and physiological activities in the human body. About seventy-two different phenolic compounds have been determined in GLs using high-performance liquid chromatography–diode array detector–quadrupole time-of-flight tandem mass spectrometry [33]. Generally, five quercetin glycosides are present in GLs. The presence of two new benzophenone galloyl glycosides (guavinosides A and B) and one quercetin galloyl glycoside (guavinoside C) was also reported [34]. Seventeen types of triterpenoids, thirty types of flavonoids, and nineteen types of sesquiterpenoids in GLs have also been reported [10]. Moreover, diphenylmethane [35] sesquiterpenoid-diphenylmethane meroterpenoids (psiguadials A and B) [36] and psiguanins A–D (1–4) [37] were also found in GLs. Epidemiological studies have established the roles of polyphenolic compounds against chronic diseases, such as diabetes, cancer, and neurodegenerative and cardiovascular diseases [38]. Phenolic compounds modulate numerous physiological processes like cell proliferation, enzymatic activity, cellular redox potential, and signal transduction pathways to fight against chronic pathologies [39]. Various phenolics reported in GLs are summarized in Table 3 and the structures can be seen in Figure 2.
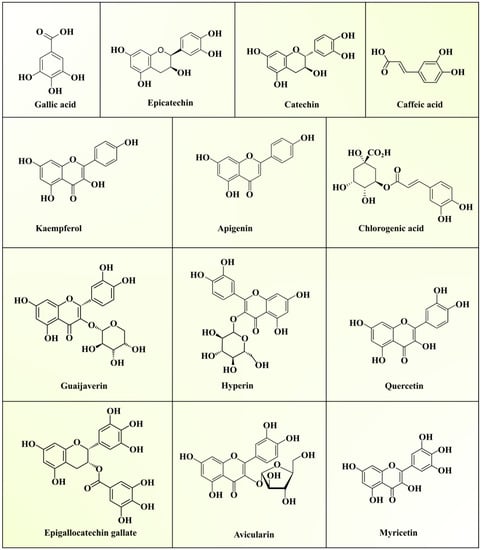
Figure 2. Structures of phenolic compounds present in guava leaf extracts.
Table 3. Phenolic compounds of guava leaves.
| Origin of Guava Leaves | Extract/Fraction | Bioactive Compounds | References |
|---|---|---|---|
| Leaves from Guangzhou (China) | Ethyl acetate-soluble fraction, n-butanol-soluble fraction, 75% ethanol extract, residual fraction, dichloromethane-soluble fraction | Quercetin, avicularin, apigenin, guaijaverin, kaempferol, hyperin, myricetin | [40] |
| Leaves from Jing-cin Farm (Tianzhong Township, Changhua County, Taiwan) | Aqueous extract | Gallic acid, catechin, epicatechin, quercetin, chlorogenic acid, epigallocatechin gallate, caffeic acid | [41] |
| Leaves from Motril (Spain) | Acetone, water, and acetic acid extract | Proanthocyanidins (PAs) | [33] |
| Leaves from Jiangmen (China) | Methanol extract | Gallic acid, chlorogenic acid, epicatechin, mono-3-hydroxyethyl-quercetin-glucuronide, rutin, isoquercitrin, quercetin-3-O-α-L-arabinofuranoside, quercetin-3-O-β-D-xylopyranoside, avicularin, quercitrin, kaempferol-3-arabofuranoside, quercetin, kaempferol | [42] |
Among phenolic compounds, quercetin is a major bioactive phenolic compound in GLs. Diets enriched with bioactive compounds have been gaining much attention in recent years due to their potential to lower the risk of the development of numerous chronic diseases. Seven pure compounds, quercetin, avicularin, apigenin, guaijaverin, kaempferol, hyperin, and myricetin, were separated from the ethyl acetate (EtOAc)-soluble GL fraction using Sephadex LH-20 column chromatography with reversed-phase thin layer chromatography (RP-TLC) to monitor separation. Mass spectrometry and nuclear magnetic resonance spectroscopy were used to elucidate the compound structures [40]. Wang et al. [43] extracted and analyzed phenolic compounds from non-fermented guava leaves (NFGLs) and fermented guava leaves (FGLs) using high-performance liquid chromatography coupled to electrospray ionization quadropole–time-of-flight mass spectrometry (HPLC–TOF–ESI/MS). The authors reported the presence of gallic acid, rutin, chlorogenic acid, avicularin, isoquercitrin, quercitrin, and kaempferol in NFGL and FGL samples. Among them, quercetin, rutin, gallic acid, avicularin, and isoquercitrin occupied about 65% of the total peak area on the chromatogram. Another study reported higher concentrations of catechin (2.25%) and epicatechin (1.45%), whereas gallic acid, chlorogenic acid, quercetin, caffeic acid, and epigallocatechin gallate were present in lower concentrations in GL extract [41]. Additionally, phenolic compounds (eugenol and isoeugenol) and alkaloids (cevadine and emetine) were detected. Díaz-de-Cerio et al. [44] optimized the extraction of proanthocyanidins, as antidiabetic and antiobesity agents [45], from GLs by HPLC–fluorimetric detector (FLD)–ESI–MS and studied their degree of polymerization in different oxidation states. Thus, the phytochemical profile of GL extract depicts the presence of numerous phytochemicals with distinct medicinal properties, suggesting its application to cure human diseases.
References
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037.
- Kumar, M.; Saurabh, V.; Tomar, M.; Hasan, M.; Changan, S.; Sasi, M.; Maheshwari, C.; Prajapati, U.; Singh, S.; Prajapat, R.K.; et al. Mango (Mangifera indica L.) leaves: Nutritional composition, phytochemical profile, and health-promoting bioactivities. Antioxidants 2021, 10, 299.
- Sharma, A.; del Carmen Flores-Vallejo, R.; Cardoso-Taketa, A.; Villarreal, M.L. Antibacterial activities of medicinal plants used in Mexican traditional medicine. J. Ethnopharmacol. 2017, 208, 264–329.
- Amat-ur-Rasool, H.; Symes, F.; Tooth, D.; Schaffert, L.N.; Elmorsy, E.; Ahmed, M.; Hasnain, S.; Carter, W.G. Potential nutraceutical properties of leaves from several commonly cultivated plants. Biomolecules 2020, 10, 1556.
- Mannino, G.; Gentile, C.; Porcu, A.; Agliassa, C.; Caradonna, F.; Bertea, C.M. Chemical profile and biological activity of cherimoya (Annona cherimola Mill.) and atemoya (Annona atemoya) leaves. Molecules 2020, 25, 2612.
- Mateos-Maces, L.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Aquino-Bolaños, E.N.; Alba-Jiménez, J.E.; Villagómez-González, B.B. Edible leafy plants from Mexico as sources of antioxidant compounds, and their nutritional, nutraceutical and antimicrobial potential: A review. Antioxidants 2020, 9, 541.
- Laily, N.; Kusumaningtyas, R.W.; Sukarti, I.; Rini, M.R.D.K. The potency of guava Psidium guajava (L.) leaves as a functional immunostimulatory ingredient. Procedia Chem. 2015, 14, 301–307.
- Chen, H.Y.; Yen, G.C. Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves. Food Chem. 2007, 101, 686–694.
- Ashraf, A.; Sarfraz, R.A.; Rashid, M.A.; Mahmood, A.; Shahid, M.; Noor, N. Chemical composition, antioxidant, antitumor, anticancer and cytotoxic effects of Psidium guajava leaf extracts. Pharm. Biol. 2016, 54, 1971–1981.
- Jiang, L.; Lu, J.; Qin, Y.; Jiang, W.; Wang, Y. Antitumor effect of guava leaves on lung cancer: A network pharmacology study. Arab. J. Chem. 2020, 13, 7773–7797.
- Dewi, P.S.; Sutjiatmo, A.B.; Nurdiansyah, A. Antidiarrheal activity of water extracts of guava leaves (Psidium guajava L.) and water extracts of green tea leaves (Camellia sinensis L.) combination in Swiss Webster mice. Acta Pharm. Indones. 2013, 38, 67–70.
- Mazumdar, S.; Akter, R.; Talukder, D. Antidiabetic and antidiarrhoeal effects on ethanolic extract of Psidium guajava (L.) Bat. leaves in Wister rats. Asian Pac. J. Trop. Biomed. 2015, 5, 10–14.
- Farag, R.S.; Abdel-Latif, M.S.; Abd El Baky, H.H.; Tawfeek, L.S. Phytochemical screening and antioxidant activity of some medicinal plants’ crude juices. Biotechnol. Rep. 2020, 28, e00536.
- Shabbir, H.; Kausar, T.; Noreen, S.; Hussain, A.; Huang, Q.; Gani, A.; Su, S.; Nawaz, A. In vivo screening and antidiabetic potential of polyphenol extracts from guava pulp, seeds and leaves. Animals 2020, 10, 1714.
- Luo, Y.; Peng, B.; Wei, W.; Tian, X.; Wu, Z. Antioxidant and anti-diabetic activities of polysaccharides from guava leaves. Molecules 2019, 24, 1343.
- Luo, Y.; Peng, B.; Liu, Y.; Wu, Y.; Wu, Z. Ultrasound extraction of polysaccharides from guava leaves and their antioxidant and antiglycation activity. Process. Biochem. 2018, 73, 228–234.
- Yu, S.; Feng, Z.; Wu, X.; Kong, F. Optimization of ultrasonic-assisted extraction of antioxidant compounds from Guava (Psidium guajava L.) leaves using response surface methodology. Pharmacogn. Mag. 2015, 11, 463.
- Kim, S.Y.; Kim, E.A.; Kim, Y.S.; Yu, S.K.; Choi, C.; Lee, J.S.; Kim, Y.T.; Nah, J.W.; Jeon, Y.J. Protective effects of polysaccharides from Psidium guajava leaves against oxidative stresses. Int. J. Biol. Macromol. 2016, 91, 804–811.
- Zhang, Z.; Kong, F.; Ni, H.; Mo, Z.; Wan, J.-B.; Hua, D.; Yan, C. Structural characterization, α-glucosidase inhibitory and DPPH scavenging activities of polysaccharides from guava. Carbohydr. Polym. 2016, 144, 106–114.
- Dutta, P.; Kundu, S.; Bauri, F.K.; Talang, H.; Majumder, D. Effect of bio-fertilizers on physico-chemical qualities and leaf mineral composition of guava grown in alluvial zone of West Bengal. J. Crop Weed 2014, 10, 268–271.
- Jassal, K.; Kaushal, S. Phytochemical and antioxidant screening of guava (Psidium guajava) leaf essential oil. Agric. Res. J. 2019, 56, 528.
- Rahman, Z.; Siddiqui, M.N.; Khatun, M.A.; Kamruzzaman, M. Effect of guava (Psidium guajava) leaf meal on production performances and antimicrobial sensitivity in commercial broiler. J. Nat. Prod. 2013, 6, 177–187.
- Thomas, L.A.T.; Anitha, T.; Lasyaja, A.B.; Suganya, M.; Gayathri, P.; Chithra, S. Biochemical and mineral analysis of the undervalued leaves—Psidium guajava L. Int. J. Adv. Sci. Res. 2017, 2, 16–21.
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Protein Function. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002.
- Lonnie, M.; Hooker, E.; Brunstrom, J.; Corfe, B.; Green, M.; Watson, A.; Williams, E.; Stevenson, E.; Penson, S.; Johnstone, A. Protein for life: Review of optimal protein intake, sustainable dietary sources and the effect on appetite in ageing adults. Nutrients 2018, 10, 360.
- Adrian, J.A.L.; Arancon, N.Q.; Mathews, B.W.; Carpenter, J.R. Mineral composition and soil-plant relationships for common guava (Psidium guajava L.) and yellow strawberry guava (Psidium cattleianum var. Lucidum) tree parts and fruits. Commun. Soil Sci. Plant Anal. 2015, 46, 1960–1979.
- Lee, W.C.; Mahmud, R.; Pillai, S.; Perumal, S.; Ismail, S. Antioxidant activities of essential oil of Psidium guajava L. leaves. APCBEE Procedia 2012, 2, 86–91.
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632.
- Smith, R.M.; Oliveros-Belardo, L. The composition of leaf essential oils of Psidium guajava L. from Manila, Philippines. Asian J. Pharm. 1977, 3, 5–9.
- Khadhri, A.; El Mokni, R.; Almeida, C.; Nogueira, J.M.F.; Araújo, M.E.M. Chemical composition of essential oil of Psidium guajava L. growing in Tunisia. Ind. Crop. Prod. 2014, 52, 29–31.
- Soliman, F.M.; Fathy, M.M.; Salama, M.M.; Saber, F.R. Comparative study of the volatile oil content and antimicrobial activity of Psidium guajava L. and Psidium cattleianum Sabine leaves. Bull. Fac. Pharm. Cairo Univ. 2016, 54, 219–225.
- El-Ahmady, S.H.; Ashour, M.L.; Wink, M. Chemical composition and anti-inflammatory activity of the essential oils of Psidium guajava fruits and leaves. J. Essent. Oil Res. 2013, 25, 475–481.
- Díaz-de-Cerio, E.; Gómez-Caravaca, A.M.; Verardo, V.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Determination of guava (Psidium guajava L.) leaf phenolic compounds using HPLC-DAD-QTOF-MS. J. Funct. Foods 2016, 22, 376–388.
- Matsuzaki, K.; Ishii, R.; Kobiyama, K.; Kitanaka, S. New benzophenone and quercetin galloyl glycosides from Psidium guajava L. J. Nat. Med. 2010, 64, 252–256.
- Shu, J.C.; Chou, G.X.; Wang, Z.T. One new diphenylmethane glycoside from the leaves of Psidium guajava L. Nat. Prod. Res. 2012, 26, 1971–1975.
- Shao, M.; Wang, Y.; Liu, Z.; Zhang, D.M.; Cao, H.H.; Jiang, R.W.; Fan, C.L.; Zhang, X.Q.; Chen, H.R.; Yao, X.S.; et al. Psiguadials A and B, two novel meroterpenoids with unusual skeletons from the leaves of Psidium guajava. Org. Lett. 2010, 12, 5040–5043.
- Shao, M.; Wang, Y.; Huang, X.J.; Fan, C.L.; Zhang, Q.W.; Zhang, X.Q.; Ye, W.C. Four new triterpenoids from the leaves of Psidium guajava. J. Asian Nat. Prod. Res. 2012, 14, 348–354.
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1–42.
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659.
- Wang, H.; Du, Y.J.; Song, H.C. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem. 2010, 123, 6–13.
- Liu, C.W.; Wang, Y.C.; Lu, H.C.; Chiang, W.D. Optimization of ultrasound-assisted extraction conditions for total phenols with anti-hyperglycemic activity from Psidium guajava leaves. Process. Biochem. 2014, 49, 1601–1605.
- Wang, L.; Wu, Y.; Bei, Q.; Shi, K.; Wu, Z. Fingerprint profiles of flavonoid compounds from different Psidium guajava leaves and their antioxidant activities. J. Sep. Sci. 2017, 40, 3817–3829.
- Wang, L.; Bei, Q.; Wu, Y.; Liao, W.; Wu, Z. Characterization of soluble and insoluble-bound polyphenols from Psidium guajava L. leaves co-fermented with Monascus anka and Bacillus sp. and their bio-activities. J. Funct. Foods 2017, 32, 149–159.
- Díaz-de-Cerio, E.; Pasini, F.; Verardo, V.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Caboni, M.F. Psidium guajava L. leaves as source of proanthocyanidins: Optimization of the extraction method by RSM and study of the degree of polymerization by NP-HPLC-FLD-ESI-MS. J. Pharm. Biomed. Anal. 2017, 133, 1–7.
- Dorenkott, M.R.; Griffin, L.E.; Goodrich, K.M.; Thompson-Witrick, K.A.; Fundaro, G.; Ye, L.; Stevens, J.R.; Ali, M.; O’Keefe, S.F.; Hulver, M.W.; et al. Oligomeric cocoa procyanidins possess enhanced bioactivity compared to monomeric and polymeric cocoa procyanidins for preventing the development of obesity, insulin resistance, and impaired glucose tolerance during high-fat feeding. J. Agric. Food Chem. 2014, 62, 2216–2227.





