
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rachael Bell | + 2022 word(s) | 2022 | 2021-04-06 08:54:08 | | | |
| 2 | Nora Tang | Meta information modification | 2022 | 2021-04-14 02:35:49 | | |
Video Upload Options
Bovine respiratory disease complex (BRDC) is a multifactorial disease of cattle which presents as bacterial and viral pneumonia. The causative agents of BRDC work in synergy to suppress the host immune response and increase the colonisation of the lower respiratory tracts by pathogenic bacteria. Environmental stress and/or viral infection predispose cattle to secondary bacterial infections via suppression of key innate and adaptive immune mechanisms. This allows bacteria to descend the respiratory tract unchallenged. BRDC is the costliest disease among feedlot cattle, and whilst vaccines exist for individual pathogens, there is still a lack of evidence for the efficacy of these vaccines and uncertainty surrounding the optimum timing of delivery.
1. Introduction
BRDC is a term used to describe severe respiratory disease in cattle and is sometimes referred to as shipping fever due to the increased risk of infection and transmission during cattle transportation [1]. BRDC is a multifactorial disease caused by both bacterial and viral infections, with high rates of re-infection [2]. BRDC is the costliest disease in the beef industry and is the biggest cause of mortality in calves aged one to five months in Ireland, accounting for between 30 and 34% of deaths in this age group [3]. A recent study estimated costs up to USD 42.15 per affected calf [4]. BRDC is also potentially responsible for up to 70% morbidity and mortality rates in US feedlot cattle [5][6]. The risk of infection and the severity of disease is determined by the infectious agents involved, their immunogenicity, genetics and microflora of the host and external environmental factors. The infectious agents involved in BRDC are opportunistic and often enhanced by stressors such as weaning, overcrowding, mycotoxins from food contamination, along with fluctuations in temperature, humidity, air, lighting, and sound. These factors can induce a transient immunosuppressive state which allows for colonisation of pathogenic bacteria and virus replication [7]. Whilst there are preventative vaccines and antibiotic treatments available against several common BRDC agents, the specific pathogens involved in individual cases of BRDC are often unknown [1], meaning prioritising a vaccination regime is difficult.
BRDC has been thoroughly reviewed previously; the immune response has been reviewed in detail [2][8], with the developments of the last decade outlined by McGill & Sacco in 2020 [9]. The exhibition of clinical signs, times of shedding, and seroconversion of each pathogen has also been outlined by Grissett et al. [10]. This review however summarises the mechanisms of immune evasion reported for each major BRDC pathogen and the current issues in the development of effective vaccines. It is worth noting however that several additional pathogens have been implicated in the development of BRDC (for example adenovirus [11], coronavirus [12][13], influenza D [13], Mycoplasma bovis [14] and Trueperella pyogenes [14]), although these will not be discussed in detail in this review.
2. BRDC Pathogenesis
Many of the bacterial agents that are associated with BRDC are present in bovine nasal passages, without illness, the most common being Mycoplasmas and Mannheimia haemolytica [15]. In healthy cattle, there exists a delicate balance between these potentially pathogenic bacteria and the commensal microflora of the upper respiratory tract (URT). Key immune mechanisms, such as mucus production and ciliated epithelial movement, actively prevent the colonisation of pathogens in the lower respiratory tract (LRT). In BRDC, there is a shift in this homeostatic balance in the URT which results in colonisation of the LRT.
Once BRDC infections reach the LRT, they are often persistent and difficult to resolve due to the unique immunosuppressive and immune avoidant mechanisms exhibited by each agent. As BRDC is a multifactorial disease, co-infections work in feedback loops to enhance viral and bacterial replication, adherence, toxicity, and persistence. This is a major challenge for vaccination development, as discussed in the second half of this review. The development of safe and effective vaccines depends on the understanding of how these infections effect each stage of the immune response, outlined herein.
3. Cellular Response
3.1. Epithelial Cells
The epithelia of the UTR are the first line of defence against respiratory and bacterial pathogens and therefore, often the initial sites of infection. Indeed, this is true for Bovine Respiratory Syncytial Virus (BRSV), Parainfluenza Virus 3 (PIV3) and Alphaherpesvirus Bovine Herpes Virus-1 (BoHV-1). BRSV initially infects both ciliated bronchial epithelia and type II pneumocytes of the respiratory mucosa [16][17]. PIV3 readily infects the respiratory ciliated epithelium [18][19]. BoHV-1 first infects mucosal epithelia of both respiratory and/or genital tracts [20][21]. Although these agents all contribute to BRDC, the mechanisms and cell type in which they initiate infection in the URT differ (depicted in Figure 1) [22].
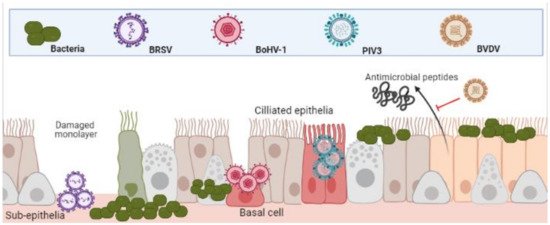
Figure 1. Differential infection of upper and lower respiratory epithelia and subsequent bacterial colonisation. Viral infection leads to inhibition of function and cell death. This both increases bacterial adherence to, and colonisation, of the lower respiratory tracts. (Created in BioRender.com, accessed on 24 March 2021).
It has been demonstrated that whilst PIV3 directly infects apical ciliated epithelium, these cells were resistant to BoHV-1 infection, which preferentially targeted basal epithelium of injured monolayers. In the same study, BRSV infected only the sub-epithelia [22]. The same patterns of infection were shown in a caprine lung slice model using BRSV, PIV3 and BoHV-1 [23]. Once infected, the physical defenses of epithelia to bacteria are weakened. BRSV infected epithelia in the bronchus and lung are significantly more susceptible to Pasteurella multocida infection [24]. BoHV-1 infected bronchial epithelium was shown to not only increase recruitment and activation of neutrophils to sites of infection but increase their susceptibility to M. haemolytica infection and subsequent cell death [25]. As well as physical barriers of defence, bovine epithelial cells mediate antimicrobial activity via beta-defensin secretions such as lingual antimicrobial peptide (LAP) and tracheal antimicrobial peptide (TAP) which is interrupted by the agents of BRDC [8]. For example, Pestivirus Bovine Viral Diarrhoea Virus (BVDV) infection has proven to decrease TAP and LAP expression after exposure to bacterial toxin lipopolysaccharide (LPS) [26][27]. This predisposes animals to bacterial infections and highlights the synergies of co-infections.
It is important to note that although BRDC is primarily a respiratory disorder, its agents are not solely pathogens of pulmonary tissue. For example, BoHV-1 infection results in not only respiratory disorders but also conjunctivitis, and genital disorders [28]. BVDV, another prominent BRDC agent, also presents clinically in multiple organs including those of the respiratory, gastrointestinal and reproductive systems [29][30][31]. Histophilis somni is not exclusively a pulmonary pathogen and it is common for more invasive, systemic infections to take hold in the weeks and months following initial exposure [14][32]. H. somni expresses an immunoglobulin binding surface protein which induces endothelial contractions, allowing for the bacteria to enter the blood stream [33]. Subsequently, infections have been found in the reproductive tract that lead to abortion. H. somni has also been associated with sudden death due to cardiac complications [34]. This multi-organ infection is largely due to the ability of the pathogenic agents to infect and replicate in not just epithelia, but in key leucocytes of the innate and adaptive immune response.
3.2. Neutrophils, Monocytes and Macrophages
Neutrophils are the first and most rapidly recruited cells to the site of infection, via chemokine signalling from damaged epithelia [35]. BoHV-1 infected ciliated and mucosal epithelia undergo apoptosis after rapidly producing inflammatory cytokines such as IL-8 [22]. This adds to tissue damage via neutrophil recruitment and subsequent degranulation and protease release [25]. Neutrophils were identified as a key target for BVDV immunosuppression. One study showed impaired phagocytosis, increased cellular toxicity, cytochrome-C reduction, iodination, oxidant production, and cytoplasmic calcium flux in neutrophils from cattle persistently infected with BVDV, compared with non-infected controls [36]. BRSV is known to evade the killing mechanisms of neutrophils [37]. Neutrophil traps (NETs) function to trap pathogens for immune clearance but are ineffective during BRSV infection and have been implicated in airway occlusion [37]. M. haemolytica also contributes heavily to the fibrosis of the lung during BRDC by inducing the release of neutrophil chemoattractant IL-8 [38][39]. The detrimental nature of neutrophil recruitment was demonstrated when infection with M. haemolytica in a neutrophil depleted model showed decreased lung pathology and reduced inflammatory cytokine release [40].
Macrophages are the most numerous immune cells in the healthy lung, with systemic monocytes being the first cells recruited during infection after neutrophils [41]. These cells play an important role in the pathogenesis of many agents of BRDC. Monocytes function as the main carrier of infection to other leukocytes for the most prominent viral agent of BRDC, BoHV-1 [21]. Like BoHV-1, BVDV readily infects macrophages and monocytes. Specifically, it invades and replicates in alveolar macrophages, impairing function and destroying these key immune cells [42]. In vivo infection of monocytes with BVDV significantly affected their ability to act as antigen presenting cells, resulting in a poor CD4+ T-cell memory response [43]. The phagocytic killing ability of both alveolar and infiltrating macrophages is inhibited by BVDV infections, seen also with PIV3 and BRSV infections [44][45][46]. Additionally, PIV3 infected alveolar macrophages are known to exhibit contact inhibition to surrounding lymphocytes [47][48][49].
Many of the immunosuppressive effects seen with BRDC infections are likely due to a decrease in overall white cell and platelet counts. Numerous studies have demonstrated this phenomenon in BVDV [50][51][52][53][54] and BoHV-1 [55] infections. A key strategy of M. haemolytica is the production of a leukocyte specific exotoxin, termed leukotoxin (LKT). This is a pore forming toxin which binds to leukocyte specific β integrin’s, resulting in inhibition of function, apoptosis and necrosis of neutrophils, macrophages and all other leukocyte subtypes [56]. The suppression of leukocyte function play a large role in pathogenesis of H. somni infection [57]. As well as inducing apoptosis [58], H. somni inhibits the production of superoxide anion by both alveolar macrophages and neutrophils [59]. As with M. haemolytica, there is an influx of neutrophils and NET formation upon initial infection, although infections persist [60]. An in vitro study using bovine neutrophils showed that any material phagocytosed is not destroyed when H. somni is present [61]. The inhibition of both macrophage and neutrophil responses are summarized in Figure 2.
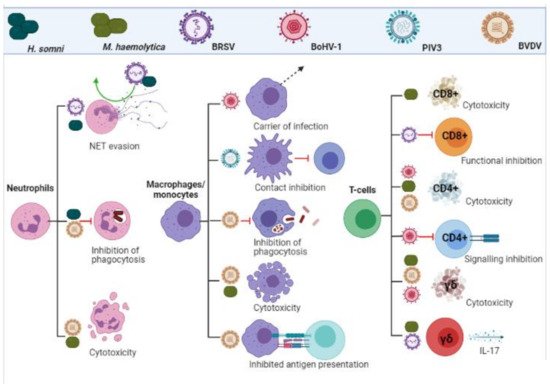
Figure 2. Leukocyte evasion and inhibition by BRDC pathogens. Viral and bacterial infections inhibit functionality, impact signalling and induce cytotoxicity in cells of the adaptive and innate immune response. (Created in BioRender.com, accessed on 24 March 2021).
3.3. T-Cells
As previously mentioned, BRDC infections exhibit leukopenia, which accounts for much of the immunosuppression. It is also well documented that BRDC infections mount a poor memory response, with persistent infection and high re-infection rates [2]. Specifically, immune memory is dependent on successful signalling from innate immune cells to the adaptive immune counterparts, arguably the most important being CD8+ and CD4+ T-cells. Cytotoxic CD8+ and helper/regulatory CD4+ T-cells are essential for bacterial and viral clearance and rapid response initiation upon re-infection [62]. BRSV is known to induce cytotoxic CD8+ T-cell inhibition [16][63]. CD8+ cells are vital for the destruction of infected cells and when depleted, correlate with disease severity [16][63]. CD4+ T-cells are known to be susceptible to BoHV-1 during acute infection, resulting in cell death [64][65] and inhibition of CD4+ signalling and regulatory functions [65]. In a model of BoHV-1 infection, both populations of CD8+ and CD4+ cells were significantly deleted throughout 14 days of infection. Furthermore, this depletion was amplified by pre-existing subclinical BVDV infection, which was particularly prominent in CD8+ populations [66].
γδ T-cells are a small subset of T-cells in humans, although make up for 60% of circulating lymphocytes in young claves [67]. These cells are thought to play a role in early pathogen detection and recognition of viral infection [68]. There is evidence for a dual role in the innate and adaptive response to BRSV infection by γδ T-cells [69]. These cells are also known to be depleted during BVDV and BoHV-1 co-infection, to a lesser extent than CD4+/CD8+ cells [66]. This phenomenon however is not seen with BRSV infections [70]. It has been suggested that γδ T-cells play a role in viral infection, specifically RSV, via IL-17 production [71], the role of which remains controversial, both acting protective and pathogenic [72]. Indeed, one study showed that calves infected with BRSV first mounted a strong IL-17 response, mediated by CD4+ and γδ T-cells, and that co-infection with M. haemolytica exacerbates this IL-17 produced primarily through γδ T-cells. The beneficial or detrimental nature of this response remains unclear [73].
The inhibition of T-cell responses are summarized in Figure 2.
4. Concluding remarks
Due to the multi-faceted nature of BRDC, it remains necessary to determine which combination of vaccines are the most effective at preventing this disease, with evidence for vaccine trials in the field currently lacking. Understanding the impact of BRDC-associated pathogens on the host immune response and their roles in the development of the disease complex is vital for deciphering which vaccination regiment offers the most protection.
References
- Urban-Chmiel, R.; Grooms, D.L. Prevention and Control of Bovine Respiratory Disease. J. Livest Sci. 2012, 3, 27–36.
- Ellis, J.A. The immunology of the bovine respiratory disease complex. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 535–550.
- Veterinary Laboratory Service; Agri-Food & Biosciences Institute. All-Island Animal Disease Surveillance 2019; Department of Agriculture, Food and the Marine: Dublin, Ireland, 2020.
- Dubrovsky, S.A.; van Eenennaam, A.L.; Aly, S.S.; Karle, B.M.; Rossitto, P.V.; Overton, M.W.; Lehenbauer, T.W.; Fadel, J.G. Preweaning cost of bovine respiratory disease (BRD) and cost-benefit of implementation of preventative measures in calves on California dairies: The BRD 10K study. J. Dairy Sci. 2020, 103, 1583–1597.
- Edwards, T.A. Control methods for bovine respiratory disease for feedlot cattle. Vol. 26, Veterinary Clinics of North America—Food Animal Practice. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 273–284.
- Loneragan, G.H.; Dargatz, D.A.; Morley, P.S.; Smith, M.A. Trends in mortality ratios among cattle in US feedlots. J. Am. Vet. Med. Assoc. 2001, 219, 1122–1127.
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The epidemiology of bovine respiratory disease: What is the evidence for predisposing factors? Can. Vet. J. 2010, 51, 1095–1102.
- Ackermann, M.R.; Derscheid, R.; Roth, J.A. Innate immunology of bovine respiratory disease. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 215–228.
- McGill, J.L.; Sacco, R.E. The Immunology of Bovine Respiratory Disease: Recent Advancements. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 333.
- Grissett, G.P.; White, B.J.; Larson, R.L. Structured Literature Review of Responses of Cattle to Viral and Bacterial Pathogens Causing Bovine Respiratory Disease Complex. J. Vet. Int. Med. 2015, 29, 770–780.
- Darbyshire, J.H.; Jennings, A.R.; Omar, A.R.; Dawson, P.S.; Lamont, P.H. Association of Adenoviruses with Bovine Respiratory Disease. Nature 1965, 208, 307–308.
- Hick, P.M.; Read, A.J.; Lugton, I.; Busfield, F.; Dawood, K.E.; Gabor, L.; Hornitzky, M.; Kirkland, P.D. Coronavirus infection in intensively managed cattle with respiratory disease. Aust. Vet. J. 2012, 90, 381–386.
- Ng, T.F.F.; Kondov, N.O.; Deng, X.; Van Eenennaam, A.; Neibergs, H.L.; Delwart, E. A Metagenomics and Case-Control Study to Identify Viruses Associated with Bovine Respiratory Disease. J. Virol. 2015, 89, 5340–5349.
- Griffin, D.; Chengappa, M.M.; Kuszak, J.; McVey, D.S. Bacterial pathogens of the bovine respiratory disease complex. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 381–394.
- McMullen, C.; Alexander, T.W.; Léguillette, R.; Workentine, M.; Timsit, E. Topography of the respiratory tract bacterial microbiota in cattle. Microbiome 2020, 10, 8.
- Guzman, E.; Taylor, G. Immunology of bovine respiratory syncytial virus in calves. Mol. Immunol. 2015, 66, 48–56.
- Viuff, B.; Uttenthal, A.; Tegtmeier, C.; Alexandersen, S. Sites of Replication of Bovine Respiratory Syncytial Virus in Naturally Infected Calves as Determined by In Situ Hybridization. Vet. Pathol. 1996, 33, 383–390.
- Goris, K.; Uhlenbruck, S.; Schwegmann-Wessels, C.; Köhl, W.; Niedorf, F.; Stern, M.; Hewicker-Trautwein, M.; Bals, R.; Taylor, G.; Braun, A.; et al. Differential Sensitivity of Differentiated Epithelial Cells to Respiratory Viruses Reveals Different Viral Strategies of Host Infection. J. Virol. 2009, 83, 1962–1968.
- Zhang, L.; Bukreyev, A.; Thompson, C.I.; Watson, B.; Peeples, M.E.; Collins, P.L.; Pickles, R.J. Infection of Ciliated Cells by Human Parainfluenza Virus Type 3 in an In Vitro Model of Human Airway Epithelium. J. Virol. 2005, 79, 1113–1124.
- Muylkens, B.; Thiry, J.; Kirten, P.; Schynts, F.; Thiry, E. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet. Res. 2007, 38, 181–209.
- Nyaga, P.N.; McKercher, D.G. Pathogenesis of Bovine herpesvirus-1 (BHV-1) infections: Interactions of the virus with peripheral bovine blood cellular components. Comp. Immunol. Microbiol. Infect Dis. 1979, 2, 587–602.
- Kirchhoff, J.; Uhlenbruck, S.; Goris, K.; Keil, G.M.; Herrler, G. Three viruses of the bovine respiratory disease complex apply different strategies to initiate infection. Vet. Res. 2014, 45, 1–12.
- Kirchhoff, J.; Uhlenbruck, S.; Keil, G.M.; Schwegmann-Wessels, C.; Ganter, M.; Herrler, G. Infection of differentiated airway epithelial cells from caprine lungs by viruses of the bovine respiratory disease complex. Vet. Microbiol. 2014, 170, 58–64.
- Sudaryatma, P.E.; Mekata, H.; Kubo, M.; Subangkit, M.; Goto, Y.; Okabayashi, T. Co-infection of epithelial cells established from the upper and lower bovine respiratory tract with bovine respiratory syncytial virus and bacteria. Vet. Microbiol. 2019, 235, 80–85.
- Rivera-Rivas, J.J.; Kisiela, D.; Czuprynski, C.J. Bovine herpesvirus type 1 infection of bovine bronchial epithelial cells increases neutrophil adhesion and activation. Vet. Immunol. Immunopathol. 2009, 131, 167–176.
- Al-Haddawi, M.; Mitchell, G.B.; Clark, M.E.; Wood, R.D.; Caswell, J.L. Impairment of innate immune responses of airway epithelium by infection with bovine viral diarrhea virus. Vet. Immunol. Immunopathol. 2007, 116, 153–162.
- Mitchell, G.B.; Al-Haddawi, M.H.; Clark, M.E.; Beveridge, J.D.; Caswell, J.L. Effect of corticosteroids and neuropeptides on the expression of defensins in bovine tracheal epithelial cells. Infect. Immun. 2007, 75, 1325–1334.
- Babiuk, L.A.; Van Drunen Littel-Van Den Hurk, S.; Tikoo, S.K. Immunology of bovine herpesvirus 1 infection. In Veterinary Microbiology; Elsevier: New York, NY, USA, 1996; pp. 31–42.
- Olafson, P.; MacCallum, A.D.; Fox, F.H. An apparently new transmissible disease of cattle. Cornell Vet. 1946, 36, 205–213.
- Childs, T. X Disease of Cattle—Saskatchewan. Can. J. Comp. Med. Vet. Sci. 1946, 10, 316–319.
- Walz, P.H.; Grooms, D.L.; Passler, T.; Ridpath, J.F.; Tremblay, R.; Step, D.L.; Callan, R.J.; Givens, M.D. Control of bovine viral diarrhea virus in ruminants. J. Vet. Intern. Med. 2010, 24, 476–486.
- Apley, M.D. Treatment of Calves with Bovine Respiratory Disease: Duration of Therapy and Posttreatment Intervals. Vet. Clin. N. Am. Food Anim. Pract. 2015, 31, 441–453.
- Corbeil, L.B. Histophilus somni host–parasite relationships. Anim. Health Res. Rev. 1996, 10, 151–160.
- Orr, J.P. Haemophilus somnus infection: A retrospective analysis of cattle necropsied at the Western College of Veterinary Medicine from 1970 to 1990. Can. Vet. J. 1992, 33, 719–722.
- Zeilhofer, H.U.; Schorr, W. Role of interleukin-8 in neutrophil signaling. Curr. Opin. Hematol. 2000, 7, 178–182.
- Brown, G.B.; Bolin, S.R.; Frank, D.E.; Roth, J.A. Defective function of leukocytes from cattle persistently infected with bovine viral diarrhea virus, and the influence or recombinant cytokines. Am. J. Vet. Res. 1991, 52, 381–387.
- Cortjens, B.; de Boer, O.J.; de Jong, R.; Antonis, A.F.G.; Sabogal Piñeros, Y.S.; Lutter, R.; van Woensel, J.B.M.; Bem, R.A. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J. Pathol. 2016, 238, 401–411.
- Singh, K.; Ritchey, J.W.; Confer, A.W. Mannheimia haemolytica: Bacterial-host interactions in bovine Pneumonia. Vet. Pathol. 2011, 48, 338–348.
- Caswell, J.L.; Middleton, D.M.; Gordon, J.R. The importance of interleukin-8 as a neutrophil chemoattractant in the lungs of cattle with pneumonic pasteurellosis. Can. J. Vet. Res. 2001, 65, 229–232.
- Radi, Z.A.; Caverly, J.M.; Dixon, R.A.; Brogden, K.A.; Ackermann, M.R. Effects of the synthetic selectin inhibitor TBC1269 on tissue damage during acute Mannheimia haemolytica-induced pneumonia in neonatal calves. Am. J. Vet. Res. 2001, 62, 17–22.
- Puttur, F.; Gregory, L.G.; Lloyd, C.M. Airway macrophages as the guardians of tissue repair in the lung. Immunol. Cell Biol. 2019, 97, 246–257.
- Adler, B.; Adler, H.; Pfister, H.; Jungi, T.W.; Peterhans, E. Macrophages infected with cytopathic bovine viral diarrhea virus release a factor(s) capable of priming uninfected macrophages for activation-induced apoptosis. J. Virol. 1997, 71, 3255–3258.
- Glew, E.J.; Carr, B.V.; Brackenbury, L.S.; Hope, J.C.; Charleston, B.; Howard, C.J. Differential effects of bovine viral diarrhoea virus on monocytes and dendritic cells. J. Gen. Virol. 2003, 84, 1771–1780.
- Schaut, R.G.; Ridpath, J.F.; Sacco, R.E. Bovine viral diarrhea virus type 2 impairs macrophage responsiveness to toll-like receptor ligation with the exception of toll-like receptor 7. PLoS ONE 2016, 11, e0159491.
- Hesse, R.A.; Toth, T.E. Effects of bovine parainfluenza-3 virus on phagocytosis and phagosome-lysosome fusion of cultured bovine alveolar macrophages. Am. J. Vet. Res. 1983, 44, 1901–1907.
- Adair, B.M.; McNulty, M.S. Effect of “in vitro” exposure of bovine alveolar macrophages to different strains of bovine respiratory syncytial virus. Vet. Immunol. Immunopathol. 1992, 30, 193–206.
- Moreno-López, J. Cell-Mediated Immunity to Parainfluenza-3 (PIV-3) in Cattle Evaluation of in vivo and in vitro tests. Zentralblatt Vet. R. B 1977, 24, 231–240.
- Johnson, K.; Morein, B. In vitro stimulation of bovine circulating lymphocytes by parainfluenza type 3 virus. Res. Vet. Sci. 1977, 22, 83–85.
- Basaraba, R.J.; Brown, P.R.; Laegreid, W.W.; Silflow, R.M.; Evermann, J.F.; Leid, R.W. Suppression of lymphocyte proliferation by parainfluenza virus type 3-infected bovine alveolar macrophages. Immunology 1993, 79, 179–188.
- Roth, J.A.; Kaeberle, M.L. Suppression of neutrophil and lymphocyte function induced by a vaccinal strain of bovine viral diarrhea virus with and without the administration of ACTH. Am. J. Vet. Res. 1983, 44, 2366–2372.
- Walz, P.H.; Bell, T.G.; Wells, J.L.; Grooms, D.L.; Kaiser, L.; Maes, R.K.; Baker, J.C. Relationship between degree of viremia and disease manifestations in calves with experimentally induced bovine viral diarrhea virus infection. Am. J. Vet. Res. 2001, 62, 1095–1103.
- Kelling, C.L.; Steffen, D.J.; Topliff, C.L.; Eskridge, K.M.; Donis, R.O.; Higuchi, D.S. Comparative virulence of isolates of bovine viral diarrhea virus type II in experimentally inoculated six- to nine-month-old calves. Am. J. Vet. Res. 2002, 63, 1379–1384.
- Carlos-Valdez, L.; Wilson, B.K.; Burciaga-Robles, L.O.; Step, D.L.; Holland, B.P.; Richards, C.J.; Montelongo, M.A.; Confer, A.W.; Fulton, R.W.; Krehbiel, C.R. Effect of timing of Mannheimia haemolytica challenge following short-term natural exposure to bovine viral diarrhea virus type 1b on animal performance and immune response in beef steers. J. Anim. Sci. 2016, 94, 4799–4808.
- Burciaga-Robles, L.O.; Step, D.L.; Krehbiel, C.R.; Holland, B.P.; Richards, C.J.; Montelongo, M.A.; Confer, A.W.; Fulton, R.W. Effects of exposure to calves persistently infected with bovine viral diarrhea virus type 1b and subsequent infection with mannheima haemolytica on clinical signs and immune variables: Model for bovinerespiratory disease via viral and bacterial interaction. J. Anim. Sci. 2010, 88, 2166–2178.
- Griebel, P.J.; Qualtiere, L.; Davis, W.C.; Gee, A.; Ohmann, H.B.; Lawman, M.J.; Babiuk, L.A. T lymphocyte population dynamics and function following a primary bovine herpesvirus type-1 infection. Viral Immunol. 1987, 1, 287–304.
- Czuprynski, C.J.; Leite, F.; Sylte, M.; Kuckleburg, C.; Schultz, R.; Inzana, T.; Behling-Kelly, E.; Corbeil, L. Complexities of the pathogenesis of Mannheimia haemolytica and Haemophilus somnus infections: Challenges and potential opportunities for prevention? Anim. Heal. Res. Rev. 2004, 5, 277–282.
- Inzana, T.J.; Balyan, R.; Howard, M.D. Decoration of Histophilus somni lipooligosaccharide with N-acetyl-5-neuraminic acid enhances bacterial binding of complement factor H and resistance to killing by serum and polymorphonuclear leukocytes. Vet. Microbiol. 2012, 161, 113–121.
- Yang, Y.F.; Sylte, M.J.; Czuprynski, C.J. Apoptosis: A possible tactic of Haemophilus somnus for evasion of killing by bovine neutrophils? Microb. Pathog. 1998, 24, 351–359.
- Howard, M.D.; Boone, J.H.; Buechner-Maxwell, V.; Schurig, G.G.; Inzana, T.J. Inhibition of bovine macrophage and polymorphonuclear leukocyte superoxide anion production by Haemophilus somnus. Microb. Pathog. 2004, 37, 263–271.
- Hellenbrand, K.M.; Forsythe, K.M.; Rivera-Rivas, J.J.; Czuprynski, C.J.; Aulik, N.A. Histophilus somni causes extracellular trap formation by bovine neutrophils and macrophages. Microb. Pathog. 2013, 54, 67–75.
- Czuprynski, C.J.; Hamilton, H.L. Bovine neutrophils ingest but do not kill Haemophilus somnus in vitro. Infect. Immun. 1985, 50, 431–436.
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: Naïve to memory and everything in between. Am. J. Physiol. Adv. Physiol. Educ. 2013, 37, 273–283.
- Gaddum, R.M.; Cook, R.S.; Furze, J.M.; Ellis, S.A.; Taylor, G. Recognition of bovine respiratory syncytial virus proteins by bovine CD8+ T lymphocytes. Immunology 2003, 108, 220–229.
- Koppers-Lalic, D.; Rychlowski, M.; van Leeuwen, D.; Rijsewijk, F.A.M.; Ressing, M.E.; Neefjes, J.J.; Bienkowska-Szewczyk, K.; Wiertz, E.J.H.J. Bovine herpesvirus 1 interferes with TAP-dependent peptide transport and intracellular trafficking of MHC class I molecules in human cells. Arch. Virol. 2003, 148, 2023–2037.
- Winkler, M.T.C.; Doster, A.; Jones, C. Bovine Herpesvirus 1 Can Infect CD4+ T Lymphocytes and Induce Programmed Cell Death during Acute Infection of Cattle. J. Virol. 1999, 73, 8657–8668.
- Molina, V.; Risalde, M.A.; Sánchez-Cordón, P.J.; Pedrera, M.; Romero-Palomo, F.; Luzzago, C.; Gómez-Villamandos, J.C. Effect of infection with BHV-1 on peripheral blood leukocytes and lymphocyte subpopulations in calves with subclinical BVD. Res. Vet. Sci. 2013, 95, 115–122.
- Guerra-Maupome, M.; Slate, J.R.; McGill, J.L. Gamma Delta T Cell Function in Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 453–469.
- McGill, J.L.; Sacco, R.E. γδ T cells and the immune response to respiratory syncytial virus infection. Vet. Immunol. Immunopathol. 2016, 181, 24–29.
- Mcgill, J.L.; Nonnecke, B.J.; Lippolis, J.D.; Reinhardt, T.A.; Sacco, R.E. Differential chemokine and cytokine production by neonatal bovine γδ T-cell subsets in response to viral toll-like receptor agonists and in vivo respiratory syncytial virus infection. Immunology 2013, 139, 227–244.
- Mcinnes, E.; Sopp, P.; Howard, C.J.; Taylor, G. Phenotypic analysis of local cellular responses in calves infected with bovine respiratory syncytial virus. Immunology 1999, 96, 396–403.
- Huang, H.; Saravia, J.; You, D.; Shaw, A.J.; Cormier, S.A. Impaired gamma delta T cell-derived IL-17A and inflammasome activation during early respiratory syncytial virus infection in infants. Immunol. Cell Biol. 2015, 93, 126–135.
- Bystrom, J.; Al-Adhoubi, N.; Al-Bogami, M.; Jawad, A.; Mageed, R. Th17 Lymphocytes in Respiratory Syncytial Virus Infection. Viruses 2013, 5, 777–791.
- McGill, J.L.; Rusk, R.A.; Guerra-Maupome, M.; Briggs, R.E.; Sacco, R.E. Bovine Gamma Delta T Cells Contribute to Exacerbated IL-17 Production in Response to Co-Infection with Bovine RSV and Mannheimia haemolytica. PLoS ONE 2016, 11, e0151083.





