
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kyung Soo Nam | + 800 word(s) | 800 | 2020-05-14 10:58:37 | | | |
| 2 | Nora Tang | + 437 word(s) | 1237 | 2020-10-30 12:38:52 | | |
Video Upload Options
Here, we investigated the effect of arctigenin (ATG) on doxorubicin (DOX)-induced cell death using MDA-MB-231 human breast cancer cells. The results showed that DOX-induced cell death was enhanced by ATG/DOX co-treatment in a concentration-dependent manner and that this was associated with increased DOX uptake and the suppression of multidrug resistance-associated protein 1 (MRP1) gene expression in MDA-MB-231 cells. ATG enhanced DOX-induced DNA damage and decreased the phosphorylation of STAT3 and the expressions of RAD51 and survivin. Cell death caused by ATG/DOX co-treatment was mediated by the nuclear translocation of apoptosis inducing factor (AIF), reductions in cellular and mitochondrial Bcl-2 and Bcl-xL, and increases in mitochondrial Bax levels. However, caspase-3 and -7 did not participate in DOX/ATG-induced cell death. We also found that DOX/ATG-induced cell death was linked with activation of the p38 signaling pathway and suppressions of the phosphorylations and expressions of Akt and c-Jun N-terminal kinase. Taken together, these results show that ATG enhances the cytotoxic activity of DOX in MDA-MB-231 human breast cancer cells by inducing prolonged p21 expression and p38-mediated AIF-dependent cell death. In conclusion, our findings suggest that ATG might alleviate the side effects and improve the therapeutic efficacy of DOX.
1. Introduction
Breast cancer is the major cause of cancer-related death among women. Although many subtypes of breast cancer have been reported, they are generally classified as hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-overexpressing, and triple-negative breast cancer (TNBC) [1]. Triple-negative breast cancer (TNBC) has a higher mortality rate than HR-positive or HER-2-overexpressing breast cancer because of its high rate of recurrence [2][3][4]. Furthermore, because of the absence of HR and HER-2 in TNBC, non-targeting anti-cancer drugs such as paclitaxel, cyclophosphamide, and doxorubicin (DOX) are being used to treat the disease [5].
2. DOX
DOX is an anthracycline antibiotic and broad spectrum anti-cancer agent [6][7]. Although DOX is useful for the treatment of triple-negative breast cancer (TNBC), in practice its use is limited because of its serious side effects, which include cardiotoxicity, diarrhea, vomiting, hair loss, and nausea. Actually, the total dose of DOX administered must not exceed 450–500 mg/m2 because of its cardiotoxicity [6]. Furthermore, reductions in DOX dosage made to address its side effects reduce its therapeutic efficacy [8].
3. Arctigenin (ATG)
Arctigenin (ATG) is a pharmaceutically active substance isolated from the seeds of Arctium lappa L. (commonly called greater burdock), and several investigators have shown it has anti-viral, anti-inflammatory, anti-cancer, and immunomodulatory activities [9][10][11][12][13]. The anti-cancer activity of ATG has been reported to due to the induction of apoptosis mediated by mitochondrial disruption and cell cycle arrest in breast, lung, bladder, gastric, hepatic, and colon cancer cells [14][15][16][17][18]. In a recent study, we showed ATG suppressed metastatic potential and induced autophagic cell death by inhibiting estrogen receptor (ER) expression in MCF-7 human breast cancer cells [19][20]. Also, Wang et al. reported human non-small cell lung cancer (NSCLC) cells treated with ATG exhibited greater chemosensitivity to cisplatin-induced apoptotic cell death mediated by the down-regulation of survivin [21].
Combination chemotherapies are being increasingly used to treat cancers to minimize toxicities and side effects based on the delivery of lower doses of the drugs responsible [22][23]. Numerous investigations have shown ATG has anti-cancer and anti-metastatic effects on different cancer cell types. Therefore, we assessed the effects of ATG/DOX co-treatment to determine whether ATG enhances the cytotoxic effect of DOX in MDA-MB-231 TNBC cells.
Although DOX is useful anti-cancer drug, its side effects severely restrict its use. However, reducing DOX dosages markedly mitigate these side effects. In the present study, we found the cytotoxic activity of DOX was significantly enhanced by co-treating it with ATG in MDA-MB-231 human triple-negative breast cancer cells and that DOX uptake was dose-dependently enhanced and MRP1 expression was dose-dependently suppressed in these cells (Figure 1B and Figure 2A,C). Furthermore, the presence of ATG synergistically increased DOX cytotoxicity (Figure 1C). These results suggest that combinatorial ATG/DOX should be considered a potential treatment for triple-negative breast cancer that reduces the side effects of DOX.
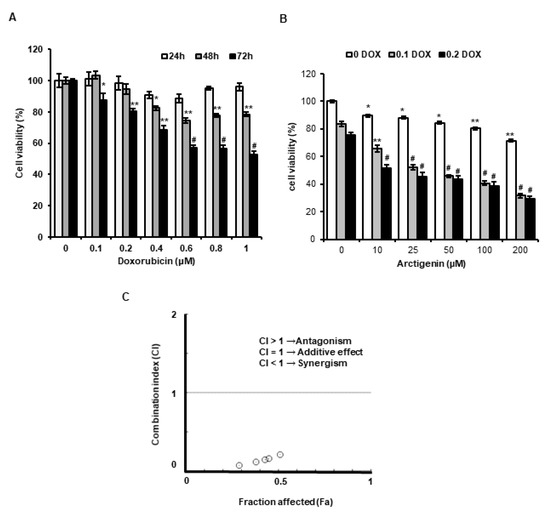
Figure 1. Effect of arctigenin (ATG) co-treatment on doxorubicin (DOX)-induced cytotoxicity in MDA-MB-231 cells. (A) Cells were incubated in Dulbecco’s Modified Eagle’s medium (DMEM) medium containing various concentrations of DOX (0–1 µM) for 24, 48, or 72 h. *, ** and # indicate p < 0.05, p < 0.01 and p < 0.001 vs. non-treated controls. (B) Cells were incubated in DMEM medium containing various concentration of ATG (0–200 μM) with or without 0.2 μM DOX for 72 h. ATG enhanced cytotoxicity of DOX in a concentration-dependent manner. * and ** indicate p < 0.05 and p < 0.01 vs. non-treated controls. ## and ### indicate p < 0.0005 and p < 0.0001 vs. non-treated controls. (A,B) Cell viabilities were determined using an MTT assay. All experiments were performed independently three times and results are presented as means ± SDs. (C) Combination indices (CI) versus fractional affected (Fa) plots for ATG/DOX co-treatment were graphically represented by Compusyn software. Synergistic cytotoxic activity of ATG/DOX co-treatment was observed in MDA-MB-231 human triple negative breast cancer cells. A CI value of < 1 indicates a synergistic cytotoxic effect.
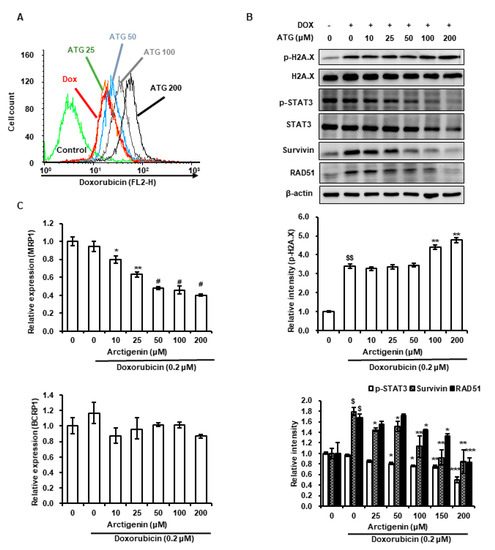
Figure 2. Effects of ATG on DOX uptake, the transcriptions of multidrug resistance-associated protein 1 (MRP1) and breast cancer resistance protein 1 (BCRP1), the phosphorylations of H2A histone family member X (H2A.X) and signal transducer and activator of transcription 3 (STAT3), and the expressions of survivin and DNA repair protein RAD51 homolog 1 isoform 1 (RAD51) in MDA-MB-231 cells. (A) Cells were grown for 48 h with various concentrations of ATG (0–200 μM) prior to being treated with 0.2 μM DOX treatment for 24 h. DOX uptake was analyzed by flow cytometry. ATG accelerated DOX uptake in a concentration-dependent manner. The X-axis shows fluorescence intensities of intracellular DOX and the Y-axis cell numbers per channel. (B) Cells were attached for 24 h and further grown for 24 h in DMEM medium supplemented 2% fetal bovine serum (FBS), 0.2 μM DOX, and various concentration of ATG (0–200 μM). MRP1 gene expression was suppressed by ATG/DOX co-treatment. Relative expressions of MRP1 and BCRP1 genes were evaluated in triplicate and normalized versus glyceraldehyde-3-phosphate dehydrogenase (GAPDH). *, ** and # indicate p < 0.05, p < 0.01, and p < 0.001 vs. DOX treated cells. –: 0.2 μM DOX-untreated, +: 0.2 μM DOX-treated (C) Cells were attached for 24 h and then co-treatment with 0.2 μM DOX and various concentration of ATG (0–200 μM) for 72 h in DMEM medium supplemented with 2% FBS. The proteins in whole cell lysates were separated by 8% or 15% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidenefluoride (PVDF) membranes. Bands were densitometrically analyzed using Scion Image and band densities were normalized versus β-actin. $ and $$ indicate p < 0.01 and p < 0.005 vs. non-treated control. *, ** and *** indicate p < 0.05, p < 0.01 and p < 0.005 vs. DOX treated cells. (B, C) All experiments were conducted independently three times and results are presented as means ± SDs.
ATG co-treatment increased DOX-induced H2A.X phosphorylation, reversed DOX-induced survivin and RAD51 protein expressions, and increased DOX uptake by MDA-MB-231 cells (Figure 2A,B). H2A.X phosphorylation is the result of DNA damage [24][25], and thus, we speculated that increased DOX-induced H2A.X phosphorylation by ATG reflects an increase in DNA, and that this enhanced MDA-MB-231 cell death (Figure 1C). Cell death by DNA damage is primarily caused by a failure to repair DNA [26][27], and survivin and RAD51 play important roles in DNA repair [26][27]. Hence, the ATG/DOX-induced suppressions of survivin and RAD51 protein levels suggests increased cell death was due to an inability to repair DNA. Consequently, the present results suggest that ATG/DOX co-treatment-induced MDA-MB-231 cell death was associated with DNA damage and failure of the DNA repair system.
References
- Prat, A.; Pineda, E.; Adamo, B.; Galvan, P.; Fernandez, A.; Gaba, L.; Diez, M.; Viladot, M.; Arance, A.; Munoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015, 24, S26–S35.
- Reddy, S.M.; Barcenas, C.H.; Sinha, A.K.; Hsu, L.; Moulder, S.L.; Tripathy, D.; Hortobagyi, G.N.; Valero, V. Long-term survival outcomes of triple-receptor negative breast cancer survivors who are disease free at 5 years and relationship with low hormone receptor positivity. Br. J. Cancer 2018, 118, 17–23.
- Liedtke, C.; Mazouni, C.; Hess, K.R.; Andre, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281.
- Lin, N.U.; Vanderplas, A.; Hughes, M.E.; Theriault, R.L.; Edge, S.B.; Wong, Y.N.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; Weeks, J.C. Clinicopathologic Features, Patterns of Recurrence, and Survival Among Women With Triple-Negative Breast Cancer in the National Comprehensive Cancer Network. Cancer 2012, 118, 5463–5472.
- Collignon, J.; Lousberg, L.; Schroeder, H.; Jerusalem, G. Triple-negative breast cancer: Treatment challenges and solutions. Breast Cancer-Targets Ther. 2016, 8, 93–107.
- Lee, D.H.; Kim, S.; Nam, K.S. Protective effects of deep sea water against doxorubicin-induced cardiotoxicity in H9c2 cardiac muscle cells. Int. J. Oncol. 2014, 45, 2569–2575.
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229.
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285.
- Hayashi, K.; Narutaki, K.; Nagaoka, Y.; Hayashi, T.; Uesato, S. Therapeutic Effect of Arctiin and Arctigenin in Immunocompetent and Immunocompromised Mice Infected with Influenza A Virus. Biol. Pharm. Bull. 2010, 33, 1199–1205.
- Swarup, V.; Ghosh, J.; Mishra, M.K.; Basu, A. Novel strategy for treatment of Japanese encephalitis using arctigenin, a plant lignan. J. Antimicrob. Chemother. 2008, 61, 679–688.
- Kim, Y.; Hollenbaugh, J.A.; Kim, D.H.; Kim, B. Novel PI3K/Akt Inhibitors Screened by the Cytoprotective Function of Human Immunodeficiency Virus Type 1 Tat. PLoS ONE 2011, 6, e21781.
- Kang, H.S.; Lee, J.Y.; Kim, C.J. Anti-inflammatory activity of arctigenin from Forsythiae Fructus. J. Ethnopharmacol. 2008, 116, 305–312.
- Lee, J.Y.; Kim, C.J. Arctigenin, a Phenylpropanoid Dibenzylbutyrolactone Lignan, Inhibits Type I-IV Allergic Inflammation and Pro-inflammatory Enzymes. Arch. Pharmacal Res. 2010, 33, 947–957.
- Li, Q.C.; Liang, Y.; Tian, Y.; Hu, G.R. Arctigenin induces apoptosis in colon cancer cells through ROS/p38MAPK pathway. J. BUON 2016, 21, 87–94.
- Hsieh, C.J.; Kuo, P.L.; Hsu, Y.C.; Huang, Y.F.; Tsai, E.M.; Hsu, Y.L. Arctigenin, a dietary phytoestrogen, induces apoptosis of estrogen receptor-negative breast cancer cells through the ROS/p38 MAPK pathway and epigenetic regulation. Free Radic. Biol. Med. 2014, 67, 159–170.
- Yang, S.C.; Ma, J.; Xiao, J.B.; Lv, X.H.; Li, X.L.; Yang, H.K.; Liu, Y.; Feng, S.J.; Zhang, Y.F. Arctigenin Anti-Tumor Activity in Bladder Cancer T24 Cell Line Through Induction of Cell-Cycle Arrest and Apoptosis. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2012, 295, 1260–1266.
- Jiang, X.X.; Zeng, L.P.; Huang, J.F.; Zhou, H.; Liu, Y.B. Arctigenin, a Natural Lignan Compound, Induces Apoptotic Death of Hepatocellular Carcinoma Cells via Suppression of PI3-K/Akt Signaling. J. Biochem. Mol. Toxicol. 2015, 29, 458–464.
- Jeong, J.B.; Hong, S.C.; Jeong, H.J.; Koo, J.S. Arctigenin induces cell cycle arrest by blocking the phosphorylation of Rb via the modulation of cell cycle regulatory proteins in human gastric cancer cells. Int. Immunopharmacol. 2011, 11, 1573–1577.
- Maxwell, T.; Lee, K.S.; Kim, S.; Nam, K.S. Arctigenin inhibits the activation of the mTOR pathway, resulting in autophagic cell death and decreased ER expression in ER-positive human breast cancer cells. Int. J. Oncol. 2018, 52, 1339–1349.
- Maxwell, T.; Chun, S.Y.; Lee, K.S.; Kim, S.; Nam, K.S. The anti-metastatic effects of the phytoestrogen arctigenin on human breast cancer cell lines regardless of the status of ER expression. Int. J. Oncol. 2017, 50, 727–735.
- Wang, H.Q.; Jin, J.J.; Wang, J. Arctigenin Enhances Chemosensitivity to Cisplatin in Human Nonsmall Lung Cancer H460 Cells through Downregulation of Survivin Expression. J. Biochem. Mol. Toxicol. 2014, 28, 39–45.
- Mayer, L.D.; Janoff, A.S. Optimizing combination chemotherapy by controlling drug ratios. Mol. Interv. 2007, 7, 216–223.
- Zoli, W.; Ricotti, L.; Tesei, A.; Barzanti, F.; Amadori, D. In vitro preclinical models for a rational design of chemotherapy combinations in human tumors. Crit. Rev. Oncol. Hematol. 2001, 37, 69–82.
- Ji, J.P.; Zhang, Y.P.; Redon, C.E.; Reinhold, W.C.; Chen, A.P.; Fogli, L.K.; Holbeck, S.L.; Parchment, R.E.; Hollingshead, M.; Tomaszewski, J.E.; et al. Phosphorylated fraction of H2AX as a measurement for DNA damage in cancer cells and potential applications of a novel assay. PLoS ONE 2017, 12, e0171582.
- Fukuchi, K.; Tomoyasu, S.; Nakamaki, T.; Tsuruoka, N.; Gomi, K. DNA damage induces p21 protein expression by inhibiting ubiquitination in ML-1 cells. Biochim. Biophys. Acta Mol. Cell Res. 1998, 1404, 405–411.
- Vequaud, E.; Desplanques, G.; Jezequel, P.; Juin, P.; Barille-Nion, S. Survivin contributes to DNA repair by homologous recombination in breast cancer cells. Breast Cancer Res. Treat. 2016, 155, 53–63.
- Gildemeister, O.S.; Sage, J.M.; Knight, K.L. Cellular Redistribution of Rad51 in Response to DNA Damage NOVEL ROLE FOR Rad51C. J. Biol. Chem. 2009, 284, 31945–31952.




