
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cristina Cacheiro Llaguno | + 1972 word(s) | 1972 | 2021-04-06 07:42:55 | | | |
| 2 | Vivi Li | -51 word(s) | 1921 | 2021-04-14 08:02:22 | | |
Video Upload Options
During canine leishmaniasis (CanL) due to Leishmania infantum (L. infantum), uncontrolled infection leads to a strong humoral immune response. As a consequence of the production of high antibody levels and the prolonged presence of parasite antigens, circulating immune complexes (CIC) are formed, which can be deposited in certain organs and tissues, inducing vasculitis, uveitis, dermatitis and especially glomerulonephritis and renal failure. A method to detect CIC, and quantify their levels in serum samples from dogs infected with L. infantum has been recently described. It allowed to demonstrate a correlation between CIC levels and disease severity. Thus, CIC measurement may be useful for diagnosis, assessment of disease progression and for monitoring response to treatment. This is an interesting finding, considering that there remains an urgent need for identification of novel biomarkers to achieve a correct diagnosis and for optimal disease staging of dogs suffering from Leishmania infection.
1. Introduction
Leishmaniases are a group of parasitic diseases caused by different species of the Leishmania parasite. Among them, canine visceral leishmaniasis (CanL), caused by Leishmania infantum (L. infantum), is a global zoonotic disease that is potentially fatal for dogs and, due to its potential transmission, to humans. The role of dogs as the main vertebrate reservoir is well-established, making visceral leishmaniasis (VL) a prime example of the importance of embracing a “One Health” approach for efficient surveillance and control of canine and human disease [1][2][3][4].
In CanL, one of the main determinants for the establishment of the infection is the host’s immune system’s ability to control the parasite. Two well-recognized types of host immune response are induced as a consequence of infection. On one hand, resistant dogs develop a robust Th1 immune response, resulting in the production of proinflammatory cytokines, such as IFN-γ and TNF-α, which limit the infection and associated inflammation by increasing the leishmanicidal activity of macrophages. In contrast, susceptible dogs develop a systemic immune response dominated by Th2 cells, regulatory T-cells (Tregs) and regulatory B cells [5][6]. The cytokines released by Th2 cells, which include interleukins IL-4 and IL-13, and the activity of Tregs and regulatory B cells via IL-10, downregulates the protective Th1 immune response, promoting “inappropriate” humoral immune responses [7][8][9][10][11][12][13]. The uncontrolled concentration of antibodies and the presence of Leishmania antigens induce the formation of circulating immune complexes (CIC) [14], composed of aggregated Leishmania proteins, anti-Leishmania IgG and IgM and, to a lesser extent, complement system fractions [15]. Macrophages activated by these immune complexes inhibit IL-12 biosynthesis, and, therefore, IFN-γ production, and secrete high levels of IL-10 [16], thus impeding the establishment of cell-mediated immunity and reducing the macrophage’s ability to kill the parasite [14]. Macrophages lose their ability to eliminate immune complexes, resulting in a deposition of CIC in the vascular walls of specific organs that leads to inflammation and tissue injury [14][17]. This deposition is responsible for some of the clinical manifestations of CanL [18], including glomerulonephritis, considered to be the most severe complication of CanL. It has variable clinical presentations, depending on the disease stage [12][14][19] and is the most frequent cause of renal failure and death.
CanL has a broad range of nonspecific clinical manifestations, ranging between subclinical, chronic and severe, sometimes reaching an acute stage that may kill the animal [20]. Therefore, the management of CanL is complex, and it is important to establish a standardized clinical staging system [12][21]. Veterinarians should use information from multiple sources, such as clinical history; examination findings; clinicopathological abnormalities; molecular tests to detect the parasite, such as the Polymerase Chain Reaction (PCR); and serological tests to evaluate the host immune response, such as the immunofluorescence antibody test (IFAT) or the Enzyme-Linked ImmunoSorbent Assay (ELISA). This evaluation is necessary to characterize the severity of the disease and determine the clinical stage, enabling the selection of an adequate treatment or to predict progression toward more serious and irreversible stages [19][20].
Although there are a wide variety of diagnostic techniques for CanL, none of them offers 100% sensitivity or specificity [22][23], and new diagnostic tools are needed to improve detection, especially in asymptomatic dogs. In this sense, Parody et al. recently described a method to isolate CIC and quantify their levels in serum samples obtained from dogs infected with L. infantum [15]. Furthermore, this study demonstrated a clear correlation between CIC levels and pathologic stage in animals infected with L. infantum. This fact has also been corroborated by other authors [14], suggesting that CIC analysis may have prognostic value. This is significant, considering that prediction of CanL clinical outcomes has been challenging to date, relying on the severity of clinicopathological abnormalities, in particular, those reflecting renal function, and the response to treatment, as prognostic indicators [12][18]. Interestingly, it has recently been published that vaccination with LetiFend® reduces CIC levels, which may be related with the mechanism of control of L. infantum infection in dogs, although the mechanism of action has not yet been defined [18].
Therefore, CIC have been revealed as biomarkers with potential diagnostic and prognostic applications, and their measurement may allow not only improved disease staging but also improvements to diagnosis of clinically healthy dogs infected by L. infantum, in order to monitor disease progression and/or response to treatments.
2. Role of CIC in CanL
CanL is characterized by a large variety of clinical signs and clinicopathological alterations, the majority of which result from immune mediated mechanisms. Many of these alterations are attributed to CIC formation and deposition in specific tissues [24], causing vasculitis, polyarthritis, uveitis, meningitis and glomerulonephritis, which can cause proteinuria and may progress to renal failure and eventually death [25][26][27][28][29].
The marked humoral response and ensuing CIC deposition in target organs of susceptible dogs constitute the mainstay of CanL pathogenesis, explaining the broad clinical spectrum observed [30][31]. The nature of the individual immune response determines whether the infection will be successfully controlled or whether the dogs will develop clinical signs due to the deposition of CIC (Figure 1).
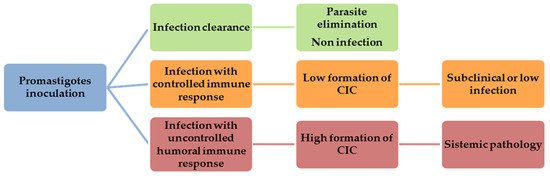
Figure 1. Canine visceral leishmaniasis (CanL) development after promastigotes inoculation.
CIC play an important role not only in leishmaniasis but also in many other infectious diseases [32][33]. Their presence has been reported in the serum of patients with a variety of pathologies caused by viral, bacterial, protozoal and helminthic agents. Immune complex-mediated pathologies can cause damage in different tissues and organs through complement activation, with or without local deposition, and can also modulate humoral and cellular immune responses through binding to surface receptors of lymphocytes and phagocytes [34].
Previous studies have highlighted the importance of CIC in the pathogenesis of a variety of systemic disorders, such as autoimmune diseases [35][36][37][38], allergic diseases [39][40], cancer [41][42] and infectious diseases [43][44][45]. In the latter, CIC have a limited capability of penetrating the basement membrane. However, in leishmaniasis, intense parasitic destruction liberates antigens, and consequently, CIC are formed, which circulate and easily penetrate the membrane, increasing subepithelial deposits [46][47]. In addition to CanL, a key role of CIC has been suggested in the pathogenesis of other canine vector- borne diseases, such as Ehrlichia canis and Dirofilaria immitis infections [48][49].
The role of CIC in the infection caused by Leishmania parasites has been studied in animal models and also in humans [16][50][51]. CIC were found in 30% of sera from human patients with VL. They were also detected in sera from patients with cutaneous leishmaniasis and persist in sera from clinically cured subjects [52]. These studies suggest that disease complications may be partly accounted by CIC deposition-related pathology, particularly nephritis [27][29][53][54][55]. In this process, it has been shown that CIC size, IgG subclasses and glycosylation of IgG are relevant [56][57]. Of all the mechanisms that lead to development of renal pathology, those of an immunologic nature are the most important and involve many processes that have in common the deposition of immune complexes on glomerular walls and/or mesangial matrix [58].
CIC have been detected in sera from Leishmania-infected dogs [14][15][18][54][59], and the pathogenesis of renal lesions has been mainly attributed to CIC deposition within capillary beds of the glomerular tuft and subsequent glomerular injury [29][60][61][62][63][64][65][66][67][68][69]. In fact, CIC related renal pathology plays a pivotal role in prognosis, and it has been adopted as a major criterion for clinical staging of the disease in dogs [19][20][70][71][72].
3. CIC as Biomarkers for Measuring CanL Progression
Over the course of its lifetime, a Leishmania-infected dog may fit into several different categories, depending on its immune response and/or the parasite challenge or exposure [73]. The complexity of parasite–host interactions has revealed that a single biomarker cannot be used alone for CanL diagnosis or prognosis, and there is a great unmet need for new biomarkers, particularly serological ones, that involve noninvasive sampling [22][74].
In this sense, recent studies have analyzed biomarkers, incorporating them into vaccine immunogenicity and protection evaluations. Different biomarkers, such as cytokines patterns, tissue parasitism, leukocytes immunophenotyping or in vitro co-culture systems using T-cells and macrophages infected with L. infantum, have been described in dog model in order to assess the immunogenicity and the protection elicited by vaccines against CanL in clinical trials [75].
Nevertheless, new biomarkers for the confirmation of Leishmania infection and useful to monitor disease progression during the treatment not only in sick animals but also in subclinical infected dogs would be a valuable tool to assist veterinarians in the control of CanL.
Although different published studies have suggested that CIC concentration is related to CanL progression [16][62], until now, this hypothesis had not been proven. Recently, different methods to isolate and quantify CIC in experimentally [18] and naturally infected dogs [14][15] have been described. These studies have established a positive correlation between CIC levels and disease stage, suggesting a potential diagnostic and prognostic value of CIC measurement in CanL. However, these results need a clinical validation for an accurate comparison of in vitro results with clinical characteristics of infected dogs.
Recent advances have been made in biomarkers related to Leishmania pathogenesis in different organs and tissues. However, all of them involve invasive sampling and most cannot be used in a laboratory setting [22]. For this reason, a noninvasive method to isolate CIC and quantify their levels in serum samples [14][15] would have several advantages, being a valuable tool to monitor disease progression (see Table 1).
Table 1. Advantages of CIC analysis in CanL.
| Advantages of CIC Analysis in CanL |
|---|
| ✓ Noninvasive sampling |
| ✓ High CIC levels in the presence of compatible clinical signs and/or clinicopathological abnormalities are conclusive of clinical leishmaniasis |
| ✓ Biomarker associated to pathology |
| ✓ Prognostic value |
| ✓ Useful tool to monitor the treatment and vaccine efficacy |
Furthermore, the measurement of CIC as biomarkers for disease progression may provide interesting information regarding the ability of vaccines or immunotherapeutic treatments to control the disease. This is a relevant point, bearing in mind that vaccination is recognized as one of the most appropriate tools for prevention and remains the most promising approach to reduce the number of CanL cases and, therefore, the incidence of leishmaniasis in humans [8][76][77].
CIC may become relevant biomarkers in prophylaxis, considering that previous studies have postulated that immunization with internal parasite antigens [78][79][80][81][82], would stimulate a powerful immune response capable of reducing the formation of CIC. This would be in agreement with the hypothesis proposed by Chang et al., according to whom, in addition to surface and secretory products of the parasite, the origin of Leishmania virulence may also involve conserved intracellular proteins, referred to as “pathoantigens” [83]. In this context, it is most likely that immunization with internal antigens would elicit a strong and quick immune response, generating specific antibodies that clear parasite intracellular antigens and, therefore, significantly reducing the amount of CIC bound to pathology.
Although a clinical validation of CIC measurement is needed to correlate in vitro observations with clinical severity, these results suggest that CIC monitoring could represent an important tool in the management and prevention of CanL, not only because of their potential as diagnostic and prognostic biomarkers, but also because it may improve the development of vaccines or strategies for immunotherapy.
References
- Adamama-Moraitou, K.K.; Rallis, T.S.; Koytinas, A.F.; Tontis, D.; Plevraki, K.; Kritsepi, M. Asymptomatic colitis in naturally infected dogs with Leishmania infantum: A prospective study. Am. J. Trop. Med. Hyg. 2007, 76, 53–57.
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446.
- Dantas-Torres, F.; Solano-Gallego, L.; Baneth, G.; Ribeiro, V.M.; de Paiva-Cavalcanti, M.; Otranto, D. Canine leishmaniosis in the Old and New Worlds: Unveiled similarities and differences. Trends Parasitol. 2012, 28, 531–538.
- Vilas, V.J.; Maia-Elkhoury, A.N.; Yadon, Z.E.; Cosivi, O.; Sanchez-Vazquez, M.J. Visceral leishmaniasis: A One Health approach. Vet. Rec. 2014, 175, 42–44.
- Day, M.J. The immunopathology of canine vector-borne diseases. Parasit Vectors 2011, 4, 48.
- Toepp, A.J.; Petersen, C.A. The balancing act: Immunology of leishmaniosis. Res. Vet. Sci. 2020, 130, 19–25.
- Abbehusen, M.M.C.; Almeida, V.D.A.; Solca, M.D.S.; Pereira, L.D.S.; Costa, D.J.; Gil-Santana, L.; Bozza, P.T.; Fraga, D.B.M.; Veras, P.S.T.; Dos-Santos, W.L.C.; et al. Clinical and immunopathological findings during long term follow-up in Leishmania infantum experimentally infected dogs. Sci. Rep. 2017, 7, 15914.
- Alvar, J.; Canavate, C.; Molina, R.; Moreno, J.; Nieto, J. Canine leishmaniasis. Adv. Parasitol. 2004, 57, 1–88.
- Guarga, J.L.; Moreno, J.; Lucientes, J.; Gracia, M.J.; Peribanez, M.A.; Alvar, J.; Castillo, J.A. Canine leishmaniasis transmission: Higher infectivity amongst naturally infected dogs to sand flies is associated with lower proportions of T helper cells. Res. Vet. Sci. 2000, 69, 249–253.
- Pinelli, E.; Killick-Kendrick, R.; Wagenaar, J.; Bernadina, W.; del Real, G.; Ruitenberg, J. Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infect. Immun. 1994, 62, 229–235.
- Quinnell, R.J.; Courtenay, O.; Shaw, M.A.; Day, M.J.; Garcez, L.M.; Dye, C.; Kaye, P.M. Tissue cytokine responses in canine visceral leishmaniasis. J. Infect. Dis. 2001, 183, 1421–1424.
- Roura, X.; Cortadellas, O.; Day, M.J.; Benali, S.L.; Zatelli, A. Canine Leishmaniosis Working Group. Canine leishmaniosis and kidney disease: Q&A for an overall management in clinical practice. J. Small Anim. Pract. 2020.
- Santos-Gomes, G.M.; Rosa, R.; Leandro, C.; Cortes, S.; Romao, P.; Silveira, H. Cytokine expression during the outcome of canine experimental infection by Leishmania infantum. Vet. Immunol. Immunopathol. 2002, 88, 21–30.
- Gizzarelli, M.; Fiorentino, E.; Ben Fayala, N.E.H.; Montagnaro, S.; Torras, R.; Gradoni, L.; Oliva, G.; Foglia Manzillo, V. Assessment of circulating immune complexes during natural and experimental canine leishmaniasis. Front. Vet. Sci. 2020, 7, 273.
- Parody, N.; Cacheiro-Llaguno, C.; Osuna, C.; Renshaw-Calderon, A.; Alonso, C.; Carnes, J. Circulating immune complexes levels correlate with the progression of canine leishmaniosis in naturally infected dogs. Vet. Parasitol. 2019, 274, 108921.
- Miles, S.A.; Conrad, S.M.; Alves, R.G.; Jeronimo, S.M.; Mosser, D.M. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J. Exp. Med. 2005, 201, 747–754.
- Wang, X.Y.; Wang, B.; Wen, Y.M. From therapeutic antibodies to immune complex vaccines. NPJ. Vaccines 2019, 4, 2.
- Cacheiro-Llaguno, C.; Parody, N.; Renshaw-Calderon, A.; Osuna, C.; Alonso, C.; Carnes, J. Vaccination with LetiFend® reduces circulating immune complexes in dogs experimentally infected with L. infantum. Vaccine 2020, 38, 890–896.
- Roura, X.; Fondati, A.; Lubas, G.; Gradoni, L.; Maroli, M.; Oliva, G.; Paltrinieri, S.; Zatelli, A.; Zini, E. Prognosis and monitoring of leishmaniasis in dogs: A working group report. Vet. J. 2013, 198, 43–47.
- Solano-Gallego, L.; Miro, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors 2011, 4, 86.
- Solano-Gallego, L.; Koutinas, A.; Miró, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 2009, 165, 1–18.
- Maia, C.; Campino, L. Methods for diagnosis of canine leishmaniasis and immune response to infection. Vet. Parasitol. 2008, 158, 274–287.
- Srivastava, P.; Dayama, A.; Mehrotra, S.; Sundar, S. Diagnosis of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 1–6.
- Nydegger, U.E. Immune complex pathophysiology. Ann. N. Y. Acad. Sci. 2007, 1109, 66–83.
- Brachelente, C.; Muller, N.; Doherr, M.G.; Sattler, U.; Welle, M. Cutaneous leishmaniasis in naturally infected dogs is associated with a T helper-2-biased immune response. Vet. Pathol. 2005, 42, 166–175.
- Chamizo, C.; Moreno, J.; Alvar, J. Semi-quantitative analysis of cytokine expression in asymptomatic canine leishmaniasis. Vet. Immunol. Immunopathol. 2005, 103, 67–75.
- Costa, F.A.; Goto, H.; Saldanha, L.C.; Silva, S.M.; Sinhorini, I.L.; Silva, T.C.; Guerra, J.L. Histopathologic patterns of nephropathy in naturally acquired canine visceral leishmaniasis. Vet. Pathol. 2003, 40, 677–684.
- Ordeix, L.; Solano-Gallego, L.; Fondevila, D.; Ferrer, L.; Fondati, A. Papular dermatitis due to Leishmania spp. infection in dogs with parasite-specific cellular immune responses. Vet. Dermatol. 2005, 16, 187–191.
- Poli, A.; Abramo, F.; Mancianti, F.; Nigro, M.; Pieri, S.; Bionda, A. Renal involvement in canine leishmaniasis. A light-microscopic, immunohistochemical and electron-microscopic study. Nephron 1991, 57, 444–452.
- Koutinas, A.F.; Koutinas, C.K. Pathologic mechanisms underlying the clinical findings in canine leishmaniasis due to Leishmania infantum/chagasi. Vet. Pathol. 2014, 51, 527–538.
- Verroust, P. The search for circulating immune complex (IC) (author’s transl). Ann. Biol. Clin. (Paris) 1980, 38, 333–343.
- Rossi, M.; Fasel, N. How to master the host immune system? Leishmania parasites have the solutions! Int. Immunol. 2018, 30, 103–111.
- Senbagavalli, P.; Hilda, J.N.; Ramanathan, V.D.; Kumaraswami, V.; Nutman, T.B.; Babu, S. Immune complexes isolated from patients with pulmonary tuberculosis modulate the activation and function of normal granulocytes. Clin. Vaccine Immunol. 2012, 19, 1965–1971.
- AL-Fakhar, S.A. Circulating immune complexes in relation to polymorphonuclear leucocytes in patients infected with toxoplasmosis. Biomed. Pharmacol. J. 2017, 10.
- Abrass, C.K.; Nies, K.M.; Louie, J.S.; Border, W.A.; Glassock, R.J. Correlation and predictive accuracy of circulating immune complexes with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 1980, 23, 273–282.
- Bernstein, K.A.; Kahl, L.E.; Balow, J.E.; Lefkowith, J.B. Serologic markers of lupus nephritis in patients: Use of a tissue-based ELISA and evidence for immunopathogenic heterogeneity. Clin. Exp. Immunol. 1994, 98, 60–65.
- Nydegger, U.E.; Davis, J.S.t. Soluble immune complexes in human disease. CRC Crit. Rev. Clin. Lab. Sci. 1980, 12, 123–170.
- Weissmann, G. Rheumatoid arthritis and systemic lupus erythematosus as immune complex diseases. Bull. NYU Hosp. Jt. Dis. 2009, 67, 251–253.
- Paganelli, R.; Levinsky, R.J.; Atherton, D.J. Detection of specific antigen within circulating immune complexes: Validation of the assay and its application to food antigen-antibody complexes formed in healthy and food-allergic subjects. Clin. Exp. Immunol. 1981, 46, 44–53.
- Park, H.S.; Nahm, D.H. Role of circulating immune complex in aspirin-sensitive asthma. Korean J. Intern. Med. 1998, 13, 51–55.
- Parveen, S.; Taneja, N.; Bathi, R.J.; Deka, A.C. Evaluation of circulating immune complexes and serum immunoglobulins in oral cancer patients—A follow up study. Indian J. Dent. Res. 2010, 21, 10–15.
- Urbaniak-Kujda, D. Circulating immune complexes as markers of Hodgkin’s disease activity. Pol. Tyg. Lek. 1996, 51, 303–304.
- Koraka, P.; Burghoorn-Maas, C.P.; Falconar, A.; Setiati, T.E.; Djamiatun, K.; Groen, J.; Osterhaus, A.D. Detection of immune-complex-dissociated nonstructural-1 antigen in patients with acute dengue virus infections. J. Clin. Microbiol. 2003, 41, 4154–4159.
- Miles, S.A.; Balden, E.; Magpantay, L.; Wei, L.; Leiblein, A.; Hofheinz, D.; Toedter, G.; Stiehm, E.R.; Bryson, Y. Rapid serologic testing with immune-complex-dissociated HIV p24 antigen for early detection of HIV infection in neonates. Southern California Pediatric AIDS Consortium. N. Engl. J. Med. 1993, 328, 297–302.
- Sengupta, K.; Ghosh, P.K.; Ganguly, S.; Das, P.; Maitra, T.K.; Jalan, K.N. Characterization of entamoeba histolytica antigens in circulating immune complexes in sera of patients with amoebiasis. J. Health Popul. Nutr. 2002, 20, 215–222.
- Soares, M.J.V.; Moraes, J.R.E.; Moraes, F.R. Renal involvement in canine leishmaniasis: A morphological and immunohistochemical study. Arq. Bras. Med. Vet. Zootec. 2009, 61, 785–790.
- DE Brito, T.; Hoshino-Shimizu, S.; Neto, V.A.; Duarte, I.S.; Penna, D.O. Glomerular involvement in human kala-azar. A light, immunofluorescent, and electron microscopic study based on kidney biopsies. Am. J. Trop. Med. Hyg. 1975, 24, 9–18.
- Harrus, S.; Day, M.J.; Waner, T.; Bark, H. Presence of immune-complexes, and absence of antinuclear antibodies, in sera of dogs naturally and experimentally infected with Ehrlichia canis. Vet. Microbiol. 2001, 83, 343–349.
- Matsumura, K.; Kazuta, Y.; Endo, R.; Tanaka, K.; Inoue, T. Detection of circulating immune complexes in the sera of dogs infected with Dirofilaria immitis, by Clq-binding enzyme-linked immunosorbent assay. J. Helminthol. 1986, 60, 239–243.
- Elshafie, A.I.; Ahlin, E.; Mathsson, L.; ElGhazali, G.; Ronnelid, J. Circulating immune complexes (IC) and IC-induced levels of GM-CSF are increased in sudanese patients with acute visceral Leishmania donovani infection undergoing sodium stibogluconate treatment: Implications for disease pathogenesis. J. Immunol. 2007, 178, 5383–5389.
- Requena, J.M.; Soto, M.; Doria, M.D.; Alonso, C. Immune and clinical parameters associated with Leishmania infantum infection in the golden hamster model. Vet. Immunol. Immunopathol. 2000, 76, 269–281.
- Al-Fakhar, S.A.; Ali, W.M.; Obaid, K.Y.; Mohammed, K.I.A.; Ali, S.H.M.; Mousa, J.M. Association of circulating immune complexes in the development of visceral leishmaniasis. Res. J. Pharm. Tech. 2020, 13, 3284–3288.
- Clementi, A.; Battaglia, G.; Floris, M.; Castellino, P.; Ronco, C.; Cruz, D.N. Renal involvement in leishmaniasis: A review of the literature. NDT Plus 2011, 4, 147–152.
- Lopez, R.; Lucena, R.; Novales, M.; Ginel, P.J.; Martin, E.; Molleda, J.M. Circulating immune complexes and renal function in canine leishmaniasis. Zentralbl. Veterinarmed. B 1996, 43, 469–474.
- Plevraki, K.; Koutinas, A.F.; Kaldrymidou, H.; Roumpies, N.; Papazoglou, L.G.; Saridomichelakis, M.N.; Savvas, I.; Leondides, L. Effects of allopurinol treatment on the progression of chronic nephritis in canine leishmaniosis (Leishmania infantum). J. Vet. Intern. Med. 2006, 20, 228–233.
- Datta, S.; Modak, D.; Sarkar, S.; Saha, B.; Mukhopadhyay, S. Identification and glycobiological characterization of circulating immune complexes in patients with visceral leishmaniasis and post kala azar dermal leishmaniasis. Indian J. Exp. Biol. 2015, 53, 321–328.
- Lux, A.; Yu, X.; Scanlan, C.N.; Nimmerjahn, F. Impact of immune complex size and glycosylation on IgG binding to human FcgammaRs. J. Immunol. 2013, 190, 4315–4323.
- Grauer, G.F. Glomerulonephritis. Semin. Vet. Med. Surg. Small Anim. 1992, 7, 187–197.
- Brandonisio, O.; Carelli, G.; Altamura, M.; Varvara, B.; Ceci, L. Circulating immune complexes and autoantibodies in canine leishmaniasis. Parassitologia 1990, 32, 275–281.
- dos Santos, J.P.; Alves, L.C.; Ramos, R.A.; Pimentel Dde, S.; de Carvalho, G.A.; Monteiro, M.F.; Faustino, M.A. Histological changes and immunolabeling of Leishmania infantum in kidneys and urinary bladder of dogs. Rev. Bras. Parasitol. Vet. 2013, 22, 420–423.
- Marcussen, N.; Vetner, M.; Kristensen, H.M. Interstitial nephritis and glomerulonephritis in visceral leishmaniasis in a dog. A case report. APMIS 1989, 97, 1137–1140.
- Margarito, J.M.; Lucena, R.; Lopez, R.; Molleda, J.M.; Martin, E.; Ginel, P.J. Levels of IgM and IgA circulating immune complexes in dogs with leishmaniasis. Zentralbl. Veterinarmed. B 1998, 45, 263–267.
- Nieto, C.G.; Navarrete, I.; Habela, M.A.; Serrano, F.; Redondo, E. Pathological changes in kidneys of dogs with natural Leishmania infection. Vet. Parasitol. 1992, 45, 33–47.
- Solano-Gallego, L.; Rodriguez, A.; Iniesta, L.; Arboix, M.; Portus, M.; Alberola, J. Detection of anti-Leishmania immunoglobulin G antibodies in urine specimens of dogs with leishmaniasis. Clin. Diagn. Lab. Immunol. 2003, 10, 849–855.
- Tafuri, W.L.; de Oliveira, M.R.; Melo, M.N. Canine visceral leishmaniosis: A remarkable histopathological picture of one case reported from Brazil. Vet. Parasitol. 2001, 96, 203–212.
- Zatelli, A.; Borgarelli, M.; Santilli, R.; Bonfanti, U.; Nigrisoli, E.; Zanatta, R.; Tarducci, A.; Guarraci, A. Glomerular lesions in dogs infected with Leishmania organisms. Am. J. Vet. Res. 2003, 64, 558–561.
- Aresu, L.; Valenza, F.; Ferroglio, E.; Pregel, P.; Uslenghi, F.; Tarducci, A.; Zanatta, R. Membranoproliferative glomerulonephritis type III in a simultaneous infection of Leishmania infantum and Dirofilaria immitis in a dog. J. Vet. Diagn. Investig. 2007, 19, 569–572.
- Costa, F.A.; Guerra, J.L.; Silva, S.M.; Klein, R.P.; Mendonca, I.L.; Goto, H. CD4(+) T cells participate in the nephropathy of canine visceral leishmaniasis. Braz. J. Med. Biol. Res. 2000, 33, 1455–1458.
- Esch, K.J.; Schaut, R.G.; Lamb, I.M.; Clay, G.; Morais Lima, A.L.; do Nascimento, P.R.; Whitley, E.M.; Jeronimo, S.M.; Sutterwala, F.S.; Haynes, J.S.; et al. Activation of autophagy and nucleotide-binding domain leucine-rich repeat-containing-like receptor family, pyrin domain-containing 3 inflammasome during Leishmania infantum-associated glomerulonephritis. Am. J. Pathol. 2015, 185, 2105–2117.
- Paltrinieri, S.; Solano-Gallego, L.; Fondati, A.; Lubas, G.; Gradoni, L.; Castagnaro, M.; Crotti, A.; Maroli, M.; Oliva, G.; Roura, X.; et al. Guidelines for diagnosis and clinical classification of leishmaniasis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1184–1191.
- Pierantozzi, M.; Roura, X.; Paltrinieri, S.; Poggi, M.; Zatelli, A. Variation of proteinuria in dogs with leishmaniasis treated with meglumine antimoniate and allopurinol: A retrospective study. J. Am. Anim. Hosp. Assoc. 2013, 49, 231–236.
- Proverbio, D.; Spada, E.; Bagnagatti de Giorgi, G.; Perego, R.; Valena, E. Relationship between Leishmania IFAT titer and clinicopathological manifestations (clinical score) in dogs. BioMed Res. Int. 2014, 2014, 412808.
- Pennisi, M.G. Leishmaniosis of companion animals in Europe: An update. Vet. Parasitol. 2015, 208, 35–47.
- Hernández, L.; Montoya, A.; Checa, R.; Dado, D.; Gálvez, R.; Otranto, D.; Latrofa, M.S.; Baneth, G.; Miró, G. Course of experimental infection of canine leishmaniosis: Follow-up and utility of noninvasive diagnostic techniques. Vet. Parasitol. 2015, 207, 149–155.
- Giunchetti, R.C.; Silveira, P.; Resende, L.A.; Leite, J.C.; Melo-Junior, O.A.O.; Rodrigues-Alves, M.L.; Costa, L.M.; Lair, D.F.; Chaves, V.R.; Soares, I.D.S.; et al. Canine visceral leishmaniasis biomarkers and their employment in vaccines. Vet. Parasitol. 2019, 271, 87–97.
- Muller, K.E.; Solberg, C.T.; Aoki, J.I.; Floeter-Winter, L.M.; Nerland, A.H. Developing a vaccine for leishmaniasis: How biology shapes policy. Tidsskr. Nor Laegeforen. 2018, 137.
- Palatnik-de-Sousa, C.B.; Day, M.J. One Health: The global challenge of epidemic and endemic leishmaniasis. Parasit Vectors 2011, 4, 197.
- Baharia, R.K.; Tandon, R.; Sahasrabuddhe, A.A.; Sundar, S.; Dube, A. Nucleosomal histone proteins of L. donovani: A combination of recombinant H2A, H2B, H3 and H4 proteins were highly immunogenic and offered optimum prophylactic efficacy against Leishmania challenge in hamsters. PLoS ONE 2014, 9, e97911.
- Soto, M.; Requena, J.M.; Quijada, L.; Perez, M.J.; Nieto, C.G.; Guzman, F.; Patarroyo, M.E.; Alonso, C. Antigenicity of the Leishmania infantum histones H2B and H4 during canine viscerocutaneous leishmaniasis. Clin. Exp. Immunol. 1999, 115, 342–349.
- Iborra, S.; Soto, M.; Carrion, J.; Alonso, C.; Requena, J.M. Vaccination with a plasmid DNA cocktail encoding the nucleosomal histones of Leishmania confers protection against murine cutaneous leishmaniosis. Vaccine 2004, 22, 3865–3876.
- Soto, M.; Requena, J.M.; Quijada, L.; Garcia, M.; Guzman, F.; Patarroyo, M.E.; Alonso, C. Mapping of the linear antigenic determinants from the Leishmania infantum histone H2A recognized by sera from dogs with leishmaniasis. Immunol. Lett. 1995, 48, 209–214.
- Iborra, S.; Soto, M.; Carrion, J.; Nieto, A.; Fernandez, E.; Alonso, C.; Requena, J.M. The Leishmania infantum acidic ribosomal protein P0 administered as a DNA vaccine confers protective immunity to Leishmania major infection in BALB/c mice. Infect. Immun. 2003, 71, 6562–6572.
- Chang, K.P.; Reed, S.G.; McGwire, B.S.; Soong, L. Leishmania model for microbial virulence: The relevance of parasite multiplication and pathoantigenicity. Acta Trop. 2003, 85, 375–390.




