
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mauricio Maldonado | + 1077 word(s) | 1077 | 2021-02-26 04:17:09 | | | |
| 2 | Camila Xu | Meta information modification | 1077 | 2021-04-13 12:51:43 | | |
Video Upload Options
The Benzodiazepines (BDZs) are a group of components that receive their name because in their chemical structure have a benzene ring joined to other seven-members heterocyclic ring called Diazepine.
1. Benzodiazepines
The Benzodiazepines (BDZs) are a group of components that receive their name because in their chemical structure have a benzene ring joined to other seven-members heterocyclic ring called Diazepine (Figure 1).
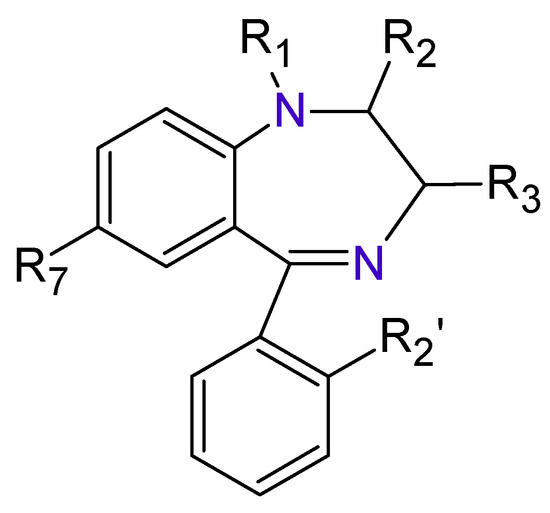
Figure 1. Benzodiazepines basic structure.
There is a wide range of medicines that belong to these types of substances. The type of BDZ depends on the component present in the basic nucleus of the compound. As an example of it, Figure 2 shows several BDZ structures, in which the termination, lam, pam, etc., indicate which is its active principle.
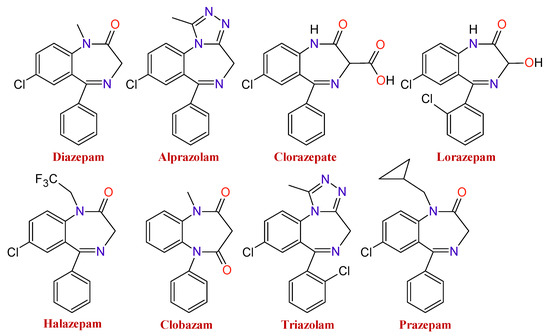
Figure 2. Chemical structures of some benzodiazepines.
2. Synthesis of Benzodiazepines
Within the BDZs group, several families of these compounds can be found differing in the position and presence of functional groups. Some of the great importance are 1,4-benzodiazepines, 1,5-benzodiazepines, 1,4-benzodiazepines-2-ones, and 1,4-benzodiazepines-3-ones. The synthesis of 1,5-benzodiazepines includes the reactions of phenylendiamines with ketones in the presence of catalysts [1][2][3][4] by condensation with α, β-unsaturated carbonyl compounds [5]. The reactions can also be carried out under solvent-free conditions [6][7] or by means of procedures under microwave conditions [8][9].
Methods for preparing 1,4-benzodiazepine derivatives include an aromatic electrophilic substitution from N-substituted anilines [10]. It is also possible via a condensation reaction between o-substituted benzylamines with activated aziridines by ring-opening reactions [11][12][13], among others. 1,4-Benzodiazepin-3-ones are synthesized by the condensation of 2-bromobenzylamines with amino acids [14], as well as by intramolecular nucleophylic aromatic substitution [15]; other alternate methods include cyclization process by Ugi reaction [16][17]. The synthesis of 1,4-benzodiazepin-2-ones includes the previous synthesis of the intermediate amino-ketone [18]. Moreover, cyclization processes involving an Ugi reaction as a first step [19][20]. Finally, other methods include the cascade coupling/ condensation process of 2-bromobenzylamines with amino acids [21] and Buchwald reaction [22] (Figure 3).
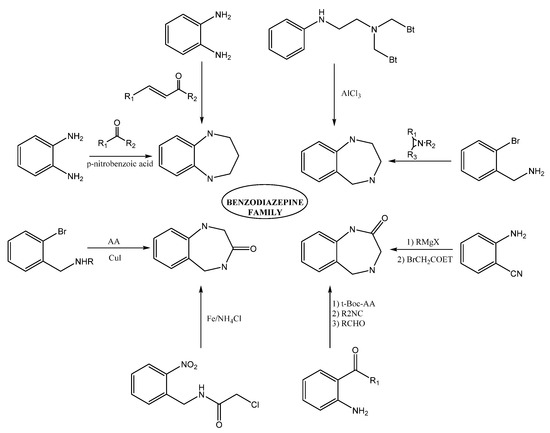
Figure 3. Some synthesis routes toward benzodiazepines.
3. Benzodiazepines Action Mechanism on the Central Nervous System
Due to its high solubility in lipids, BDZ can penetrate the brain and cause rapid and effective action through interference with the nerve impulse transmission mechanism [23]. In order to understand this, it is necessary to keep in mind that the main inhibitory neurotransmitter of the central nervous system is the γ-aminobutyric acid (GABA), which has a reception place in the neurons acquiesced by a heteropentameric glycoprotein. This protein is coupled in the central nervous system, more abundant in ionic channels through which chloride ions selectively transit, they also have a site for the reception of the BDZ, known as Benzodiazepine Union Place. The BDZ bind to the binding site and modify the three-dimensional disposition of the receptor (allosteric modulation) [24][25], favoring the opening of the chloride channel by the action of GABA (Figure 4). The former process is the potentiation of the inhibitory effect of the GABA neurotransmitter so that the neuron becomes less excitable and begins to be in a neuronal inhibition state. In addition, the permanent activation of GABAA receptors causes adaptative changes that result in tolerance [26].
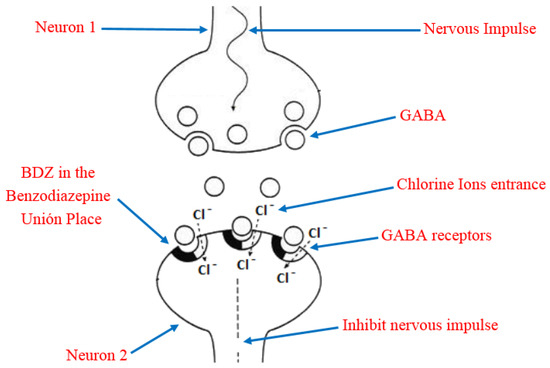
Figure 4. Representation of the benzodiazepines (BDZ) action mechanism and their role in the nervous impulse inhibition.
The GABA neurotransmitter action is decisive in the central nervous system because approximately 40% of neurons respond to its effects, so the reassuring effect is significant. The inconvenience with altering this process is that by consuming BDZ neurons get overloaded and they do not respond in the same way to the exciters neurotransmitters, and even reduces noradrenaline, serotonin, acetylcholine, and dopamine which are important in processes of wakefulness, alert, memory, coordination, in the production of glandular secretions, the cardiac rhythm control and blood pressure, among others, which in fact leads to a general sedative-hypnotic effect [27]. To avoid these inconveniences, for several decades molecules capable of being used as anxiolytics have been developed as an alternative to benzodiazepines such as bretazeni, imidazenil [28], alpidem, and ocinaplon and although in the last two cases, these were toxic to the liver, they showed that it is possible to develop anxiolytics based on GABAA receptors [29].
4. Pharmacological Differences between the Different Types of Benzodiazepines
Differences in action between the different types of BDZs in the brain are associated with several sub-units (α1, α2, α3, α5) present at the Benzodiazepine Union Place of the heteropentameric glycoprotein at the γ-aminobutyric acid reception site [30][31]. Although it is not yet completely elucidated, it is believed that each sub-unit regulates different actions. For example, Rosas-Gutierrez et al. in 2012 indicated that the sub-unit α1 is the most abundant and regulates the anti-convulsant, hypnotic, and sedative actions in the body, the α2 sub-unit regulates the anxiolytic effects, and the sub-units α3 and α5 are related to the relaxing muscle effect. These authors also indicate that, to a greater or lesser degree, each BDZ joins to an α sub-unit. Thus, classic BDZs like Diazepam get joined to the α1, α2, α3, α5 sub-units, while other medicines such Zoldipem has a specific affinity for the α1 sub-unit and barely any affinity for the α5 sub-unit [25]. On the other hand, Engin et al. indicate that positive allosteric modulators of α5 sub-units might be useful to attenuate interference-related cognitive symptoms and hippocampal hyperactivity in some psychiatric disorders [31]. While Zhiqiang indicates a possible role of α3 in the abuse potential and sedative effects of benzodiazepine-type drugs according to studies carried out in rhesus monkeys [32].
Regarding the pharmacological sphere, the BDZ action can vary slightly depending on its nature [33] and its selectivity with respect to the receptors. These differences are mainly manifested in the hypnotic/anxiolytic sedative effect, in the time of life, in the administered doses [34], in the elimination velocity [27], in the hepatic metabolism [35], in the number of doses (single or multiple), in the drug liposolubility, in the administration mechanism (oral, intramuscular), in the potency, in the action onset (fast <1 h, medium 1–2 h, slow >2 h) [34], and in the accumulation and myorelaxing effects, among others [36]. As an example of this, we can cite BDZs with different half-life times; among them, it is possible to find Bentazepam, Midazolam, and Loprazolam, with a half-life time of less than six hours; Alprazolam, Bromazepam, Nitrazepam, with a half-life time between 6 and 24 h; and Diazepam, Ketazolam, and Flurazepam, with a half-life time greater than 24 h [37].
5. Degradation of Benzodiazepines in the Organism
BDZs are metabolized in the liver with the intervention of microsomal enzyme systems. The first stage of the process (Figure 5) is the hydrolysis of the Diazepine ring at positions 2 or 5 in order to obtain the intermediates 1 and 2; afterward, a hydrolysis process takes place to produce 2-aminobenzofenona and glycine, which are excreted through the urine.
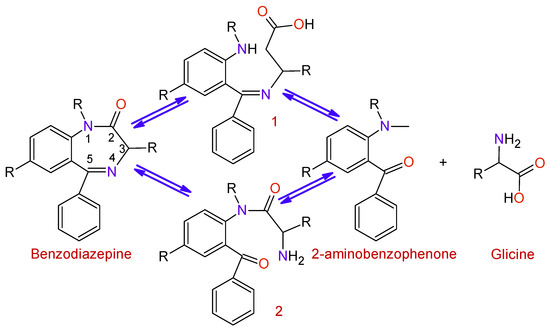
Figure 5. Degradation of benzodiazepines into 2-aminobenzophenone and glycine.
References
- De, S.K.; Gibbs, R.A. Scandium (III) triflate as an efficient and reusable catalyst for synthesis of 1,5-benzodiazepinevderivatives. Tetrahedron Lett. 2005, 46, 1811–1813.
- Jarikote, D.V.; Siddiqui, S.A.; Rajagopal, R.; Daniel, T.; Lahoti, R.J.; Srinivasan, K.V. Roomtemperature ionic liquid promoted synthesis of 1,5-benzodiazepine derivatives under ambient conditions. Tetrahedron Lett. 2003, 44, 1835–1838.
- Jung, D.I.; Choi, T.W.; Kim, Y.Y.; Kim, I.S.; Park, Y.M.; Lee, Y.G.; Jung, D.H. Synthesis of 1,5-benzodiazepine derivatives. Synth. Commun. 1999, 29, 1941–1951.
- Pasha, M.A.; Jayashankara, V.P. Synthesis of 1,5-benzodiazepine derivatives catalyzed by zinc chloride. Heterocycles 2006, 68, 1017–1023.
- Claramunt, R.M.; Sanz, D.; Aggarwal, S.; Kumar, A.; Prakash, O.; Singh, S.P.; Elgueroc, J. The reaction of o-phenylenediamine with α,β-unsaturated carbonyl compounds. Arkivoc 2006, 2006, 35–45.
- An, L.-T.; Ding, F.-Q.; Zou, J.-P.; Lu, X.-H. Montmorillonite K10: An efficient catalyst forsolvent-free synthesis of 1,5-benzodiazepine derivatives. Synth. Commun. 2008, 38, 1259–1267.
- Yadav, J.S.; Reddy, B.V.S.; Kumar, S.P.; Nagaiah, K. Indium(III) bromide: A novel and efficient reagent for the rapid synthesis of 1,5-benzodiazepines under solvent-free conditions. Synthesis 2005, 36, 480–484.
- Kaboudin, B.; Navaee, K. Alumina/phosphorus pentoxide (APP) as an efficient reagent for the synthesis of 1,5-benzodiazepines under microwave irradiation. Heterocycles 2001, 55, 1443–1446.
- Pozarentzi, M.; Stephanatou, J.S.; Tsoleridis, C.A. An efficient method for the synthesis of 1,5-benzodiazepine derivatives under microwave irradiation wihout solvent. Tetrahedron Lett. 2002, 43, 1755–1758.
- Katritzky, A.R.; Xu, Y.-J.; He, H.-Y. Convenient Synthesis of 2,3,4,5-tetrahydro-1,4-benzodiazepines, 1,4-beozoxazepines and 1,4-benzotiazepines. J. Chem. Soc. Perkin Trans. 1 2002, 2, 592–598.
- Ghorai, M.K.; Shahi, C.K.; Bhattacharyya, A.; Sayyad, M.; Mal, A.; Wani, I.A.; Chauhan, N. Synthesis of tetrahydrobenzodiazepines via ring-opening of activated aziridines with 2-bromoaniline Followed by Copper-Powder-Mediated C-N bond Formation. Asian J. Org. Chem. 2015, 4, 1103–1111.
- Pop, T.A.; Uhl, E.; Ong, D.N.; Dittrich, S.; Bracher, F. A new appoach to monoprotected 1,4-benzodiazepines via a one pot N-deprotection/Reductive cyclization Porcedure. Tetrahedron 2016, 72, 1668–1674.
- Shahi, C.K.; Bhattacharyya, A.; Nanaji, Y.; Ghorai, M.K. A stereoselective Route to tetrahydrobenzoxazepines and tetrahydrobenzodiazepines via ring-opening and Aza Michael addition of activated aziridines with 2-hydroxyphenyl and 2-aminophenyl acrylates. J. Org. Chem. 2017, 82, 37–47.
- Wang, H.; Jiang, Y.; Gao, K.; Ma, D. Facile Synthesis of 1,4-benzodiazepin-3-ones from o-bromobenzylamines and amino acids via cascade coupling/condensation process. Tetrahedron 2009, 65, 8956–8960.
- Clement, E.C.; Carlier, P.R. A simple route to tetrahydro-1,4-benzodiazepin-3-ones bearing diverse N1, N4, and C10 functionalization. Tetrahedron Lett. 2005, 46, 3633–3636.
- Dawod, S.V.; Gerber, S.N.; Liang, X.; Biron, E. Convenient two-step synthesis of highly functionalized venzo-fused 1,4-diazepin-3-ones and 1,5-diazocin-4-ones by sequential Ugi and intramolecular SNAr reactions. Tetrahedron 2017, 73, 6347–6355.
- de Silva, R.A.; Santra, S.; Andreana, P.R. A tándem one-pot microwave-assisted synthesis of regiochemically differentieted 1,2,4,5-tetrahydro-1,4-benzodiazepin-3-ones. Org. Lett. 2008, 10, 4541–4545.
- Dörr, A.A.; Lubell, W.D. γ-Turn Mimicry with Benzodiazepinones and pyrrolobenzodiazepinoes synthesized from a common amino ketone intermediate. Org. Lett. 2015, 17, 3592–3595.
- Mossetti, R.; Saggiorato, G.C. Tron Exploiting the acylating nature of the imide-ugi intermediate: A straightforward synthesis of tetrahydro-1,4-benzodiazepin -2-ones. J. Org. Chem. 2011, 76, 10258–10262.
- Azuaje, J.; Rubio, J.M.P.; Yaziji, V.; Maatougui, A.E.; Gómez, J.C.G.; Pedregal, V.M.S.; Vásquez, A.N.; Masaguer, C.F. Integrated Ugi-Based Assembly of Functionally, Skeletally, and Stereochemically Diverse 1,4-Benzodiazepin-2-ones. J. Org. Chem. 2015, 80, 1533–1549.
- Mishra, J.K.; Panda, G. A Convenient two-step synthesis of amino acid derived chiral 3-substituted [1,4]benzodiazepin-2-ones. Synthesis 2005, 11, 1881–1887.
- Salomé, C.; Schmitt, M.; Bourguignon, J.-J. Novel access to 1,4-benzodiazepin-2-ones via the Buchwald reaction and application to the synthesis of novel heterocyclics. Tetrahedron Lett. 2012, 53, 1033–1035.
- Jacobson, A.F.; Goldstein, B.J.; Dominguez, R.A.; Steinbook, R.M. A placebo-controlled, double-blind comparison of clobazam and diazepam in the treatment of anxiety. J. Clin. Psychiatry 1983, 44, 296–300.
- Cheng, S.Z.; Fei, W.P.; Fang, W.; Xuan, X.Z.; Qiao, D.; Lei, N.T.; Qi, Z.S.; Ling, Z.H.; Hui, L.W.; Jing, L.J.; et al. Gephyrin Palmitoylation in basolateral amygdala mediates the anxiolytic action of benzodiazepine. Biol. Psychiatry 2019, 85, 202–213.
- Cornett, E.M.; Novitch, M.B.; Brunk, A.J.; Davidson, K.; Menard, B.; Urman, R.; Kaye, A.D. New benzodiazepines for sedation. Best Pract. Res. Clin. Anaesthesiol. 2018, 32, 149–164.
- Foitzick, M.F.; Medina, N.B.; Iglesias, L.C.; Gravielle, M.C. Benzodiazepine exposure induces trnscriptional down-regulation of GABAA receptor α1 gene via L-type voltaje-gated calcium channel activation in rat cerebrocortical neurons. Neurosci. Lett. 2020, 721, 134801.
- Ashton, H.C. Benzodiazepine abuse. Drugs Depend. 2002, 197–212.
- Gogas, K.R.; Lechner, S.M.; Markison, S.; Wlliams, J.P.; McCarthy, W.; Grigoriadis, D.E.; Foster, A.C. Anxiety. Compr. Med. Chem. II 2006, 6, 85–115.
- Skolnick, P. Anxioselective anxiolytics: On a quest for the holy grail. Trends Pharmacol. Sci. 2012, 33, 611–620.
- Berro, L.F.; Rowlett, J.K. GABAA receptor subtypes and the reinforcing effects of benzodiazepines in remifentanil-experienced Rhesus monkeys. Drug Alcohol. Depend. 2020, 213, 108076.
- Engin, E.; Benham, R.; Rudolph, U. An emerging circuit pharmacology of GABAA receptors. Trends Pharmacol. Sci. 2018, 39, 710–732.
- Meng, Z.; Berro, L.; Sawyer, E.; Bettschen, D.; Cook, J.R.; Guanguan, P.; Cook, D.; James, M.; Rowlett, J. Evaluation of the anti-conflict, reinforcing, and sedative effects of YT-III-31, a ligand functionally selective for α3 subunit-containing GABAA receptors. J. Psychopharmacol. 2020, 34, 348–357.
- Langer, E.; Einat, H.; Stukalin, Y. Similarities and dissimilarities in the effects of benzodiazepines and specific serotonin reuptake inhibitors (SSRIs) in the defensive marble burying test: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2020, 36, 38–49.
- Lechuga, G.; Indart, C. Selection of benzodiazepines: Bases for its use in the hospital. Farm. Hosp. 1996, 21, 117–122.
- Danza, A.; Cristiani, F.; Tamosiunas, G. Benzodiazepine-related risks. Arch. Med. Intern. 2009, 31, 103–107.
- Holstege, C.P. Benzodiazepines. In Encyclopedia of Toxicology, 2nd ed.; Wexler, P., Ed.; Elsevier Ltd.: Oxford, UK, 2005; Volume 1, pp. 260–262.
- Ashrafi, H.; Mobed, A.; Hasanzadeh, M.; Babaie, P.; Ansarin, K. Monitoring of five benzodiazepines using a novel polymeric interface prepared by layer by layer strategy. Microchem. J. 2019, 146, 121–125.





