
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gabriela Lima | + 1989 word(s) | 1989 | 2021-03-17 05:57:29 | | | |
| 2 | Peter Tang | Meta information modification | 1989 | 2021-04-12 07:12:27 | | |
Video Upload Options
Plasma is an electrically conducting medium that responds to electric and magnetic fields. Most of the visible matter in the universe (about 99%), such as stars, nebulas and interstellar medium, is in the state of plasma. It consists of large quantities of highly reactive species, such as ions, energetic electrons, exited atoms and molecules, ultraviolet photons, and active radicals in different temperatures. Non-thermal or cold plasmas are partially ionized gases whose electron temperatures usually exceed several tens of thousand degrees K, while the ions and neutrals have much lower temperatures. Due to the presence of reactive species at low temperature, the biological effects of non-thermal plasmas have been studied for application in the medical area with promising results.
1. Introduction
Plasma is frequently referred to as the fourth state of the matter and can be described as a gaseous mixture of neutral particles, electrons and ions at different densities and temperatures. Most of the visible matter in the universe (about 99%), such as stars, nebulas and interstellar medium, is in the state of plasma. Plasma can be generated by heating a gas or by subjecting it to strong electromagnetic fields to the point that the gas particles become ionized. Thus, plasma is an electrically conducting medium that responds to electric and magnetic fields, which is also a source of large quantities of highly reactive species, such as ions, energetic electrons, excited atoms and molecules, ultraviolet photons, metastable, and active radicals [1]. In laboratory conditions, plasma is generally produced by an electric discharge in noble or molecular gases, such as argon (Ar), helium (He), oxygen (O2) and nitrogen (N2), using different excitation schemes, such as microwaves, radiofrequency and DC or AC electric fields [2].
Normally, the electron and ion densities in plasmas are approximately equal (a condition called quasi-neutrality), but the respective electron and ion temperatures can be quite different. Plasmas are usually classified as thermal and non-thermal plasmas. Thermal plasmas are in thermal equilibrium, which means that their temperatures are relatively homogenous throughout the heavy particles (i.e., atoms, molecules, and ions) and electrons that usually span in the range of thousands of K [1]. The so-called non-thermal or cold plasmas are partially ionized gases and their electron temperatures exceed several tens of thousands K, while the heavy particles (ions and neutrals) have a much lower temperature [2]. Plasma can be also generated under different pressure conditions, including atmospheric pressure [1]. In the last decade, atmospheric pressure plasmas have become a very attractive tool for material processing applications because they are generated in an open environment and can be easily implemented in online processing. However, working at atmospheric pressure has some disadvantages. For instance, gas breakdown at atmospheric pressure occurs at much higher electric field, typically in the order of ten of kV cm−1 [1]. Additionally, if special precautions are not taken, the atmospheric plasmas have the tendency to become thermal i.e., hot plasmas that can damage heat sensitive materials or burn living tissues [2].
A gas discharge in which a dielectric layer covers one or both electrodes is called Dielectric Barrier Discharge (DBD) and it was first introduced by Siemens—in 1857—for the production of ozone. Large number of DBD systems has been reported [3] with the planar and cylindrical geometries as the most common employed configurations. Figure 1a depicts a typical planar DBD reactor, while Figure 1b shows the so-called floating electrode DBD (FE-DBD) [4][5]. The plasma, in this case, is formed between an insulated high voltage electrode and a target (human skin or living tissue), which acts as a floating counter electrode.
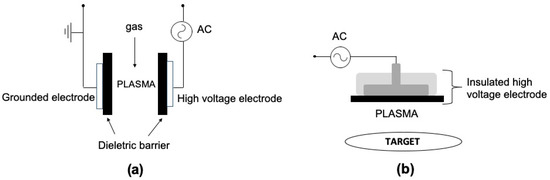
Figure 1. Schematic representation of DBD reactors in planar geometry: (a) conventional two electrodes DBD and (b) floating electrode DBD (FE-DBD). Adapted from [5].
Due to the current limitation caused by the charge accumulation on the dielectric surface, the gas temperature in DBD can be quite low (about the room temperature), which makes it adequate for biological applications [4]. However, the plasma in DBD devices is confined into small gaps between two electrodes (usually in the order of few mm), which is a disadvantage for some applications. For instance, FE-DBD was successfully used in a number of clinical trials for the treatment of skin diseases, to control melanoma development, blood coagulation and antisepsis of open wounds [5]. However, it is not suitable for plasma application inside the human body’s cavities, such as tooth root canals or internal organs.
On the other hand, in the so-called atmospheric pressure plasma jets (APPJs) the plasma generated into a dielectric enclosure (tube or syringe) is expelled through a small orifice into the ambient atmosphere by gas flow (usually a noble gas). The ejected plasma forms a plasma plume that extended several cm into the air and can be easily directed to a target. Figure 2 shows a drawing of the APPJ concept. In the past decade many APPJ configurations, different electrodes arrangements and excitation schemes have been reported in the literature. More details about APPJs and their characteristics can be found in some recent review papers [6][7][8].
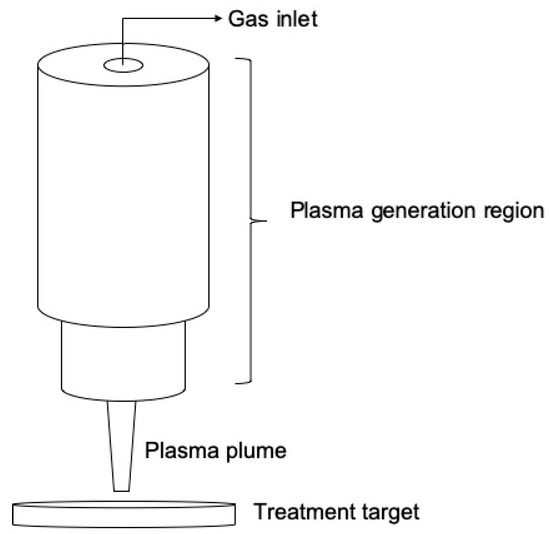
Figure 2. Schematic representation of an APPJ.
Depending on the jet’s operating conditions, the plasma plume tip can be maintained below 40 °C, enabling the contact with living tissues without any risk of burns and electric shock. Thus, cold atmospheric pressure plasma (CAPP), such as FE-DBD and APPJ, have been appointed as the most promising tools for biomedical and hospital applications [9][10][11][12].
Since CAPPs are generated in ambient air, large quantities of reactive oxygen and nitrogen species (RONS) are produced. Therefore, when CAPP enters in contact with living tissues, the synergistic action of several plasma components, such RONS, energetic (UV) photons, and charge particles should be considered. The biochemical mechanisms involved in the interaction of plasma species with microorganisms and cells, as well as the plasma application for tissue healing and disinfection are extensively studied in a novel interdisciplinary field called Plasma Medicine [9][10]. Recent studies have demonstrated that RONS are the main factor responsible for plasma antimicrobial and tissue healing effects, while the UV photons have only minor effect [13].
CAPP can be applied directly on living tissues and, in this case, RONS reach directly the target. Alternatively, CAPP can be applied indirectly, by previous exposure of liquids (i.e., water, liquid culture media) to the plasma, creating solutions containing RONS, known as plasma-activated media (PAM) or plasma-activated water (PAW) [14][15]. In general, the plasma-liquid interaction generates hydrogen peroxide, nitrites, nitrates and others RONS [16]. However, the composition of PAM depends not only on the plasma-liquid interactions but also on the subsequent chemical reactions in the liquid phase that can cause further changes into PAM composition [15].
2. CAPP Biological Activities
The biological effects of CAPP enable several applications in the medical area [9]. Laroussi [17] was the first author to report on the antibacterial effect of CAPP. After, an expressive number of manuscripts, review articles, contributions to conference and books on the antimicrobial potential of CAPP and on the physicochemical mechanisms for antimicrobial inactivation have been published. The growth control of several pathogenic microorganisms, such as Gram-positive and Gram-negative bacteria, fungal species and bacterial spores have been reported [18][19][20][21][22][23]. Additionally, an antibiofilm effect has also been observed for bacteria and fungi [24][25][26][27][28].
Interesting data point out to the anti-inflammatory and tissue repair effect induced by CAPP [29][30]. CAPP improved wound healing in mice with induction of type I collagen and MCP-1 protein production in keratinocytes and fibroblasts [31][32]. Brun et al. [33] observed increased migration and proliferation of fibroblasts in response to the production of RONS during CAPP exposure. Similar effects were observed by Bourdens et al. [34] and Haralambiev et al. [35]. Stimulation of keratinocytes by antioxidant pathways was also reported [30][36]. CAPP showed positive effect in the cutaneous microcirculation, increasing the tissue oxygen saturation and radial blood flow [37][38] that can contribute to improved tissue repair.
A remarkable feature of CAPP is its highly selective toxicity that highlights the potential for clinical treatment of infectious diseases [9][39][40]. This differential activity is based on differences in the cellular metabolism in presence of RONS. Eukaryotic cells exhibit protection to RONS, while prokaryotic ones do not demonstrate such protective mechanism [39][41][42][43]. The disparity in the cell sizes influences response to CAPP. For instance, bacterial cells (typically between 0.2 and 10 μm) have higher surface-volume ratio, which favours the plasma action, while eukaryotic cells are much bigger, from 10 to 100 µm [10][39][42]. Moreover, the organization of eukaryotic cells into tissues additionally increases their resistance to CAPP effects. Therefore, by adjusting the treatment parameters, plasma can be used to eliminate bacteria in planktonic or biofilm forms without damage to the surrounding host tissues [9][44][45][46].
In this context, treating fungal diseases has an additional challenge, as both fungal and host cells are eukaryotic. Hence, in this particular situation, the simultaneous study of fungal inhibition and toxicity to host cells is extremely important. Interestingly, there have already been some encouraging results in the literature. Borges et al. [24] reported that CAPP treatment for 5 min has antibiofilm effects on Candida albicans with low cytotoxicity to Vero cells [47]. In the same study, CAPP was also applied in vivo to treat oral candidiasis in mice without damaging the surrounding tissues.
The ability of CAPP to induce cell death by inducing apoptosis [48] can be also very useful for therapeutic purposes, and it has been applied in the control of cancer cells. In this case, metabolic differences between healthy and malignant cells favour the selectivity of CAPP. Constant cell replications, observed in malignant cells, can expose their DNA to CAPP more frequently, favouring to the cell structural damage [39][42][49][50][51].
RONS generated by CAPP, including hydroxyl radicals (OH−), hydrogen peroxide (H2O2), singlet oxygen (1O2), superoxide anion (O−2), atomic oxygen (O), atomic nitrogen (N), nitric oxide (NO), nitrogen trioxide (NO3), influence redox-regulated cell processes [9][11][18][42][52][53][54][55][56]. In particular, reactive oxygen species (ROS) can react with many biological macromolecules, causing oxidative structural modification and the loss of their biological function [57]. At the cellular level, ROS regulate growth, apoptosis and other signalling processes, while at the system level, they contribute to complex functions, including regulation of immune response [58]. In addition, ROS are emerging as the most important agents in the bacterial response to lethal stress. Currently, the effects of superoxide, hydrogen peroxide and hydroxyl radical have been studied. Superoxide and hydrogen peroxide arise when molecular oxygen oxidizes redox enzymes that transfer electrons to other substrates. Hydrogen peroxide that can be produced from the dismutation of superoxide serves as a substrate for the formation of hydroxyl radicals. If this oxidative process is not controlled, an accumulation of hydroxyl radicals can occur. The hydroxyl radical breaks down nucleic acids, carbonylated proteins and peroxidised lipids which can lead to cell death [59].
Reactive nitrogen species (RNS) can be both harmful or beneficial to living systems. At low concentrations, RNS can play an important role as a regulatory mediator in signalling. On the other hand, at moderate or high concentrations, RNS are harmful to living organisms and can inactivate important cellular molecules [60]. Nitric oxide is an important regulator of physiological processes [61] and can mediate the harmful cellular toxicity of metabolic enzymes, generating nitrite peroxide as a final product of reaction with superoxide [62].
The exact mechanism of CAPP and microbial cell interaction is not fully understood yet, but presently it is widely accepted that its antimicrobial activity is associated with synergetic action of two major CAPP components, UV radiation and RONS. They can break covalent bonds of stable compounds, such as the peptidoglycan from the bacterial cell walls and peroxidation of lipids in the cell membrane [17][44][56][63][64][65]. Also, CAPP interaction with prokaryotic cells can cause cellular rupture by electro erosion with formation of ionic pores and subsequent loss of cellular content [11][63]. CAPP can also break covalent bonds in the polymeric matrix of microbial biofilms, favouring their disruption [28][45][46][56][66].
3. Conclusions
Cold atmospheric pressure plasma (CAPP) has antimicrobial and anti-inflammatory effects that are useful in several areas of Dentistry, such as in Cariology, Periodontology and Endodontics. Additionally, CAPP has been showing potential to be used in the treatment of oral fungal diseases and to control oral cancer. However, additional in vivo studies to standardize clinical protocols are still needed.
References
- von Keudell, A.; Schulz-von der Gathen, V. Foundations of low-temperature plasma physics—An introduction. Plasma Sources Sci. Technol. 2017, 26, 113001.
- Bruggeman, P.J.; Iza, F.; Brandenburg, R. Foundations of atmospheric pressure non-equilibrium plasmas. Plasma Sources Sci. Technol. 2017, 26, 123002.
- Gibalov, V.I.; Pietsch, G.J. The development of dielectric barrier discharges in gas gaps and on surfaces. J. Phys. D Appl. Phys. 2000, 33, 2618–2636.
- Chirokov, A.; Gutsol, A.; Fridman, A. Atmospheric pressure plasma of dielectric barrier discharges. Pure Appl. Chem. 2005, 77, 487–495.
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of production and application in dentistry and oncology. Medical Gas Res. 2013, 3, 21.
- Winter, J.; Brandenburg, R.; Weltmann, K.D. Atmospheric pressure plasma jets: An overview of devices and new directions. Plasma Sources Sci. Technol. 2015, 24, 064001.
- Lu, X.; Reuter, S.; Laroussi, M.; Liu, D. Nonequlibrium Atmospheric Pressure Plasma Jets. Fundamentals, Diagnostics and Medical Applications; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019.
- Fanelli, F.; Fracassi, F. Atmospheric pressure non-equilibrium plasma jet technology: General features, specificities and applications in surface processing of materials. Surf. Coat. Technol. 2017, 322, 174–201.
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8.
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Physi. 2009, 11, 115012.
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324.
- Neyts, E.C.; Brault, P. Molecular Dynamics Simulations for Plasma-Surface Interactions. Plasma Process. Polym. 2017, 14, 1600145.
- Nicol, M.J.; Brubaker, T.R.; Honish, B.J.; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D.; et al. Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species. Sci. Rep. 2020, 10, 3066.
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62.
- Bradu, C.; Kutasi, K.; Magureanu, M.; Puač, N.; Živković, S. Reactive nitrogen species in plasma-activated water: Generation, chemistry and application in agriculture. J. Phys. D Appl. Phys. 2020, 53, 223001.
- Tanaka, H.; Nakamura, K.; Mizuno, M.; Ishikawa, K.; Takeda, K.; Kajiyama, H.; Utsumi, F.; Kikkawa, F.; Hori, M. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci. Rep. 2016, 6, 36282.
- Laroussi, M. Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans. Plasma Sci. 1996, 24, 1188–1191.
- Laroussi, M.; Mendis, D.A.; Rosenberg, M. Plasma interaction with microbes. New J. Phys. 2003, 5, 41.
- Kostov, K.G.; Rocha, V.; Koga-Ito, C.Y.; Matos, B.M.; Algatti, M.A.; Honda, R.Y.; Kayama, M.E.; Mota, R.P. Bacterial sterilization by a dielectric barrier discharge (DBD) in air. Surf. Coat. Technol. 2010, 204, 2954–2959.
- Klämpfl, T.G.; Isbary, G.; Shimizu, T.; Li, Y.F.; Zimmermann, J.L.; Stolz, W.; Schlegel, J.; Morfill, G.E.; Schmidt, H.U. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl. Environ. Microbiol. 2012, 78, 5077–5082.
- Nishime, T.M.C.; Borges, A.C.; Koga-Ito, C.Y.; Machida, M.; Hein, L.R.O.; Kostov, K.G. Non-thermal atmospheric pressure plasma jet applied to inactivation of different microorganisms. Surf. Coat. Technol. 2017, 312, 19–24.
- Liao, X.; Muhammad, A.I.; Chen, S.; Hu, Y.; Ye, X.; Liu, D.; Ding, T. Bacterial spore inactivation induced by cold plasma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2562–2572.
- Borges, A.C.; Nishime, T.M.C.; de Moura Rovetta, S.; Lima, G.M.G.; Kostov, K.G.; Thim, G.P.; de Menezes, B.R.C.; Machado, J.P.B.; Koga-Ito, C.Y. Cold Atmospheric Pressure Plasma Jet Reduces Trichophyton rubrum Adherence and Infection Capacity. Mycopathologia 2019, 184, 585–595.
- Borges, A.C.; Castaldelli Nishime, T.M.; Kostov, K.G.; de Morais Gouvêa Lima, G.; Lacerda Gontijo, A.V.; de Carvalho, J.N.M.M.; Yzumi Honda, R.; Yumi Koga-Ito, C. Cold atmospheric pressure plasma jet modulates Candida albicans virulence traits. Clin. Plasma Med. 2017, 7, 9–15.
- Rao, Y.; Shang, W.; Yang, Y.; Zhou, R.; Rao, X. Fighting Mixed-Species Microbial Biofilms With Cold Atmospheric Plasma. Front. Microbiol. 2020, 11, 1000.
- Jiang, C.; Schaudinn, C.; Jaramillo, D.E.; Webster, P.; Costerton, J.W. In Vitro Antimicrobial Effect of a Cold Plasma Jet against Enterococcus faecalis Biofilms. ISRN Dent. 2012, 2012, 295736.
- Idlibi, A.N.; Al-Marrawi, F.; Hannig, M.; Lehmann, A.; Rueppell, A.; Schindler, A.; Jentsch, H.; Rupf, S. Destruction of oral biofilms formed in situ on machined titanium (Ti) surfaces by cold atmospheric plasma. Biofouling 2013, 29, 369–379.
- Delben, J.A.; Zago, C.E.; Tyhovych, N.; Duarte, S.; Vergani, C.E. Effect of Atmospheric-Pressure Cold Plasma on Pathogenic Oral Biofilms and In Vitro Reconstituted Oral Epithelium. PLoS ONE 2016, 11, e0155427.
- Rutkowski, R.; Schuster, M.; Unger, J.; Seebauer, C.; Metelmann, H.R.; Woedtke, T.V.; Weltmann, K.D.; Daeschlein, G. Hyperspectral imaging for in vivo monitoring of cold atmospheric plasma effects on microcirculation in treatment of head and neck cancer and wound healing. Clin. Plasma Med. 2017, 7–8, 52–57.
- Shome, D.; von Woedtke, T.; Riedel, K.; Masur, K. The HIPPO Transducer YAP and Its Targets CTGF and Cyr61 Drive a Paracrine Signalling in Cold Atmospheric Plasma-Mediated Wound Healing. Oxid. Med. Cell Longev. 2020, 2020, 4910280.
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.K.; et al. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS ONE 2013, 8, e79325.
- Xu, D.; Wang, S.; Li, B.; Qi, M.; Feng, R.; Li, Q.; Zhang, H.; Chen, H.; Kong, M.G. Effects of Plasma-Activated Water on Skin Wound Healing in Mice. Microorganisms 2020, 8, 1091.
- Brun, P.; Pathak, S.; Castagliuolo, I.; Palù, G.; Zuin, M.; Cavazzana, R.; Martines, E. Helium generated cold plasma finely regulates activation of human fibroblast-like primary cells. PLoS ONE 2014, 9, e104397.
- Bourdens, M.; Jeanson, Y.; Taurand, M.; Juin, N.; Carrière, A.; Clément, F.; Casteilla, L.; Bulteau, A.-L.; Planat-Bénard, V. Short exposure to cold atmospheric plasma induces senescence in human skin fibroblasts and adipose mesenchymal stromal cells. Sci. Rep. 2019, 9, 8671.
- Haralambiev, L.; Bandyophadyay, A.; Suchy, B.; Weiss, M.; Kramer, A.; Bekeschus, S.; Ekkernkamp, A.; Mustea, A.; Kaderali, L.; Stope, M.B. Determination of Immediate. Anticancer Res. 2020, 40, 3743–3749.
- Schmidt, A.; Dietrich, S.; Steuer, A.; Weltmann, K.D.; von Woedtke, T.; Masur, K.; Wende, K. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J. Biol. Chem. 2015, 290, 6731–6750.
- Kisch, T.; Helmke, A.; Schleusser, S.; Song, J.; Liodaki, E.; Stang, F.H.; Mailaender, P.; Kraemer, R. Improvement of cutaneous microcirculation by cold atmospheric plasma (CAP): Results of a controlled, prospective cohort study. Microvasc. Res. 2016, 104, 55–62.
- Busco, G.; Robert, E.; Chettouh-Hammas, N.; Pouvesle, J.M.; Grillon, C. The emerging potential of cold atmospheric plasma in skin biology. Free Radic. Biol. Med. 2020, 161, 290–304.
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020.
- Weltmann, K.D.; von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Control. Fusion 2016, 59, 014031.
- Kumar, N.; Attri, P.; Yadav, D.K.; Choi, J.; Choi, E.H.; Uhm, H.S. Induced apoptosis in melanocytes cancer cell and oxidation in biomolecules through deuterium oxide generated from atmospheric pressure non-thermal plasma jet. Sci. Rep. 2014, 4, 7589.
- Xu, D.; Liu, D.; Wang, B.; Chen, C.; Chen, Z.; Li, D.; Yang, Y.; Chen, H.; Kong, M.G. In Situ OH Generation from O2- and H2O2 Plays a Critical Role in Plasma-Induced Cell Death. PLoS ONE 2015, 10, e0128205.
- Lin, A.; Truong, B.; Patel, S.; Kaushik, N.; Choi, E.H.; Fridman, G.; Fridman, A.; Miller, V. Nanosecond-Pulsed DBD Plasma-Generated Reactive Oxygen Species Trigger Immunogenic Cell Death in A549 Lung Carcinoma Cells through Intracellular Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 966.
- Lunov, O.; Zablotskii, V.; Churpita, O.; Lunova, M.; Jirsa, M.; Dejneka, A.; Kubinová, Š. Chemically different non-thermal plasmas target distinct cell death pathways. Sci. Rep. 2017, 7, 600.
- Alkawareek, M.Y.; Algwari, Q.T.; Gorman, S.P.; Graham, W.G.; O’Connell, D.; Gilmore, B.F. Application of atmospheric pressure nonthermal plasma for the in vitro eradication of bacterial biofilms. FEMS Immunol. Med. Microbiol. 2012, 65, 381–384.
- Puligundla, P.; Mok, C. Potential applications of nonthermal plasmas against biofilm-associated micro-organisms in vitro. J. Appl. Microbiol. 2017, 122, 1134–1148.
- Borges, A.C.; Lima, G.M.G.; Nishime, T.M.C.; Gontijo, A.V.L.; Kostov, K.G.; Koga-Ito, C.Y. Amplitude-modulated cold atmospheric pressure plasma jet for treatment of oral candidiasis: In vivo study. PLoS ONE 2018, 13, e0199832.
- Boehm, D.; Bourke, P. Safety implications of plasma-induced effects in living cells—A review of in vitro and in vivo findings. Biol. Chem. 2018, 400, 3–17.
- Han, X.; Kapaldo, J.; Liu, Y.; Stack, M.S.; Alizadeh, E.; Ptasinska, S. Large-Scale Image Analysis for Investigating Spatio-Temporal Changes in Nuclear DNA Damage Caused by Nitrogen Atmospheric Pressure Plasma Jets. Int. J. Mol. Sci. 2020, 21, 4127.
- Van der Paal, J.; Hong, S.-H.; Yusupov, M.; Gaur, N.; Oh, J.-S.; Short, R.D.; Szili, E.J.; Bogaerts, A. How membrane lipids influence plasma delivery of reactive oxygen species into cells and subsequent DNA damage: An experimental and computational study. Phys. Chem. Chem. Phys. 2019, 21, 19327–19341.
- Bengtson, C.; Bogaerts, A. On the Anti-Cancer Effect of Cold Atmospheric Plasma and the Possible Role of Catalase-Dependent Apoptotic Pathways. Cells 2020, 9, 2330.
- VON Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma Medicine: A Field of Applied Redox Biology. In Vivo 2019, 33, 1011–1026.
- Zhao, S.; Xiong, Z.; Mao, X.; Meng, D.; Lei, Q.; Li, Y.; Deng, P.; Chen, M.; Tu, M.; Lu, X.; et al. Atmospheric pressure room temperature plasma jets facilitate oxidative and nitrative stress and lead to endoplasmic reticulum stress dependent apoptosis in HepG2 cells. PLoS ONE 2013, 8, e73665.
- Zhao, J.; Nie, L. Five gaseous reactive oxygen and nitrogen species (RONS) density generated by microwave plasma jet. Phys. Plasmas 2019, 26, 073503.
- Kim, S.J.; Chung, T.H. Cold atmospheric plasma jet-generated RONS and their selective effects on normal and carcinoma cells. Sci. Rep. 2016, 6, 20332.
- Kurita, H.; Haruta, N.; Uchihashi, Y.; Seto, T.; Takashima, K. Strand breaks and chemical modification of intracellular DNA induced by cold atmospheric pressure plasma irradiation. PLoS ONE 2020, 15, e0232724.
- Bartosz, G. Reactive oxygen species: Destroyers or messengers? Biochem. Pharmacol. 2009, 77, 1303–1315.
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly 2012, 142, w13659.
- Imlay, J.A. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003, 57, 395–418.
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247.
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916.
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424.
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610.
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86.
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 5.
- Brelles-Mariño, G. Challenges in biofilm inactivation: The use of cold plasma as a new approach. J. Bioprocess. Biotech. 2012, 2, 4.




