| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Edoardo Longo | + 2125 word(s) | 2125 | 2021-01-11 04:38:51 | | | |
| 2 | Peter Tang | -96 word(s) | 2029 | 2021-04-12 03:41:39 | | |
Video Upload Options
Phenolic compounds are secondary metabolites present in grapes and wine that can be formed and transformed during the winemaking process. Phenolics can be classified as flavonoids (e.g., anthocyanins, flavan-3-ols, flavonols) and non-flavonoids (e.g., phenolic acids, stilbenes).
1. Introduction
Wine is a product with high commercial value and relevant cultural aspects. Its desirability on the market, combined with high prices that consumers are willing to pay for top quality bottles, is a cause for food frauds [1][2], also with very recent examples [3]. Recently, over one million liters of counterfeit wine were discovered by the European Anti-Fraud Office [4]. Mislabeling of variety, geographical origin, or vintage year and adulteration with ethanol, sugar, and colorants are typical examples of frauds related to wine [5]. Therefore, the wine industry and consumers are highly concerned about the quality and authenticity of wine [6].
Wine quality is determined by several factors such as the type (or blend) of grape varieties, the terroir, the viticultural practices, the winemaking techniques, and the aging conditions [7][8][9]. The variety of grapes is a key factor in determining the wine flavor, especially during the production of premium wines. Thus, the adulteration of these types of wines with cheaper grape varieties is common [10][11][12]. Terroir is a French term that defines the very specific combination of geographical, climatic, and pedological factors, characterizing the growth and quality of the grapes. Terroir is mainly influenced by the climate and soil conditions, and it is strongly related to viticultural practices and vintage year [13][14][15].
One of the aspects related to wine authenticity is based on the (blend of) grape varieties used in winemaking, their geographical origin, and vintage. In Europe, the authenticity related to the terroir is guaranteed by strict guidelines adopted by the European Union also based on the national rules and the indications of The International Organization of Vine and Wine [16].
Wine quality evaluation is based on sensory and chemical analyses. In the sensory tasting, wine quality indicators, such as color, mouthfeel, and taste are largely, but not exclusively, influenced by the phenolic profile. Thus, phenolic compounds are widely used for the wine quality and authenticity assessment [17][18][19].
2. Classification of Phenolic Compounds of Wine
Phenolic compounds are secondary metabolites present in grapes and wine that can be formed and transformed during the winemaking process. In addition, wine phenolics can derive from wood products (containers or derived products) used for the winemaking.
Phenolics can be classified as non-flavonoids (e.g., phenolic acids, stilbenes, volatile phenols) [20][21] and flavonoids (e.g., anthocyanins, flavan-3-ols, flavonols). The phenolic compounds mostly applied for the quality and authenticity assessment of wine are phenolic acids, flavonoids, tannins, and stilbenes.
2.1. Phenolic acids
There are two main groups of phenolic acids that are used for the quality and authenticity assessment and are significant for white grapes and wines: hydroxybenzoic acids (containing seven carbon atoms) and hydroxycinnamic acids (nine carbon atoms, phenylpropanoid derivatives). Model structures are reported in Figure 1.
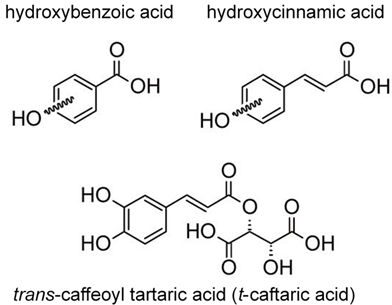
Figure 1. Model structure for common natural hydroxybenzoic acids (upper left), hydroxycinnamic acids (upper right), and an example of a cinnamic acid derivative present in grape and wine (lower). For the simple phenols, the hydroxylation substitutions on aromatic rings are generically shown by curled bonds.
Hydroxycinnamic acids (e.g., caffeic, coumaric, ferulic, and sinapic) can be found in two isomeric forms (cis and trans) because of the presence of an α,β-unsaturated carboxylic acid functional unit. Hydroxybenzoic and hydroxycinnamic acids do not only occur in their free forms but also as derivatives in conjugated or esterified forms as well, such as caftaric acid (trans-caffeoyl tartaric acid) (Figure 1) and its adduct with glutathione, which leads to 2-S-glutathionylcaftaric acid (grape reaction product)[22][23], or fertaric acid (an ester formed from ferulic acid bound to tartaric acid) and coutaric acid (an ester formed from coumaric acid and tartaric acid). Hydroxycinnamic acids in wine originate during fermentation from the hydrolysis of hydroxycinnamic tartaric esters [13][24]. They can be an oxidation substrate and precursors of browning of white wines and may give a bitter flavor [13][25].
2.2. Flavonoids
Flavonoids comprise a wide range of 15-carbon compounds, having in their structures two aromatic rings bound through a three-carbon chain. Model general structures for the most common families of natural flavonoids are reported in Figure 2.

Figure 2. Model structures for common natural flavonoids. Hydroxylation substitutions and stereogenic configuration patterns are not explicitly shown for brevity.
Wine flavonoids occur both in free and conjugated forms, as for example glucosides. The most important classes of flavonoids that have been applied as chemical markers are anthocyanins, flavonols, and flavan-3-ols. The most common mono-glycosylated anthocyanin forms are summarized in Figure 3. Anthocyanins can be classified into mono-, di- and trisubstituted congeners according to the total number of hydroxyl and methoxy groups present in the lateral ring (they can be 2 or 3 considering R1, R2, and R3 in Figure 3). Anthocyanins are not only found in simple mono-glycosylated or di-glycosylated forms, but they can also be esterified on the glycosidic moiety, such as acetyl-glucosides, p-coumaroyl-glucosides, and caffeoyl-glucosides (acylated anthocyanins).

Figure 3. Structures of mono-glycosylated anthocyanins.
Each grape variety presents a typical anthocyanin pattern [26][27]; thus, anthocyanins are the most applied flavonoids for the assessment of authenticity and quality of red wines. These protocols are based on the differentiation between (a) different anthocyanidin congeners, (b) anthocyanins mono- and di-glucosides (3-O-glucoside derivatives are shown in Figure 3), and (c) acylated or non-acylated anthocyanins.
Aged red wines (and aged fruit juices in general) may contain pyranoanthocyanins, a class of anthocyanin-derived pigments. Pyranoanthocyanins usually originate from the condensation of anthocyanins with phenolic (cinnamic) acids or organic acids occurring during aging [28]. The main congeners identified so far are A-type and B-type vitisins, methylpyranoanthocyanins, pinotins, flavanol-pyranoanthocyanins, oxovitisins and A-type portisins and are responsible for the color stabilisation of red wines [29].
Flavonols show an unsaturation between C2 and C3. They are hydroxylated in the position C3 and have a carbonyl group in C4. Flavonols are present in wine as aglycones and glycosylated forms [30]. The most abundant flavonols in wines are quercetin and myricetin [31]. Differently from flavonols, flavan-3-ols have a saturated carbon bond between C2 and C3 and no carbonyl group. Catechin, epicatechin, and their derivatives (e.g., gallocatechin, epicatechin gallate) are the most abundant flavan-3-ols in wine. Some derivatives show also the presence of an ester with gallic acid in C3 [32]. A particular type of flavonoids, condensed tannins (or proanthocyanidins), are oligomeric or polymeric forms of flavan-3-ols.
2.3. Tannins
The general definition of tannins include two very different chemical classes of phenolic compounds: hydrolyzable tannins and condensed tannins; tannins give the astringency perception to wines [33]. Hydrolizable tannins are named as gallotannins (derivatives of gallic acid) or ellagitannins (derivatives of ellagic acid) depending in which acid they are converted upon hydrolysis [34]. Unlike hydrolysable tannins, condensed tannins are oligomers or polymers of flavan-3-ols, depending on their degree of polymerization. Condensed tannins are also called proanthocyanidins and their degree of polymerization (DP) may range from 2 to about 20 in wine; their solubility tends to decrease with the increasing number of monomeric units [35][36]. They show a great variability of isomers, depending on the geometry of their bindings and the type of monomers involved (Figure 4). They influence the taste (bitterness and astringency) of wine [37] and the color stabilization by combining with anthocyanins in red wines [38].

Figure 4. Example of isomerism for dimeric procyanidins: procyanidin B1 (A), procyanidin B2 (B), procyanidin B3 (C), procyanidin B4 (D). They are precursors of condensed tannins.
Recently, tetra-, penta- and hexameric proanthocyanidins with a cyclic macromolecular structure have been identified in small amounts both in white and red wines. An example is given in Figure 5 [39][40]. They can be subclassified into cyclic procyanidins and cyclic prodelphinidins; the latter ones may contain a different number of (epi)gallocatechin monomers [41]. Remarkably, the proportion of cyclic species vs the total amount (per number of monomeric units) was shown to be affected by the grape varieties of the wine [42]. Notably, the actual extent of the natural distribution of these compounds is yet not completely known; some of these compounds have been also found in cranberries (Vaccinium macrocarpon) [43].
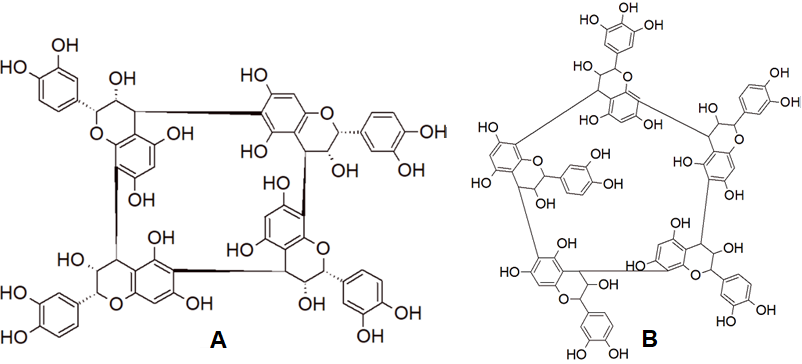
Figure 5. Resolved molecular structure of cyclic tetrameric procyanidin (A) [44] and structure of cyclic pentameric prodelphinidin with one (epi)gallocatechin unit (B) [45].
In enology, also the use of exotic tannins is allowed, such as those extracted from quebracho (Schinopsis balansae), mimosa or myrabolan [46]. The tannins obtained from quebracho and mimosa can be classified as condensed tannins with profisetidin as monomeric unit. However, studies reporting the concentration of exotic tannins in wine are missing or incomplete.
2.4. Stilbenes
The main stilbenes present in grapes and wine are resveratrol and piceid (resveratrol glucoside) in cis and trans isomeric forms (Figure 6). According to the scientific literature, stilbenes have proved to be good discriminants of the grape variety [10][21][47][48][49], grape species [5] and terroir [7][8][13][50] in white, rosé, and red wines. Usually, cis-resveratrol and cis-piceid are found at lower concentrations and are less biologically active than their trans forms [51]. The oxidation products of resveratrol are viniferins, such as, ε-, α-, β-, and γ-viniferin, respectively, as a di-, tri-, tetramer, and a more highly polymerized oligomer [52]. An example of dehydrodimer of resveratrol is presented in Figure 6.
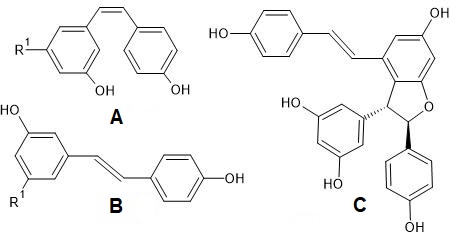
Figure 6. Structures of stilbenes: cis-piceid (A) and trans-piceid (B), when R1 = O-glucose; and cis-resveratrol (A) and trans-resveratrol (B), when R1 = OH; trans-ε-viniferin (C).
2.5. Volatile phenols
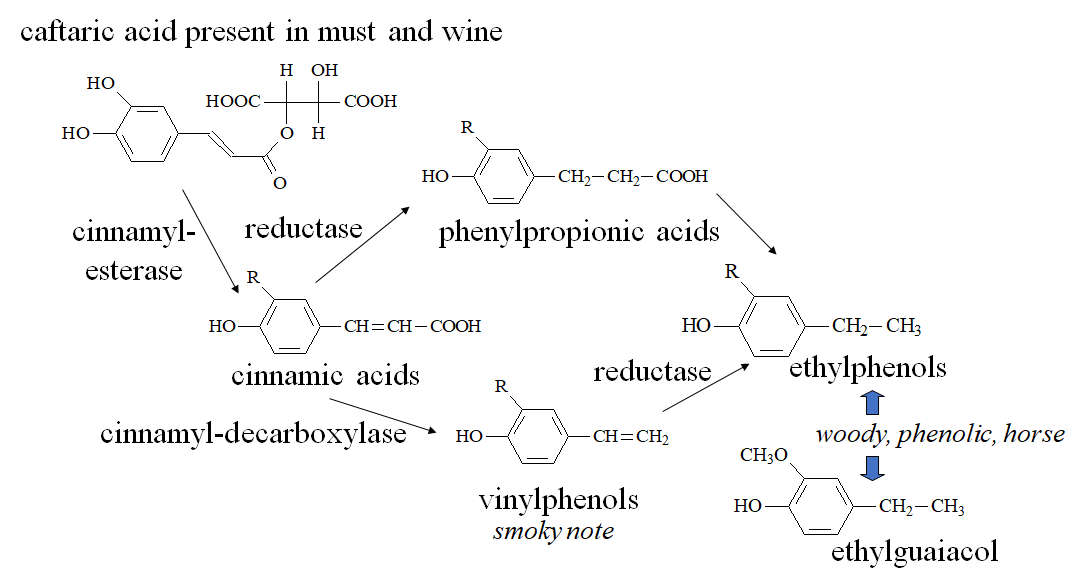
Volatile phenols are odorants produced by yeasts which can be occasionally found in wine and are often related to undesired fermentations such as the Brett character (from Brettanomyces spp.). Although volatile phenols may enrich the complexity of the wine flavor in low concentrations, their excess is related to undesired odors described as horsey, phenolic, pharmaceutical, stable, leather, typical of some red wines aged in oak barrels contaminated with Brettanomyces. The main congeners are ethylphenols (4-ethylphenol and 4-ethylguaiacol, usually occurring as a 10:1 mixture in Bordeaux wines [53]) and vinylphenols (4-vinylphenol, 4-vinylguaiacol [54]). In Figure 7, a possible pathway of formation of volatile phenols in wine is displayed.
In addition, vanillin, which is often present in wine, could be technically included in the family of volatile phenols. However, due to the different origin and sensory characteristics, it will be discussed in the section describing wine phenols coming from the storage or contact with wood.
Figure 7. Formation of volatile phenols (vinylphenols and ethylphenols) in wine.
2.6. Wine phenols coming from the storage or contact with wood
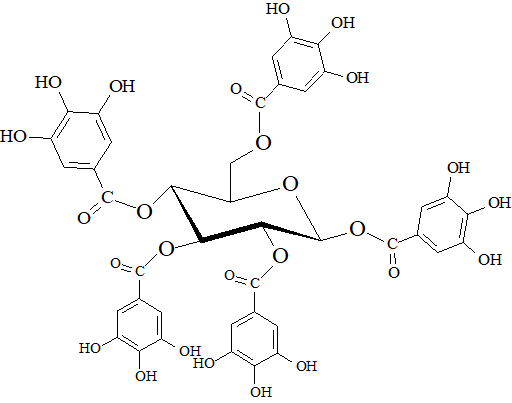
Wine contains phenols that are not only coming from the grape variety, grape varietal combination or the yeasts used for the winemaking, but also from the wood containers that are often used at different stages of the winemaking process (usually during the fermentation or maturation steps). In many cases, wood derived products, such as staves, chips or powders may have been in contact with wine. Usually, the preferred wood type is oak, due to its compact texture of fibers (it is a slow growing wood) that reduces the permeability to air (but also chestnut or acacia might be used in specific wine styles); the main species used in cooperage are Quercus robur, Q. petraea and Q. alba. The main phenols that are diffusing from the oak into the wine are hydrolyzable tannins which are in the form of polygalloyl esters of glucose. Oak tannins are a plethora of different congeners; one of the most common methods used to study them is an acidic hydrolysis in which two families of compounds are released: gallotannins and ellagitannins [55]. Figure 8 shows the molecular structure of pentagalloylglucose, as a precursor of gallotannins. The oxidative coupling of gallotannins give the origin to ellagitannins, the main tannins extracted from the oak heartwood. Two monomers (castalagin and vescalagin) account for about 40-65% of the total extractable ellagitannins, however also pentosylated monomers (roburin E and grandinin), dimers (roburin A and D) and pentosylated dimers (roburin B and C) have been identified so far.
Figure 8. β-1,2,3,4,6-pentagalloyl-O-D-glucose.
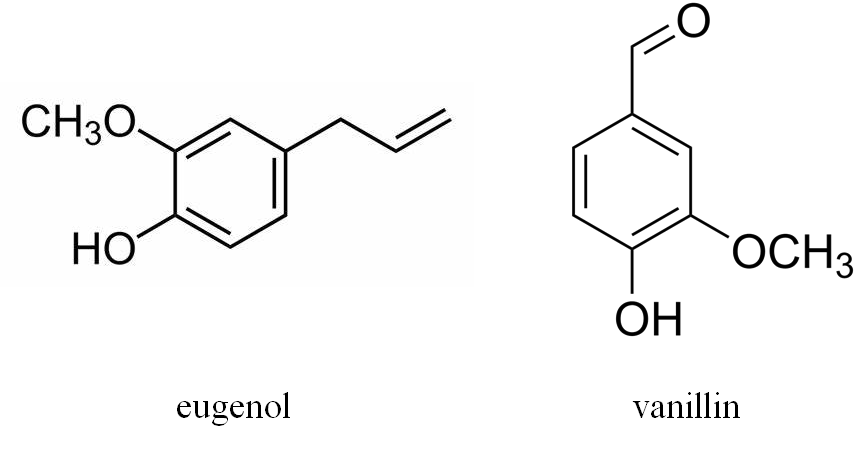
In addition, vanillin, eugenol and other volatile phenols [56] are formed as lignin degradation products in oak barrels, mainly during coopering and then diffused into wines during aging (Figure 9). Since vanillin is the main aroma-active constituent of natural vanilla, its presence strongly influences the perceived wine flavor of barrel-aged wines or wines which were in contact with wood derived products. The same applies to eugenol, which is described by sensory note of cloves.
Figure 9. Structures of eugenol and vanillin.
References
- Geană, E. I.; Ciucure, C. T.; Apetrei, C.; Artem, V.; Application of Spectroscopic UV-Vis and FT-IR Screening Techniques Coupled with Multivariate Statistical Analysis for Red Wine Authentication: Varietal and Vintage Year Discrimination. Molecules 2019, 24, 4166, 10.3390/molecules24224166.
- ICQRF Activity Report . Ministero delle politiche agricole alimentari e forestali. Retrieved 2021-4-6
- Italian police put cork on ‘world’ best wine’ fakes . Reuters. Retrieved 2021-4-6
- More than 1m litres of counterfeit wine and alcoholic beverages seized under OLAF’s lead . European Commission. Retrieved 2021-4-6
- Stój, A.; Kapusta, I.; Domagała, D.; Classification of Red Wines Produced from Zweigelt and Rondo Grape Varieties Based on the Analysis of Phenolic Compounds by UPLC-PDA-MS/MS. Molecules 2020, 25, 1342, 10.3390/molecules25061342.
- Costa, N. L.; García Llobodanin, L. A.; Castro, I. A.; Barbosa, R.; Using Support Vector Machines and neural networks to classify Merlot wines from South America. Information Processing in Agriculture 2019, 6, 265-278, 10.1016/j.inpa.2018.10.003.
- Anastasiadi, M.; Zira, A.; Magiatis, P.; Haroutounian, S. A.; Skaltsounis, A. L.; Mikros, E.; 1H NMR-Based Metabonomics for the Classification of Greek Wines According to Variety, Region, and Vintage. Comparison with HPLC Data. Journal of Agricultural and Food Chemistry 2009, 57, 11067-11074, 10.1021/jf902137e.
- Kumšta, M.; Pavloušek, P.; Kupsa, J.; Phenolic profile in Czech white wines from different terroirs. Food Science and Biotechnology 2012, 21, 1593-1601, 10.1007/s10068-012-0212-0.
- Parpinello, G. P.; Ricci, A.; Arapitsas, P.; Curioni, A.; Moio, L.; Riosegade, S.; Ugliano, M.; Versari, A.; Multivariate characterisation of Italian monovarietal red wines using MIR spectroscopy. OENO One 2019, 53, 741–751, 10.20870/oeno-one.2019.53.4.2558.
- Agatonovic-Kustrin, S.; Milojković-Opsenica, D.; Morton, D. W.; Ristivojević, P.; Chemometric characterization of wines according to their HPTLC fingerprints. European Food Research and Technology 2016, 243, 659-667, 10.1007/s00217-016-2779-9.
- Sartor, S.; Malinovski, L. I.; Caliari, V.; Lima Da Silva, A.; Bordignon-Luiz, M. T.; Particularities of Syrah wines from different growing regions of Southern Brazil: grapevine phenology and bioactive compounds. Journal of Food Science and Technology 2017, 54, 1414-1424, 10.1007/s13197-017-2557-0.
- Li, S. Y.; Zhu, B. Q.; Reeves, M. J.; Duan, C. Q.; Phenolic Analysis and Theoretic Design for Chinese Commercial Wines’ Authentication. Journal of Food Science 2017, 83, 30-38, 10.1111/1750-3841.13961.
- Pavloušek, P.; Kumšta, M.; Authentication of Riesling wines from the Czech Republic on the basis of the non-flavonoid phenolic compounds. Czech Journal of Food Sciences 2013, 31, 474-482, 10.17221/40/2013-cjfs.
- Pisano, P. L.; Silva, M. F.; Olivieri, A. C.; Anthocyanins as markers for the classification of Argentinean wines according to botanical and geographical origin. Chemometric modeling of liquid chromatography–mass spectrometry data. Food Chemistry 2015, 175, 174-180, 10.1016/j.foodchem.2014.11.124.
- Urvieta, R.; Buscema, F.; Bottini, R.; Coste, B.; Fontana, A.; Phenolic and sensory profiles discriminate geographical indications for Malbec wines from different regions of Mendoza, Argentina. Food Chemistry 2018, 265, 120-127, 10.1016/j.foodchem.2018.05.083.
- International Standard for the Labelling of Wines . OIV. Retrieved 2021-4-6
- Chira, K.; Pacella, N.; Jourdes, M.; Teissedre, P. L.; Chemical and sensory evaluation of Bordeaux wines (Cabernet-Sauvignon and Merlot) and correlation with wine age. Food Chemistry 2011, 126, 1971-1977, 10.1016/j.foodchem.2010.12.056.
- Heras-Roger, J.; Díaz-Romero, C.; Darias-Martín, J.; A comprehensive study of red wine properties according to variety. Food Chemistry 2016, 196, 1224-1231, 10.1016/j.foodchem.2015.10.085.
- Lukić, I.; Radeka, S.; Budić-Leto, I.; Bubola, M.; Vrhovsek, U.; Targeted UPLC-QqQ-MS/MS profiling of phenolic compounds for differentiation of monovarietal wines and corroboration of particular varietal typicity concepts. Food Chemistry 2019, 300, 125251, 10.1016/j.foodchem.2019.125251.
- Cejudo-Bastante, M. J.; Vicario, A.; Guillén, D. A.; Hermosín-Gutiérrez, I.; Pérez-Coello, M. S.; Phenolic characterization of minor red grape varieties grown in Castilla-La Mancha region in different vinification stages. European Food Research and Technology 2014, 240, 595-607, 10.1007/s00217-014-2360-3.
- Ragusa, A.; Centonze, C.; Grasso, M. E.; Latronico, M. F.; Mastrangelo, P. F.; Sparascio, F.; Fanizzi, F. P.; Maffia, M.; A Comparative Study of Phenols in Apulian Italian Wines. Foods 2017, 6, 24, 10.3390/foods6040024.
- Cheynier, V. F.; Trousdale, E. K.; Singleton, V. L.; Salgues, M. J.; Wylde, R.; Characterization of 2-S-glutathionyl caftaric acid and its hydrolysis in relation to grape wines. Journal of Agricultural and Food Chemistry 1986, 34, 217-221, 10.1021/jf00068a016.
- Emanuele Boselli; Massimo Minardi; Andrea Giomo; Natale G. Frega; Phenolic composition and quality of white d.o.c. wines from Marche (Italy). Analytica Chimica Acta 2006, 563, 93-100, 10.1016/j.aca.2005.10.024.
- Di Lecce, G.; Boselli, E.; D'ignazi, G.; Frega, N. G.; Evolution of phenolics and glutathione in Verdicchio wine obtained with maceration under reductive conditions. LWT - Food Science and Technology 2013, 53, 54-60, 10.1016/j.lwt.2013.03.006.
- Boselli, E.; Di Lecce, G.; Alberti, F.; Frega, N. G.; Nitrogen gas affects the quality and the phenolic profile of must obtained from vacuum-pressed white grapes. LWT - Food Science and Technology 2010, 43, 1494-1500, 10.1016/j.lwt.2010.03.006.
- Berente, B.; De La Calle Garcı́a, D.; Reichenbächer, M.; Danzer, K.; Method development for the determination of anthocyanins in red wines by high-performance liquid chromatography and classification of German red wines by means of multivariate statistical methods. Journal of Chromatography A 2000, 871, 95-103, 10.1016/s0021-9673(99)01272-8.
- Revilla, E.; Garcı́a-Beneytez, E.; Cabello, F.; Martı́n-Ortega, G.; Ryan, J. M.; Value of high-performance liquid chromatographic analysis of anthocyanins in the differentiation of red grape cultivars and red wines made from them. Journal of Chromatography A 2001, 915, 53-60, 10.1016/s0021-9673(01)00635-5.
- Rentzsch, M.; Schwarz, M.; Winterhalter, P.; Pyranoanthocyanins – an overview on structures, occurrence, and pathways of formation. Trends in Food Science & Technology 2007, 18, 526-534, 10.1016/j.tifs.2007.04.014.
- Quaglieri, C.; Jourdes, M.; Waffo-Teguo, P.; Teissedre, P. L.; Updated knowledge about pyranoanthocyanins: Impact of oxygen on their contents, and contribution in the winemaking process to overall wine color. Trends in Food Science & Technology 2017, 67, 139-149, 10.1016/j.tifs.2017.07.005.
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I.; Flavonol Profiles ofVitis viniferaRed Grapes and Their Single-Cultivar Wines. Journal of Agricultural and Food Chemistry 2007, 55, 992-1002, 10.1021/jf062800k.
- Vergara, C.; Von Baer, D.; Mardones, C.; Gutiérrez, L.; Hermosín-Gutiérrez, I.; Castillo-Muñoz, N.; FLAVONOL PROFILES FOR VARIETAL DIFFERENTIATION BETWEEN CARMÉNÈRE AND MERLOT WINES PRODUCED IN CHILE: HPLC AND CHEMOMETRIC ANALYSIS. Journal of the Chilean Chemical Society 2011, 56, 827-832, 10.4067/s0717-97072011000400001.
- Mattivi, F.; Vrhovsek, U.; Masuero, D.; Trainotti, D.; Differences in the amount and structure of extractable skin and seed tannins amongst red grape varieties. Australian Journal of Grape and Wine Research 2009, 15, 27-35, 10.1111/j.1755-0238.2008.00027.x.
- Chira, K.; Jourdes, M.; Teissedre, P. L.; Cabernet sauvignon red wine astringency quality control by tannin characterization and polymerization during storage. European Food Research and Technology 2011, 234, 253-261, 10.1007/s00217-011-1627-1.
- Basalekou, M.; Kallithraka, S.; Tarantilis, P. A.; Kotseridis, Y.; Pappas, C.; Ellagitannins in wines: Future prospects in methods of analysis using FT-IR spectroscopy. LWT - Food Science and Technology 2019, 101, 48-53, 10.1016/j.lwt.2018.11.017.
- Motta, S.; Guaita, M.; Cassino, C.; Bosso, A.; Relationship between polyphenolic content, antioxidant properties and oxygen consumption rate of different tannins in a model wine solution. Food Chemistry 2020, 313, 126045, 10.1016/j.foodchem.2019.126045.
- Sun, B.; Leandro, C.; Da Silva, A. J. M. R.; Spranger, I.; Separation of Grape and Wine Proanthocyanidins According to Their Degree of Polymerization. Journal of Agricultural and Food Chemistry 1998, 46, 1390-1396, 10.1021/jf970753d.
- Llaudy, M. C.; Canals, R.; Canals, J. M.; Rozès, N.; Arola, L.; Zamora, F.; New Method for Evaluating Astringency in Red Wine. Journal of Agricultural and Food Chemistry 2004, 52, 742-746, 10.1021/jf034795f.
- Mazza, G.; Francis, F. J.; Anthocyanins in grapes and grape products. Critical Reviews in Food Science and Nutrition 1995, 35, 341-371, 10.1080/10408399509527704.
- Longo, E.; Rossetti, F.; Scampicchio, M.; Boselli, E.; Isotopic Exchange HPLC-HRMS/MS Applied to Cyclic Proanthocyanidins in Wine and Cranberries. Journal of the American Society for Mass Spectrometry 2018, 29, 663-674, 10.1007/s13361-017-1876-8.
- Zeng, L.; Pons-Mercadé, P.; Richard, T.; Krisa, S.; Teissèdre, P. L.; Jourdes, M.; Crown Procyanidin Tetramer: A Procyanidin with an Unusual Cyclic Skeleton with a Potent Protective Effect against Amyloid-β-Induced Toxicity. Molecules 2019, 24, 1915, 10.3390/molecules24101915.
- Longo, E.; Merkyte, V.; Rossetti, F.; Teissedre, P.L.; Jourdes, M.; Boselli, E.; Relative abundances of novel cyclic prodelphinidins in wine depending on the grape variety. Journal of Mass Spectrometry 2018, 53, 1116-1125, 10.1002/jms.4280.
- Longo, E.; Rossetti, F.; Jouin, A.; Teissedre, P. L.; Jourdes, M.; Boselli, E.; Distribution of crown hexameric procyanidin and its tetrameric and pentameric congeners in red and white wines. Food Chemistry 2019, 299, 125125, https://www.sciencedirect.com/science/article/abs/pii/S0308814619312312.
- Longo, E.; Rossetti, F.; Scampicchio, M.; Boselli, E.; Isotopic Exchange HPLC-HRMS/MS Applied to Cyclic Proanthocyanidins in Wine and Cranberries. Journal of The American Society for Mass Spectrometry 2018, 29, 663-674, https://link.springer.com/article/10.1007/s13361-017-1876-8.
- Zeng, L.; Pons-Mercadé, P.; Richard, T.; Krisa, S.; Teissèdre, P. L.; Jourdes, M.; Crown procyanidin tetramer: A procyanidin with an unusual cyclic skeleton with a potent protective effect against amyloid-β-induced toxicity. Molecules 2019, 24(10), 1915, https://www.mdpi.com/1420-3049/24/10/1915.
- Longo, E.; Merkyte, V.; Rossetti, F.; Teissedre, P. L.; Jourdes, M.; Boselli, E.; Relative abundances of novel cyclic prodelphinidins in wine depending on the grape variety. Journal of Mass Spectrometry 2018, 53, 1116-1125, 10.1002/jms.4280.
- Versari, A.; Du Toit, W.; Parpinello, G. P.; Oenological tannins: a review. Australian Journal of Grape and Wine Research 2012, 19, 1-10, 10.1111/ajgw.12002.
- Baron, M.; Sochor, J.; Tomaskova, L.; Prusova, B.; Kumsta, M.; Study on Antioxidant Components in Rosé Wine Originating from the Wine Growing Region of Moravia, Czech Republic. Erwerbs-Obstbau 2017, 59, 253-262, 10.1007/s10341-016-0317-3.
- Martelo-Vidal, M. J.; Vázquez, M.; Determination of polyphenolic compounds of red wines by UV–VIS–NIR spectroscopy and chemometrics tools. Food Chemistry 2014, 158, 28-34, 10.1016/j.foodchem.2014.02.080.
- Ragusa, A.; Centonze, C.; Grasso, M. E.; Latronico, M. F.; Mastrangelo, P. F.; Sparascio, F.; Maffia, M.; HPLC Analysis of Phenols in Negroamaro and Primitivo Red Wines from Salento. Foods 2019, 8, 45, 10.3390/foods8020045.
- Lampíř, L.; Pavloušek, P.; Influence of locality on content of phenolic compounds in white wines. Czech Journal of Food Sciences 2013, 31, 619-626, 10.17221/337/2013-cjfs.
- Vitrac, X.; Bornet, A.; Vanderlinde, R.; Valls, J.; Richard, T.; Delaunay, J. C.; Mérillon, J. M.; Teissédre, P. L.; Determination of Stilbenes (δ-viniferin,trans-astringin,trans-piceid,cis- andtrans-resveratrol, ε-viniferin) in Brazilian Wines. Journal of Agricultural and Food Chemistry 2005, 53, 5664-5669, 10.1021/jf050122g.
- Pezet, R.; Perret, C.; Jean-Denis, J. B.; Tabacchi, R.; Gindro, K.; Viret, O.; δ-Viniferin, a Resveratrol Dehydrodimer: One of the Major Stilbenes Synthesized by Stressed Grapevine Leaves. Journal of Agricultural and Food Chemistry 2003, 51, 5488-5492, 10.1021/jf030227o.
- Tempere, S.; Cuzange, E.; Schaaper, M. H.; De Lescar, R.; De Revel, G.; Sicard, G.; “Brett character” in wine: Is there a consensus among professional assessors? A perceptual and conceptual approach. Food Quality and Preference 2014, 34, 29-36, 10.1016/j.foodqual.2013.12.007.
- Cibrario, A.; Sertier, C. M.; Riquier, L.; De Revel, G.; Masneuf-Pomarède, I.; Ballestra, P.; Dols-Lafargue, M.; Cellar Temperature Affects Brettanomyces bruxellensis Population and Volatile Phenols Production in Aging Bordeaux Wines. American Journal of Enology and Viticulture 2019, 71, 1-9, 10.5344/ajev.2019.19029.
- Hornedo-Ortega, R.; González-Centeno, M. R.; Chira, K.; Jourdes, M.; Teissedre, P. L.; Phenolic Compounds of Grapes and Wines: Key Compounds and Implications in Sensory Perception. Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging 2020, June 30, 1, 10.5772/intechopen.93127.
- Fernández De Simón, B.; Cadahía, E.; Del Álamo, M.; Nevares, I.; Effect of size, seasoning and toasting in the volatile compounds in toasted oak wood and in a red wine treated with them. Analytica Chimica Acta 2010, 660, 211-220, 10.1016/j.aca.2009.09.031.