
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Inês Serrenho | + 1727 word(s) | 1727 | 2021-04-06 09:52:29 | | | |
| 2 | Vicky Zhou | -1 word(s) | 1726 | 2021-04-12 04:42:45 | | |
Video Upload Options
Neonatal hypoxic-ischemic encephalopathy (HIE) is an important cause of mortality and morbidity in the perinatal period. This condition results from a period of ischemia and hypoxia to the brain of neonates, leading to several disorders that profoundly affect the daily life of patients and their families. Currently, therapeutic hypothermia (TH) is the standard of care in developing countries; however, TH is not always effective, especially in severe cases of HIE. Addressing this concern, several preclinical studies assessed the potential of stem cell therapy (SCT) for HIE.
1. Introduction
Hypoxic-ischemic encephalopathy (HIE) is one of the major causes of neonatal death and long-term disability, leading to chronic motor and cognitive impairments [1]. Several disorders are associated with HIE, namely epilepsy, cerebral palsy [2], attention deficit hyperactivity disorder, seizures, hearing and vision loss, language disorders, and cognitive delay. The different outcomes of this condition can be severe, profoundly affecting patients and their families daily lives. This condition also represents a significant economic burden to the government and caretakers [3].
About a quarter of neonatal deaths worldwide occur due to perinatal asphyxia [4]. The estimated incidence of HIE is variable across studies, ranging from 1 to 8 cases per 1000 live births [5]. In developed countries, neonatal HIE incidence is approximately 0.5 to 1 case per 1000 live births [6]; however, the global estimate is highly influenced by the higher incidence found in developing countries [7]. Infants diagnosed with HIE have a reserved prognosis since the HIE mortality rate is about 25%, and 20% of the survivors develop moderate to severe long-term impairment [7].
Neonatal HIE is originated from an insult that involves a period of reduced blood flow and oxygen delivery to the brain of neonates—ischemia and hypoxia, respectively. This hypoxic-ischemic (HI) event can occur due to placental abruption, uterine rupture, and cord prolapse, among others [5]. Research shows that this type of injury comprises different stages: energy depletion, inflammation, excitotoxicity, oxidative stress, and apoptosis [8]. Months after the HI insult, alterations caused by this injury are still occurring, namely late cell death, remodeling of the injured brain, astrogliosis, as well as epigenetic changes [1]. Magnetic resonance imaging studies of term newborns diagnosed with HIE revealed characteristic patterns of brain injury that can relate to mechanisms, severity, and duration of the HI insult, such as the parasagittal cerebral injury pattern or watershed injury, involving cortical gray matter and subcortical white matter, which can be related with cerebral hypoperfusion and low sustained systemic blood pressure. The selective neuronal necrosis pattern either involving basal ganglia and brain stem in severe acute events or in a diffuse global injury when severe but also prolonged HI events occur [9].
Due to poor antioxidant defenses and higher fatty acid concentrations, the developing brain is more susceptible to oxidative stress, therefore being highly vulnerable to hypoxic-ischemic insults [10]. The HI insult affects the preterm and term brain differently, originating different types of injury [11][12].
One of the most widely used animal models for the assessment of hypoxic-ischemic brain damage in the neonatal brain is the Rice–Vannucci (RV) murine model [1][13]. The RV model was first described in postnatal day-seven (P7) rats, and the protocol consists of the unilateral permanent occlusion of the common carotid artery (CCA)—ischemia—followed by exposure to a variable period of reduced oxygen levels (usually 8%)—hypoxia. The degree of brain damage severity is highly dependent on both the duration of the hypoxic period [14] and the arteries occluded, since the ligation of both the common and external carotid arteries, instead of only CCA ligation, results in a more significant and more consistent brain lesion [15]. Concerning the rodent age when the HI insult is inflicted, the majority of the studies use P7 rodents, which present a brain development equivalent to the human fetus at 32–34 weeks of gestation, while other use P10 rodents, which present a brain development equivalent to the human newborn at term [16].
Besides supportive intensive care, therapeutic hypothermia (TH) is currently the standard of care in developing countries for neonates presenting HIE. Therapeutic hypothermia consists of internal body temperature cooling to 33.5 °C for 72 h, beginning in the first six h after birth [17]. The hypothermic treatment timing seems critical since neonates that underwent TH up to 3 h after birth presented better outcomes [18].
According to a meta-analysis performed by Jacob et al. (2013) [6], TH reduces the absolute risk of mortality or neurodevelopmental disability in children with 18 to 24 months of age diagnosed with HIE at term by 15%. However, in newborns diagnosed with severe HIE, TH does not improve the significant neurodevelopmental disabilities and neuromotor delays. Therefore, it is essential to uncover therapies that will enhance the outcome of newborns diagnosed with HIE, including those classified as severe.
2. Stem Cell Therapy for Hypoxic-ischemic Encephalopathy
Stem cell therapy (SCT) has been investigated as a novel therapy for HIE. The human body has several sources of multipotent stem cells, such as bone marrow, adipose tissue, and skin. Moreover, the umbilical cord blood (UCB) and umbilical cord tissue (UCT) are excellent sources of stem cells, such as hematopoietic stem cells (HSCs) and mesenchymal stem/stromal cells (MSCs), and other cells such as endothelial progenitor cells (EPCs) [19][20]. Stem cells isolated from the UCB and UCT show a great potential to be used in the context of HIE. Indeed, Cotten et al. (2014) and Tsuji et al. (2020) demonstrated that the collection, preparation, and intravenous infusion of a non-cryopreserved mononuclear fraction of cord blood cells is safe and feasible within the first postnatal days of newborns diagnosed with HIE [21][22].
In the last ten years, several studies focused on assessing the potential of SCT in preclinical models of HIE. These studies show that stem cell therapy could have positive effects after a hypoxic-ischemic insult in the perinatal period, with several positive outcomes identified: improved functional outcome, increased angiogenesis, increased neurotrophic and growth factors levels, and cell proliferation; reduction in the extension of brain damage, translated in decreased apoptosis; decreased microglial activation and/or astrogliosis, and neuroinflammation [23](Figure 1).
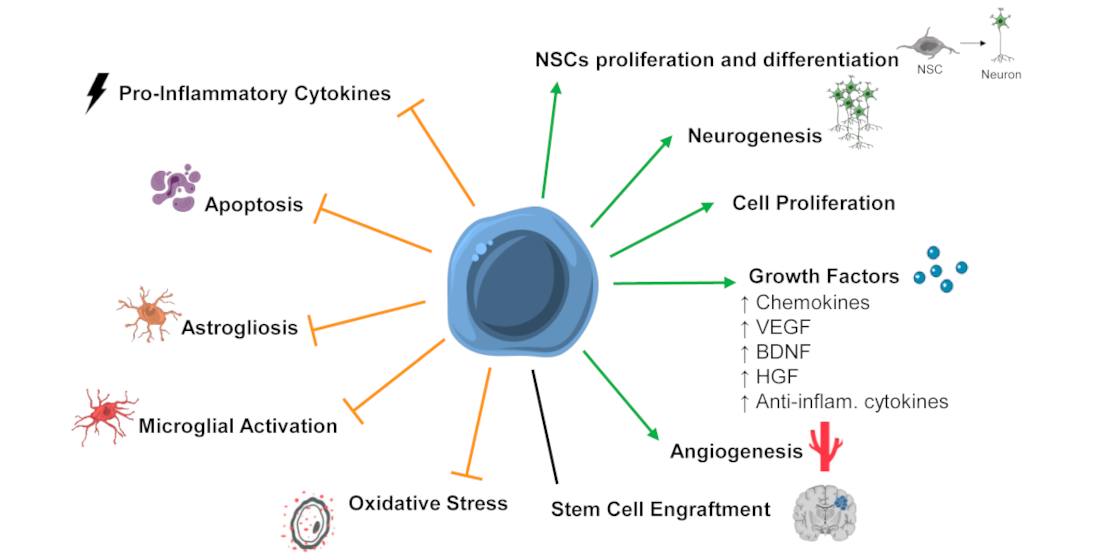
Figure 1. Mechanisms of stem cells' action that might be mediating the positive functional outcomes observed after SCT in preclinical models of neonatal hypoxic-ischemic encephalopathy (HIE). Stem cell therapy (SCT) was associated with the promotion or upregulation (green arrows) of neuronal stem cells (NSCs) proliferation and differentiation, neurogenesis, cell proliferation, growth factors levels/secretion, angiogenesis, and inhibition or downregulation (orange truncated arrows) of pro-inflammatory cytokines, apoptosis, astrogliosis, microglial activation, and oxidative stress. Also, some studies report stem cell engraftment after SCT, while other report low or no engraftment. Abbreviations: Anti-inflam—anti-inflammatory; BDNF—brain-derived neurotrophic factor; HGF—hepatocyte growth factor; VEGF—vascular endothelial growth factor.
3. Therapeutic Hypothermia and Stem Cell Therapy
Although TH is the current standard of care for HIE in term neonates in developing countries, it is not entirely effective in preventing mortality or neurodevelopmental disabilities in HIE patients, especially those diagnosed with severe HIE[6]. Therefore, it is crucial to find safe and effective therapies that will enhance TH’s neuroprotective effects and improve these patients' outcomes.
To our knowledge, few preclinical studies assessed the potential of combining TH with SCT [24][25][26] to treat severe HIE. These studies present some contradictory results. Two studies revealed that hypothermia alone did not improve the animals’ functional outcome following severe HIE [24][25]; however, hypothermia and MSCs infusion two days after insult had a positive effect, improving the animal’s functional outcome while decreasing brain damage, cytokine levels, microgliosis, and astrogliosis [24][25]. Interestingly, both studies reported that combined therapy was more effective than MSC administration alone [24][25].
In contrast, a study performed by Herz et al. (2018) showed that animals treated either with TH or MSCs had a better outcome than animals treated with the combined therapy of TH followed by MSC administration three days post insult [26]. This study revealed that, after HI insult in the neonatal period, only the MSC treatment improved cognitive function and decreased white matter injury, and MSC or TH treatment improved motor function. However, the combined therapy, TH followed later by MSC administration, reversed the protective effects observed with each therapy alone, resulting in increased, long-lasting functional deficits, brain damage, endothelial cells infiltration, peripheral immune cell infiltration, and pro-inflammatory cytokine levels, as well as decreased levels of growth factor expression. One potential mechanism pointed out by the authors is an alteration of the cerebral microenvironment after TH, resulting in modifying the MSCs phenotype after their administration. This alteration may induce pro-inflammatory cytokine expression and block the expression of growth factors, thus interfering with the rescuing of the injured brain.
4. Conclusions and Future Perspectives
The positive effects reported included improved functional outcome, cognitive and motor function, decreased brain damage, translated by a decrease in apoptotic cells and prevention of neuronal loss, microglial activation, astrogliosis, inflammation, and increased angiogenesis and cell proliferation, among others. Thus, stem cell therapy appears to have significant therapeutic potential and could become a new therapy for HIE. Nonetheless, there is a high variability regarding the SCT protocol used, namely the dose of stem cells applied, route, and administration timing. Therefore, it would be critical to perform studies assessing different amounts of stem cells, considering the clinical setting, and determining the optimal time for stem cell administration (e.g., if during the secondary or tertiary phase of the injury) to increase the chance of successful translating stem cell therapy into the clinical practice.
A new possible therapeutic combination would be adding SCT to the current standard of care for HIE, TH, thus improving the effectiveness of TH in treating infants diagnosed with HIE, especially those diagnosed with severe HIE. However, the lack of studies addressing the effect of combining TH with SCT in HIE and the existing heterogeneity in the few studies that were performed until today stresses the importance of exploring this research line.
In conclusion, there is increasing evidence in the literature that SCT could, in combination with TH, be the next standard of care for HIE patients, addressing the lack of effectiveness of therapeutic hypothermia. Infusion of human umbilical cord blood cells was already demonstrated to be safe and feasible in newborns diagnosed with HIE. However, it is still necessary to optimize the protocol for SCT, namely determining the optimal dose, route of administration, and timing, as well as assessing which stem cell types provide the maximal neuroprotection. This is where translational research and animal models become extremely useful, allowing them to explore multiple therapeutic interventions and unravel which ones have the potential to be applied in the clinic.
References
- Millar, L.J.; Shi, L.; Hoerder-Suabedissen, A.; Molnar, Z. Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges. Front. Cell Neurosci. 2017, 11, 78.
- Zhang, S.; Li, B.; Zhang, X.; Zhu, C.; Wang, X. Birth Asphyxia Is Associated With Increased Risk of Cerebral Palsy: A Meta-Analysis. Front. Neurol. 2020, 11, 704.
- Honeycutt, A.A.; Grosse, S.D.; Dunlap, L.J.; Schendel, D.E.; Chen, H.; Brann, E.; Homsi, G.a. Economic Costs of Mental Retardation, Cerebral Palsy, Hearing Loss, and Vision Impairment. In Using Survey Data to Study Disability: Results from the National Health Survey on Disability (USA); Emerald Group Publishing Limited: Bingley, UK, 2003; pp. 207–228.
- Lawn, J.E.; Cousens, S.; Zupan, J.; Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: When? Where? Why? Lancet 2005, 365, 891–900.
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338.
- Jacobs, S.E.; Berg, M.; Hunt, R.; Tarnow-Mordi, W.O.; Inder, T.E.; Davis, P.G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, 1, CD003311.
- Lee, A.C.; Kozuki, N.; Blencowe, H.; Vos, T.; Bahalim, A.; Darmstadt, G.L.; Niermeyer, S.; Ellis, M.; Robertson, N.J.; Cousens, S.; et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 2013, 74, 50–72.
- Nabetani, M.; Shintaku, H.; Hamazaki, T. Future perspectives of cell therapy for neonatal hypoxic-ischemic encephalopathy. Pediatr. Res. 2018, 83, 356–363.
- Volpe, J.J.; Kinney, H.C. Volpe’s Neurology of the Newborn, 6th ed.; Elsevier: Philadelphia, PA, USA, 2018; Unit IV, Chapter 20; 1224p.
- Ikonomidou, C.; Kaindl, A.M. Neuronal death and oxidative stress in the developing brain. Antioxid. Redox. Signal. 2011, 14, 1535–1550.
- Patel, S.D.; Pierce, L.; Ciardiello, A.J.; Vannucci, S.J. Neonatal encephalopathy: Pre-clinical studies in neuroprotection. Biochem. Soc. Trans. 2014, 42, 564–568.
- Miller, S.P.; Ferriero, D.M. From selective vulnerability to connectivity: Insights from newborn brain imaging. Trends Neurosci. 2009, 32, 496–505.
- Rice, J.E., 3rd; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141.
- Vannucci, R.C.; Christensen, M.A.; Yager, J.Y. Nature, time-course, and extent of cerebral edema in perinatal hypoxic-ischemic brain damage. Pediatr. Neurol. 1993, 9, 29–34.
- Edwards, A.B.; Feindel, K.W.; Cross, J.L.; Anderton, R.S.; Clark, V.W.; Knuckey, N.W.; Meloni, B.P. Modification to the Rice-Vannucci perinatal hypoxic-ischaemic encephalopathy model in the P7 rat improves the reliability of cerebral infarct development after 48hours. J. Neurosci. Methods 2017, 288, 62–71.
- Huang, L.; Zhao, F.; Qu, Y.; Zhang, L.; Wang, Y.; Mu, D. Animal models of hypoxic-ischemic encephalopathy: Optimal choices for the best outcomes. Rev. Neurosci. 2017, 28, 31–43.
- Joanne O. Davidson; Guido Ewassink; Lotte G. Van Den Heuij; Laura Ebennet; Alistair Jan Gunn; Therapeutic Hypothermia for Neonatal Hypoxic–Ischemic Encephalopathy – Where to from Here?. Frontiers in Neurology 2015, 6, 198, 10.3389/fneur.2015.00198.
- Marianne Thoresen; James Tooley; Xun Liu; Sally Jary; Peter Fleming; Karen Luyt; Anoopam Jain; Pamela Cairns; David Harding; Hemmen Sabir; et al. Time Is Brain: Starting Therapeutic Hypothermia within Three Hours after Birth Improves Motor Outcome in Asphyxiated Newborns. Neonatology 2013, 104, 228-233, 10.1159/000353948.
- Mark L. Weiss; Deryl L. Troyer; Stem cells in the umbilical cord. Stem Cell Reviews and Reports 2006, 2, 155-162, 10.1007/s12015-006-0022-y.
- Pham Van Phuc; Vu Bich Ngoc; Dang Hoang Lam; Nguyen Thanh Tam; Pham Quoc Viet; Phan Kim Ngoc; Isolation of three important types of stem cells from the same samples of banked umbilical cord blood. Cell and Tissue Banking 2011, 13, 341-351, 10.1007/s10561-011-9262-4.
- C. Michael Cotten; Amy P. Murtha; Ronald N. Goldberg; Chad A. Grotegut; P. Brian Smith; Ricki F. Goldstein; Kimberley A. Fisher; Kathryn E. Gustafson; Barbara Waters-Pick; Geeta K. Swamy; et al.Benjamin RattraySiddhartha TanJoanne Kurtzberg Feasibility of Autologous Cord Blood Cells for Infants with Hypoxic-Ischemic Encephalopathy. The Journal of Pediatrics 2014, 164, 973-979.e1, 10.1016/j.jpeds.2013.11.036.
- Masahiro Tsuji; Mariko Sawada; Shinichi Watabe; Hiroyuki Sano; Masayo Kanai; Emi Tanaka; Satoshi Ohnishi; Yoshiaki Sato; Hisanori Sobajima; Takashi Hamazaki; et al.Rintaro MoriAkira OkaHiroyuki IchibaMasahiro HayakawaSatoshi KusudaMasanori TamuraMakoto NabetaniHaruo Shintaku Autologous cord blood cell therapy for neonatal hypoxic-ischaemic encephalopathy: a pilot study for feasibility and safety. Scientific Reports 2020, 10, 1-10, 10.1038/s41598-020-61311-9.
- Inês Serrenho; Miguel Rosado; Alexandra Dinis; Carla M. Cardoso; Mário Grãos; Bruno Manadas; Graça Baltazar; Stem Cell Therapy for Neonatal Hypoxic-Ischemic Encephalopathy: A Systematic Review of Preclinical Studies. International Journal of Molecular Sciences 2021, 22, 3142, 10.3390/ijms22063142.
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Park, W.S. Hypothermia broadens the therapeutic time window of mesenchymal stem cell transplantation for severe neonatal hypoxic ischemic encephalopathy. Sci. Rep. 2018, 8, 7665.
- Park, W.S.; Sung, S.I.; Ahn, S.Y.; Yoo, H.S.; Sung, D.K.; Im, G.H.; Choi, S.J.; Chang, Y.S. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS ONE 2015, 10, e0120893.
- Herz, J.; Koster, C.; Reinboth, B.S.; Dzietko, M.; Hansen, W.; Sabir, H.; van Velthoven, C.; Bendix, I.; Felderhoff-Muser, U. Interaction between hypothermia and delayed mesenchymal stem cell therapy in neonatal hypoxic-ischemic brain injury. Brain Behav. Immun. 2018, 70, 118–130.
- Masahiro Tsuji; Mariko Sawada; Shinichi Watabe; Hiroyuki Sano; Masayo Kanai; Emi Tanaka; Satoshi Ohnishi; Yoshiaki Sato; Hisanori Sobajima; Takashi Hamazaki; et al.Rintaro MoriAkira OkaHiroyuki IchibaMasahiro HayakawaSatoshi KusudaMasanori TamuraMakoto NabetaniHaruo Shintaku Autologous cord blood cell therapy for neonatal hypoxic-ischaemic encephalopathy: a pilot study for feasibility and safety. Scientific Reports 2020, 10, 1-10, 10.1038/s41598-020-61311-9.
- Inês Serrenho; Miguel Rosado; Alexandra Dinis; Carla M. Cardoso; Mário Grãos; Bruno Manadas; Graça Baltazar; Stem Cell Therapy for Neonatal Hypoxic-Ischemic Encephalopathy: A Systematic Review of Preclinical Studies. International Journal of Molecular Sciences 2021, 22, 3142, 10.3390/ijms22063142.




