
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michalis Konsolakis | + 1709 word(s) | 1709 | 2021-04-08 10:53:58 | | | |
| 2 | Michalis Konsolakis | + 13 word(s) | 1722 | 2021-04-08 19:52:12 | | | | |
| 3 | Michalis Konsolakis | + 12 word(s) | 1721 | 2021-04-08 20:02:19 | | |
Video Upload Options
The rational design and fabrication of highly-active and cost-efficient catalytic materials constitutes the main research pillar in catalysis field. In this context, the fine-tuning of size and shape at nanometer scale can exert an intense impact not only on the inherent reactivity of catalyst’s counterparts but also on their interfacial interactions, opening up new horizons for the development of highly active and robust materials. The main implications of ceria nanoparticles’ shape engineering (rods, cubes, polyhedra) in catalysis are revealed, on the ground of some of the most pertinent heterogeneous reactions, such as CO2 hydrogenation, CO oxidation, and N2O decomposition. It is clearly revealed that shape functionalization can remarkably affect the intrinsic features and in turn the reactivity of ceria nanoparticles. More importantly, by combining ceria nanoparticles (CeO2 NPs) of specific architecture with various transition metals (e.g., Cu, Fe, Co, Ni) remarkably active multifunctional composites can be obtained due mainly to the synergistic metal-ceria interactions, providing the design principles of earth-abundant metal oxide catalysts for various real-life environmental and energy applications.
1. Background
Catalysis has gained ongoing attention from academic and industrial community, since it is massively involved in numerous energy and environmental processes, including, among others, the manufacture of value-added fuels and chemicals, hydrocarbons processing, fuel cell applications, photocatalytic degradation, abatement of hazardous substances, etc. To this end, the development of cost-efficient catalysts with improved activity and durability constitutes the main research pillar in the field of catalysis [1][2][3].
Although, noble metals (NMs) hold an integral role in catalysis for chemical bond activation and formation, their scarcity and high cost constitutes a major and inherent drawback. In this regard, the development of catalysts by combining the low cost with the high activity is a major driving force in contemporary research [1][2][3][4]. Metal oxides, composed of earth-abundant transition metals (TMs) have gained particular attention towards replacing the rare and costly NMs, owing to their unique characteristics, such as improved redox properties and thermal stability [1][2][5]. More importantly, through admixing different metal oxides (MOs) in an exact proportion, mixed metal oxides (MMOs) can be obtained with unique physicochemical properties, which are mainly linked to interfacial phenomena. Moreover, several reducible oxides (e.g., CeO2, ZnO, TiO2), could serve as TMs supporting carriers, exerting a beneficial impact on the inherent activity through peculiar metal-support interactions [6][7][8][9].
Among the different MOs, cerium oxide or ceria has gained tremendous interest in heterogeneous catalysis due to its exceptional redox properties driven by the high oxygen mobility and the rapid redox interplay between Ce3+ and Ce4+ [1][10][11]. In addition to these physicochemical advantages of cerium oxide, its average 2020 price stands at very low levels (ca. 1815 USD/metric ton), with a relative cost trend of ZrO2 > ZnO > SiO2 > TiO2 > CeO2, revealing the economic benefits of ceria-based catalytic materials [12]. Furthermore, the cost of base middle-late 3d metals, such as Cu, Ni, is about 3–4 orders of magnitude lower as compared to NMs, rendering extremely attractive, from a financial perspective, the development of ceria-based transition metal catalysts. More importantly, the combination of reducible oxides (e.g., CeO2) with TMs (e.g., Fe, Co, Ni, Cu) could offer novel catalyst formulations with exceptional properties, arising mainly from the multifaceted electronic and geometric interactions amongst the different counterparts [6][7][8][13][14]. In this respect, it was recently appraised on account of both experimental and theoretical studies that various interconnected phenomena could be at work at metal-support interface, which in turn exert a profound influence on the catalytic activity, such as [6][7][8][13][14]:
- electronic perturbations linked to bonding interactions between TMs and Ceria nanoparticles
- facilitation of oxygen vacancies’ formation resulting in enhanced reducibility and oxygen exchange kinetics
- high intrinsic activity of interfacial sites
However, it is well known today—thanks to the huge progress in cutting-edge characterization techniques—that the individual characteristics of catalyst’s counterparts can notably affect not only their own activity but also the interaction between them with great consequences in catalysis. In specific, by adjusting the geometrical and electronic features of the different counterparts through suitable synthetic and promotional routes highly active materials are obtained [7][10][15][16].
2. Synthesis and Characterization of CeO2 NPs of Different Morphology
As shown in Figure 1, the hydrothermal method was successfully used in order to prepare three distinct ceria morphologies of well-defined shape, i.e., nanocubes (NC), nanorods (NR) and nanopolyhedra (NP), which are then employed as supporting carriers for various transition metal catalysts (Fe, Co, Ni, Cu) through the wet impregnation method (Figure 1).
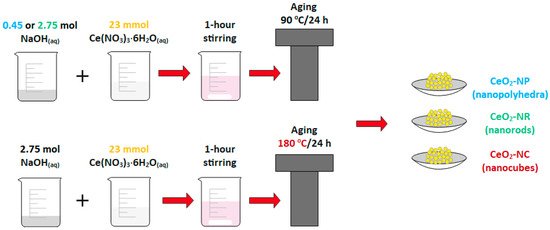
Figure 1. Schematic illustration of synthetic procedure of ceria nanoparticles of different shape (nanocubes = NC, nanorods = NR, and nanopolyhedra = NP).
3. Ceria Shape Effects on the Structural Defects and Redox Properties
Figure 2 shows indicative TEM images of ceria nanorods (NR), nanopolyhedra (NP), and nanocubes (NC). The CeO2-NR sample (Figure 2a,d) shows a rod-like morphology with a length from 25 to 200 nm. In the case of CeO2-NC (Figure 2c,f), a well-defined cube-like morphology is obtained (of about 20–30 nm), whereas nanopolyhedra of irregular size/shape are observed for CeO2-NP (Figure 2b,e). Among the different ceria nanostructures, ceria nanocubes exhibit the lowest BET surface area, along with the highest mean particle size: NC (40 m2/g) < NR (92 m2/g) < NP (109 m2/g).
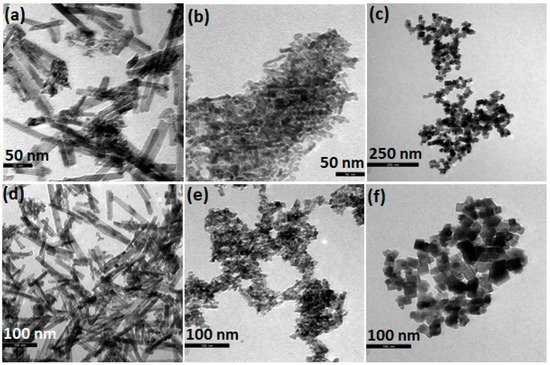
Figure 2. Indicative transmission electron microscopy (TEM) images of ceria (a,d) nanorods, (b,e) nanopolyhedra, and (c,f) nanocubes.
The research showed that the rod-shaped ceria nanoparticles possess the highest population of weakly bound labile oxygen species, which coincides with the oxygen storage capacity (OSC): NC (0.21 mmol/g) < NP (0.24 mmol/g) < NR (0.29 mmol/g). Hence, on the basis of the aforementioned results, it can be argued that, although ceria nanorods do not display the optimum textural properties (e.g., surface area), they possess the optimum redox characteristics in terms of population and facile reduction of loosely bound oxygen species, which is of paramount importance in several reaction processes obeying a redox mechanism.
4. Implication in Catalysis
The effect of ceria nanoparticles geometry on the catalytic behaviour of TMs/CeO2 oxides is demonstrated on the grounds of indicative oxidation/reduction reactions, such as CO oxidation, nitrous oxide (N2O) decomposition and CO2 hydrogenation to value-added products [17][18][19][20][21].
Figure 3 depicts the CO oxidation performance of the different bare ceria nanoshapes (NR, NC, NP) in comparison to that of transition metal-based catalysts, i.e., Fe/CeO2 and Cu/CeO2 samples. Regarding, at first, the CO conversion of bare ceria, it is evident that significant differences are obtained between the samples, demonstrating the key role of morphology. On the basis of T50, i.e., the required temperature for 50% conversion, the following order is observed for bare ceria: NR (320 °C) < NP (350 °C) < NC (385 °C). It is noteworthy that the conversion order of bare ceria samples totally coincides with their relative concentration in labile oxygen species (expressed by OSC) and oxygen vacancies (expressed by the ratio of ID/IF2g). The latter clearly reveals the major role of ceria shape towards determining the redox/structural properties and in consequence the catalytic behaviour [17][18]. Most significantly, the incorporation of transition metals (Fe, Cu) to ceria drastically improves the catalytic performance, without affecting, however, the activity order obtained for single CeO2 samples. This clearly implies the guided role of support morphology on the catalytic performance of ceria-based transition metal catalysts. Apparently, the rod-shaped samples exhibit the optimum catalytic efficiency, demonstrating complete CO conversion at 250 °C and 100 °C for Fe/CeO2 and Cu/CeO2 samples, respectively.
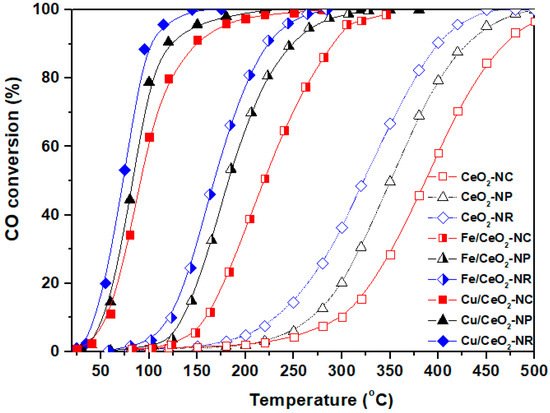
Figure 3. Ceria nanoparticles morphological effects (nanorods = NR, nanocubes = NC, nanopolyhedra = NP) on the CO oxidation performance of bare CeO2, Cu/CeO2, and Fe/CeO2 samples.
The superior reactivity of samples with rod-like shape was further verified by a kinetic study under differential reaction conditions, which revealed the following order in relation to apparent activation energy (Ea): CeO2-NR (44.2 kJ/mol) < CeO2-NP (46.7 kJ/mol) < CeO2-NC (49.8 kJ/mol) [18]. A similar trend was acquired for TMs/Ceria samples, indicating once again the pivotal role of ceria morphology towards determining the activity of ceria-based transition metal samples [18].
Perfect relationships between the reaction rate (rco, mmol s−1 g−1) and the following activity descriptors were disclosed: (i) population of oxygen vacancies expressed by ID/IF2g ratio, (ii) oxygen storage capacity (OSC), as shown in Figure 4. Similar relationships have been obtained for Cu- and Fe-based samples, revealing the pivotal role of support morphology [17][18].
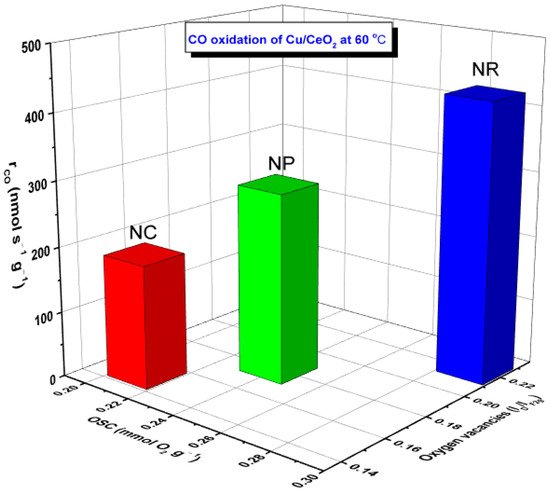
Figure 4. Relationship between the CO oxidation performance (rCO, nmol s−1 g−1) of Cu/CeO2 samples and the oxygen storage capacity (OSC, mmol O2 g−1) as well as the abundance of oxygen vacancies (in terms of ID/IF2g ratio) of ceria nanorods (NR), nanopolyhedra (NP) and nanocubes (NC).
Figure 5a,b demonstrates the conversion of CO2 as well as the selectivity to methane, respectively, for bare CeO2-NR samples as well as for TMs (Fe, Co, Ni, Cu)/CeO2-NR. It is obvious that there are significant differences between the samples, mostly depending on metal’s nature. Regarding CO2 conversion, the following order is obtained: CeO2 < Fe/CeO2 < Cu/CeO2 < Co/CeO2 < Ni/CeO2, signifying the pivotal role of metal-support combination. Ni/CeO2-NR exhibits by far the optimum performance, offering ca. 92% conversion at 300 °C. In other words, by combining Ni with ceria nanorods extremely active CO2 methanation catalysts can be obtained, which practically reach the equilibrium at very low temperatures of ca. 300 °C. More importantly, the high conversion performance of Ni catalysts is followed by 100% selectivity to CH4 (Figure 5b) in the entire temperature range studied. Interestingly, Cu/CeO2-NR catalyst is totally selective to CO (rWGS reaction), reaching the equilibrium at temperatures higher than 400 °C. Hence, the combination of Ni with CeO2-NR favours the CO2 methanation reaction, whereas the rWGS reaction is favoured over Cu/CeO2-NR.
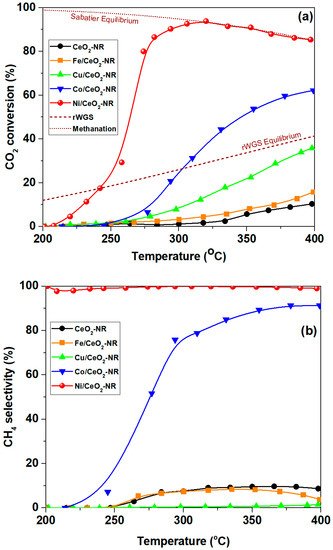
Figure 5. CO2 conversion (a) and selectivity to CH4 (b) for bare CeO2-NR and various metal-based catalysts (Fe, Cu, Co, Ni). Reaction conditions: Weight Hourly Space Velocity (WHSV) = 30,000 mL g−1 h−1, H2:CO2 = 4:1, P = 1 atm [21]. Dotted lines represent the corresponding equilibrium for rWGS and Sabatier reactions.
From the practical point of view, novel catalyst formulations with similar or even superior reactivity to that of noble metals can be obtained by co-adjusting the shape and composition of mixed oxides, such as Cu/Ceria nanorods for CO oxidation and Ni/Ceria nanorods for CO2 hydrogenation.
References
- M. Melchionna; P. Fornasiero; The role of ceria-based nanostructured materials in energy applications. Materials Today 2014, 17, 349-357, 10.1016/j.mattod.2014.05.005.
- Tiziano Montini; Michele Melchionna; Matteo Monai; Paolo Fornasiero; Fundamentals and Catalytic Applications of CeO2-Based Materials. Chemical Reviews 2016, 116, 5987-6041, 10.1021/acs.chemrev.5b00603.
- José A. Rodriguez; Ping Liu; Jesús Graciani; Sanjaya D. Senanayake; David C. Grinter; Dario Stacchiola; Jan Hrbek; Javier Fernández-Sanz; Inverse Oxide/Metal Catalysts in Fundamental Studies and Practical Applications: A Perspective of Recent Developments. The Journal of Physical Chemistry Letters 2016, 7, 2627-2639, 10.1021/acs.jpclett.6b00499.
- Weiting Yang; Xiao Wang; Shuyan Song; Hongjie Zhang; Syntheses and Applications of Noble-Metal-free CeO2-Based Mixed-Oxide Nanocatalysts. Chem 2019, 5, 1743-1774, 10.1016/j.chempr.2019.04.009.
- Yarong Fang; Yanbing Guo; Copper-based non-precious metal heterogeneous catalysts for environmental remediation. Chinese Journal of Catalysis 2018, 39, 566-582, 10.1016/s1872-2067(17)62996-6.
- Michalis Konsolakis; The role of Copper–Ceria interactions in catalysis science: Recent theoretical and experimental advances. Applied Catalysis B: Environmental 2016, 198, 49-66, 10.1016/j.apcatb.2016.05.037.
- Matteo Cargnello; Vicky V. T. Doan-Nguyen; Thomas R. Gordon; Rosa E. Diaz; Eric A. Stach; Raymond J. Gorte; Paolo Fornasiero; Christopher B. Murray; Control of Metal Nanocrystal Size Reveals Metal-Support Interface Role for Ceria Catalysts. Science 2013, 341, 771-773, 10.1126/science.1240148.
- Gianfranco Pacchioni; Electronic interactions and charge transfers of metal atoms and clusters on oxide surfaces. Physical Chemistry Chemical Physics 2013, 15, 1737-1757, 10.1039/c2cp43731g.
- Marçal Capdevila-Cortada; Gianvito Vilé; Detre Teschner; Javier Pérez-Ramírez; Núria López; Reactivity descriptors for ceria in catalysis. Applied Catalysis B: Environmental 2016, 197, 299-312, 10.1016/j.apcatb.2016.02.035.
- Ke Wu; Ling-Dong Sun; Chun-Hua Yan; Recent Progress in Well-Controlled Synthesis of Ceria-Based Nanocatalysts towards Enhanced Catalytic Performance. Advanced Energy Materials 2016, 6, 1600501, 10.1002/aenm.201600501.
- José A. Rodriguez; David C. Grinter; Zongyuan Liu; Robert M. Palomino; Sanjaya D. Senanayake; Ceria-based model catalysts: fundamental studies on the importance of the metal–ceria interface in CO oxidation, the water–gas shift, CO2 hydrogenation, and methane and alcohol reforming. Chemical Society Reviews 2017, 46, 1824-1841, 10.1039/c6cs00863a.
- Kourosh Razmgar; Mohammednoor Altarawneh; Ibukun Oluwoye; Gamini Senanayake; Ceria-Based Catalysts for Selective Hydrogenation Reactions: A Critical Review. Catalysis Surveys from Asia 2021, 25, 27-47, 10.1007/s10563-020-09319-z.
- M. Ahmadi; H. Mistry; B. Roldan Cuenya; Tailoring the Catalytic Properties of Metal Nanoparticles via Support Interactions. The Journal of Physical Chemistry Letters 2016, 7, 3519-3533, 10.1021/acs.jpclett.6b01198.
- Eric D. Hermes; Glen R. Jenness; J.R. Schmidt; Decoupling the electronic, geometric and interfacial contributions to support effects in heterogeneous catalysis. Molecular Simulation 2014, 41, 123-133, 10.1080/08927022.2014.926549.
- Yan Zhou; Yong Li; Wenjie Shen; Shape Engineering of Oxide Nanoparticles for Heterogeneous Catalysis. Chemistry – An Asian Journal 2016, 11, 1470-1488, 10.1002/asia.201600115.
- Michalis Konsolakis; Maria Lykaki; Recent Advances on the Rational Design of Non-Precious Metal Oxide Catalysts Exemplified by CuOx/CeO2 Binary System: Implications of Size, Shape and Electronic Effects on Intrinsic Reactivity and Metal-Support Interactions. Catalysts 2020, 10, 160, 10.3390/catal10020160.
- Maria Lykaki; Eleni Pachatouridou; Sónia A.C. Carabineiro; Eleni Iliopoulou; Chrysanthi Andriopoulou; N. Kallithrakas-Kontos; Soghomon Boghosian; Michalis Konsolakis; Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Applied Catalysis B: Environmental 2018, 230, 18-28, 10.1016/j.apcatb.2018.02.035.
- Maria Lykaki; Sofia Stefa; Sόnia A. C. Carabineiro; Pavlos K. Pandis; Vassilis N. Stathopoulos; Michalis Konsolakis; Facet-Dependent Reactivity of Fe2O3/CeO2 Nanocomposites: Effect of Ceria Morphology on CO Oxidation. Catalysts 2019, 9, 371, 10.3390/catal9040371.
- Maria Lykaki; Eleni Papista; Nikolaos Kaklidis; Sόnia A. C. Carabineiro; Michalis Konsolakis; Ceria Nanoparticles’ Morphological Effects on the N2O Decomposition Performance of Co3O4/CeO2 Mixed Oxides. Catalysts 2019, 9, 233, 10.3390/catal9030233.
- Michalis Konsolakis; Maria Lykaki; Sofia Stefa; Sόnia A. C. Carabineiro; Georgios Varvoutis; Eleni Papista; Georgios E. Marnellos; CO2 Hydrogenation over Nanoceria-Supported Transition Metal Catalysts: Role of Ceria Morphology (Nanorods versus Nanocubes) and Active Phase Nature (Co versus Cu). Nanomaterials 2019, 9, 1739, 10.3390/nano9121739.
- Georgios Varvoutis; Maria Lykaki; Sofia Stefa; Eleni Papista; Sónia A.C. Carabineiro; Georgios E. Marnellos; Michalis Konsolakis; Remarkable efficiency of Ni supported on hydrothermally synthesized CeO2 nanorods for low-temperature CO2 hydrogenation to methane. Catalysis Communications 2020, 142, 106036, 10.1016/j.catcom.2020.106036.




