
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michael Giretzlehner | + 1433 word(s) | 1433 | 2021-03-26 04:05:22 | | | |
| 2 | Karina Chen | Meta information modification | 1433 | 2021-04-12 07:40:38 | | | | |
| 3 | Karina Chen | Meta information modification | 1433 | 2021-04-15 10:09:06 | | | | |
| 4 | Michael Giretzlehner | + 509 word(s) | 1942 | 2021-05-10 18:47:16 | | |
Video Upload Options
In burn medicine, the percentage of the burned body surface area (TBSA-B) to the total body surface area (TBSA) is a crucial parameter to ensure adequate treatment and therapy. Inaccurate estimations of the burn extent can lead to wrong medical decisions resulting in considerable consequences for patients. These include, for instance, over-resuscitation, complications due to fluid aggregation from burn edema, or non-optimal distribution of patients. Due to the frequent inaccurate TBSA-B estimation in practice, objective methods allowing for precise assessments are required.
1. Introduction
An accurate assessment of both the burn depth and the burn extent is essential for adequate and successful treatment. In order to determine the burn depth, German-speaking countries differentiate between so-called burn degrees correlating with an increasing depth of a burn of the skin [1][2]:
“First-Degree” burns affect the epidermis and lead to redness and severe pain but do not cause cell death.
“Second-Degree” burns are distinguished between “second-degree superficial” (2a) and “second-degree deep” (2b). Whereas the first type involves damage to the epidermis and the superficial dermis, with blisters, a rosy and recapillarizing wound base, severe pain, and firmly anchored hair, the second type is characterized by injuries to the deep dermis and skin appendages. The wound is comparatively pale and has little or no recapillarization, and the pain receptors are partially destroyed, the pain perception of the patient is reduced, and the hairs are easy to remove.
“Third-Degree” burns lead to complete epidermal and dermal destruction and are accompanied by hair absence and a dry, white, leathery hard wound base without pain.
Historically, a “fourth-degree” burn was also distinguished. In this type of burn, charring occurs, and in addition to the epidermis and dermis, other layers are destroyed, such as the subcutaneous fatty tissue, muscles, tendons, bones, or joints.
In comparison, English-speaking countries classify the burn depth into “superficial”, “superficial partial thickness” burn (equal to second-degree superficial), “deep partial thickness” burn (equal to second-degree deep), and “full thickness” burn (equal to a third-degree or fourth-degree) [2]. Clinical evaluations are never reliable, and “the accuracy of bedside depth assessment is widely considered to be far from optimal” [3] (p. 762).
While in clinical practice, an assessment of burn depth based on these classifications tends to be difficult, a classification based on healing time is usually of higher practical value [3]. Usually, burns healing within one week are categorized as “first-degree” or “superficial” burns; those healing within two weeks are referred to as “second-degree superficial” or “superficial partial thickness” burns. If healing occurs within three weeks or more, the burns are classified as “second-degree deep” or “deep partial thickness” burns, while burns taking even longer to heal but still heal spontaneously from the skin’s appendages are classified in the same group. “Full thickness” burns do not heal from regenerative tissue in the wound but the margins [3]. An exact classification of which burn degree correlates with healing time is not given. It is challenging to differentiate between partial deep or 2b burns and full thickness or third-degree burns.
As described above, in addition to the depth of a burn injury, the burn extent is the second important criterion to be assessed to determine adequate treatment methods. The latter is defined as the percentage of the burned body surface area (TBSA-B) to the total body surface area (TBSA), whereby first-degree burns are excluded. According to different studies, an accurate determination of the extent of a burn injury often proves to be challenging in practice. In most cases, TBSA-B is overestimated [2].
2. Methods for Burn Size Estimation
Due to the frequently occurring inaccurate estimation of the burn size in practice and the associated far-reaching consequences for patients and medical resources, objective methods allowing for a more accurate burn size assessment are required. Over time, various methods have been established, whose development has been influenced by contemporary technical standards. The following is an extract of burn size estimation history and shows methods proven in practice or with high practical potential. Figure 1 shows this extract of TBSA-B determination methods as a timeline.
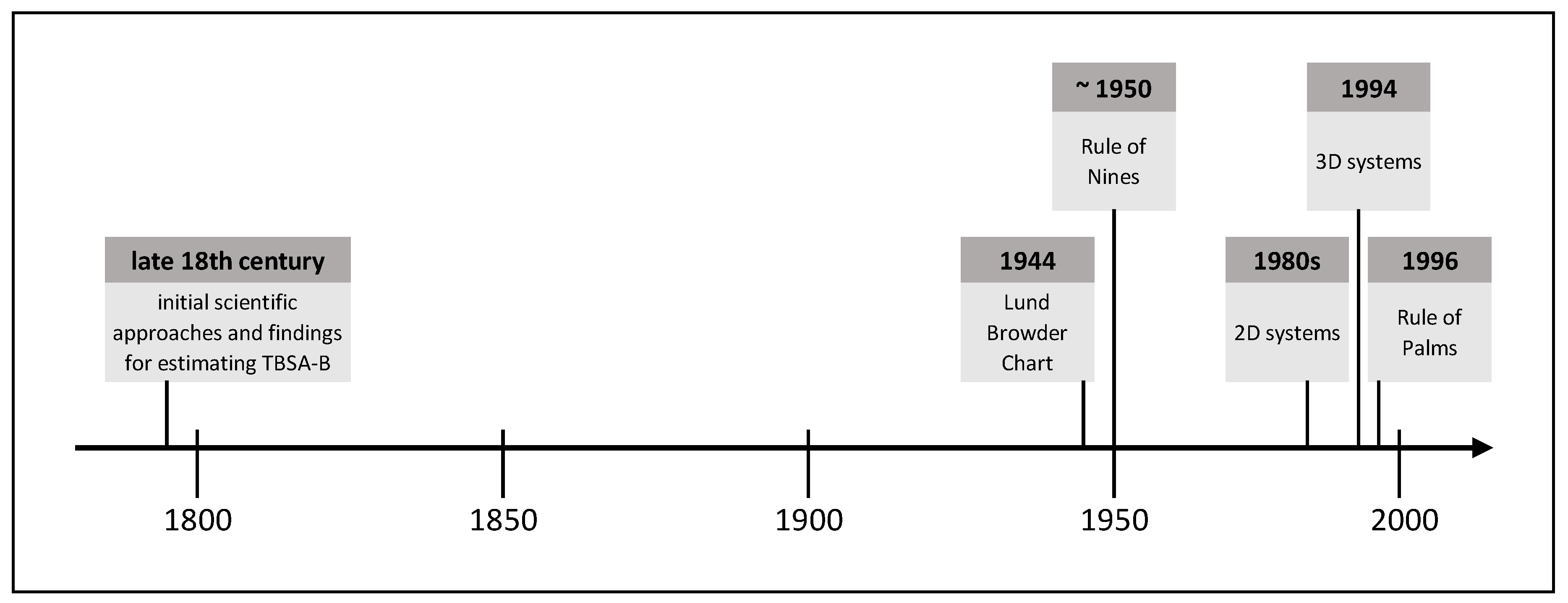
2.1. Initial Scientific Approaches and Findings for Estimating TBSA-B
Documented knowledge about the link between the severity of burns and the survival probability begun in Europe in the late 18th century with Richter’s report in the year 1788. Schjerning gave a rough description of this connection in 1884. The correlation of burn size and mortality was questioned at that time, for example, by Liman [4]. Based on Meeh’s calculations in 1879, Weidenfeld from the University of Vienna established a constant ratio of well-defined body areas to the TBSA. He defined the ratio as proportions and not yet as percentages. Besides, Weidenfeld demonstrated the correlation between burn size and time of early death [4]. Riehl confirmed these findings in 1925 [5].
Without being aware of the work by Weidenfeld, Berkow recalculated the surface area of the body parts of five people with different physiques according to Dubois and Dubois [6]. He observed a mean error rate of 15% in Meeh’s calculations and reported an average error below 5% in his owns. Further, Berkow found that children’s body proportions differ from those of adults and suggested to take this into account. He proposed to name the method of calculating burn size as a percentage of the TBSA according to Weidenfeld and himself, but this was rejected [4].
In 1942, the scientifically based burn treatment became the focus of national interest, not only due to the ongoing World War but also because of the “coconut grove nightclub disaster”. A Burn Research Service was established at Boston City Hospital, and a “National Committee for Burn and Trauma Research” begun its work. TBSA-B determination and the feasibility of a high-quality method became increasingly important. Treating burn shock on the basis of TBSA-B was suggested at the “National Research Council Conference” in 1942 [4].
2.2. Lund Browder Chart
Two years later, in 1944, C.C. Lund and N.C. Browder [7] from Harvard Medical School published the so-called “Lund Browder Chart” to improve the calculation of body proportions and to reduce errors. This chart was based on Boyd’s [8] surface area calculations. Lund and Browder defined clear boundaries of body regions and considered different proportions during human growth. Even though many authors have modified the “Lund Browder Chart” [9][10], it has remained in use in its original form until the present. The “Lund Browder Chart” illustrates the human body’s front and back in a graphic model and assigns different body proportions to different age groups. It shows the boundaries of specific body regions for which different percentages of TBSA are defined. For the calculation procedure, an adapted planimetry is used. Even though the “Lund Browder Chart” does not differentiate depending on sex, weight, height, or body shape, many authors consider it “the most accurate” method [11] (p. 58).
2.3. Rule of Nines
Continuing with the history of burn size estimation, another method still used in practice is the so-called “Rule of Nines”. In his publication in 1951, Wallace [12] assumed for different body parts the following proportions of the TBSA: arms 9% of the TBSA each, legs 18% each, chest and back 18% each, head and face 9%, neck 1%, and genital area 1%. Like the “Lund Browder Chart”, the “Rule of Nines” ignores differences in sex, weight, height, and body shapes. When using this rule, the burn extent is overestimated in many cases [13][14]. For example, Giretzlehner et al. [15] reported a mean overestimation rate of 138% using the “Rule of Nines” and the “Lund Browder Chart”.
2.4. Rule of Palms
The “Rule of Palms” by Rossiter et al. [16] relies on the original “Lund Browder Chart”. It can be applied either alone or in combination with other methods such as the “Lund Browder Chart” to estimate the TBSA-B of a specific body region. In its simplest form, the “Rule of Palms” states that the patient’s hand’s surface accounts for approximately 1% of the TBSA. Due to different understandings on whether the palm should be calculated including or excluding fingers, this rule is used inconsistently in practice [17]. It is mainly applied to measure the size of reasonably small burn injuries [18].
2.5. Two-Dimensional Computer-Aided Systems
In the course of technical advances, the development and implementation of IT-based systems started at the beginning of the 1980s. Wachtel et al. [19] and Nichter et al. [20] were among the first to employ electronic systems. Two-dimensional (2D) computer models rely on simple human body drawings on a computer screen. These models do not consider the human body’s three-dimensionality, and in many applications, it is not possible to capture the lateral and other parts of the body. Moreover, 2D models usually do not depict individual differences in sex, weight, height, and body shape. Nevertheless, they are easy to handle but can only provide a rather rough overview of the burn type and the affected areas, particularly on the lateral parts of the body. In many cases, they miss the actual extent of the burned area. The planimetry type can be distinguished between simple planimetry and adapted planimetry. While the former is related to a simple pixel count in a 2D image and is used in some electronic devices, the latter is a pixel count in a 2D image that is additionally corrected by a particular percentage recommended for a specific body region.
2.6. Three-Dimensional Computer-Aided Systems
In 1994, Lee et al. were the first to develop and describe 3D (three-dimensional) systems [21][22]. The significant advantages of 3D models are the lateral areas’ presence and the possibility of adapting to the patients’ characteristics and better representing the real patient. Three-dimensional systems prevent the methodological error of reduction to a 2D drawing. Typically, 3D systems allow for an adaptation to sex, weight, height, and body shape. Available systems achieve a high validity in adults with a BMI below 30, but there are some limitations with very obese burn patients and unusual body proportions. The more accurate the model is, the more precise the adaptation to the individual body shape and body characteristics of the patient [23].
Systems based on individual measurements should provide an exact picture of the patient’s body to be assessed, considering the individual body characteristics. A precise 3D scan appears to be a complete individual and accurate 3D model. However, due to the time-consuming process, they were mainly applied in studies, having deficiencies, and showing other methods’ weaknesses [24].
Model-based systems use models that are modified to sex, height, weight, age, and body shape. Based on the measurements, the model is selected from a library either by the user or partially or fully computer-aided. As far as the computer system allows, the model is then adapted to the individual characteristics.
3. Burn Size Documentation
Any documentation aims to make the documented facts available. In most cases, such documentations focus on gathering data itself rather than making existing data available.
Although paper-based documentation has significant shortcomings compared to electronic wound documentation [25], many institutions still use paper forms (or free-text electronic forms). The literature shows that most clinical systems do not meet the known requirements for successful burn documentation. Many existing documentation systems use predefined terms without indicating their sources or have deficiencies in capturing a patient’s complete medical history due to the lack of standards. Besides, most of them do not include the ability to perform statistical analysis of the collected data simultaneously, and only a few systems can collect data via mobile devices [26].
Electronic documentation systems proved qualitative and quantitative advantages in several studies. They enhance documentation quality, reduce documentation errors, and result in positive attitudes among medical staff. Advantages like better availability and evaluability of the collected data, the more direct exchange of information (for consultation of experts), easier access to resources, and creation of new medical knowledge were described by Törnvall et al. [27] and Kinnunen et al. [28].
References
- Deutsche Gesellschaft für Verbrennungsmedizin (DGV). Leitlinie Behandlung Thermischer Verletzungen des Erwachsenen. Klasse: S2k. AWMF-Register-Nr.: 044-001 2018. Available online: (accessed on 30 November 2020).
- Haller, H. Verbrennungstiefe und Ausmaß. In Verbrennungen; Springer: Berlin/Heidelberg, Germany, 2009; pp. 159–167.
- Monstrey, S.; Hoeksema, H.; Verbelen, J.; Pirayesh, A.; Blondeel, P. Assessment of Burn Depth and Burn Wound Healing Potential. Burns 2008, 34, 761–769.
- Klasen, H.J. Chapter I: Classification of burns. In History of Burns; Erasmus Publishing: Rotterdam, The Netherlands, 2004; pp. 21–66. ISBN 90 5235 168 6.
- Riehl, G. Zur Therapie Schwerer Verbrennungen. Wien Klin Wochenschr. 1925, 37, 833–834.
- Dubois, D.; Dubois, E. A Formula to Estimate the Approximate Surface Area If Height and Weight Be Known. Arch. Intern. Med. 1916, 17, 863–871.
- Lund, C.C.; Browder, N.C. The Estimation of Areas of Burns. Surg. Gynecol. Obstet. 1944, 79, 352–358.
- Boyd, E. The Growth of the Surface Area of the Human Body; University of Minnesota Press: Minnesota, MN, USA, 1935.
- Neaman, K.C.; Andres, L.A.; McClure, A.M.; Burton, M.E.; Kemmeter, P.R.; Ford, R.D. A New Method for Estimation of Involved BSAs for Obese and Normal-Weight Patients with Burn Injury. J. Burn Care Res. 2011, 32, 421–428.
- Wilson, G.R.; Fowler, C.A.; Housden, P.L. A New Burn Area Assessment Chart. Burns 1987, 13, 401–405.
- Miminas, D.A. A Critical Evaluation of the Lund and Browder Chart. Wounds 2007, 3, 58–68. [Google Scholar]
- Wallace, A.B. The Exposure Treatment of Burns. Lancet 1951, 257, 501–504. [Google Scholar] [CrossRef]
- Wachtel, T.L.; Berry, C.C.; Wachtel, E.E.; Frank, H.A. The Inter-Rater Reliability of Estimating the Size of Burns from Various Burn Area Chart Drawings. Burn. J. Int. Soc. Burn Inj. 2000, 26, 156–170. [Google Scholar] [CrossRef]
- Berry, C.C.; Wachtel, T.; Frank, H.A. Differences in Burn Size Estimates Between Community Hospitals and a Burn Center. J. Burn Care Rehabil. 1982, 3, 176–178. [Google Scholar] [CrossRef]
- Giretzlehner, M.; Dirnberger, J.; Owen, R.; Haller, H.L.; Lumenta, D.B.; Kamolz, L.-P. The Determination of Total Burn Surface Area: How Much Difference? Burn. J. Int. Soc. Burn Inj. 2013, 39, 1–7. [Google Scholar] [CrossRef]
- Rossiter, N.D.; Chapman, P.; Haywood, I.A. How Big Is a Hand? Burn. J. Int. Soc. Burn Inj. 1996, 22, 230–231. [Google Scholar] [CrossRef]
- Rossiter, N.D.; Chapman, P.; Haywood, I.A. How Big Is a Hand? Burn. J. Int. Soc. Burn Inj. 1996, 22, 230–231. [Google Scholar] [CrossRef]
- Wachtel, T.L.; Berry, C.C.; Wachtel, E.E.; Frank, H.A. The Inter-Rater Reliability of Estimating the Size of Burns from Various Burn Area Chart Drawings. Burn. J. Int. Soc. Burn Inj. 2000, 26, 156–170. [Google Scholar] [CrossRef]
- Nichter, L.S.; Williams, J.; Bryant, C.A.; Edlich, R.F. Improving the Accuracy of Burn-Surface Estimation. Plast. Reconstr. Surg. 1985, 76, 428–433. [Google Scholar] [CrossRef]
- Lee, R.C.; Kieska, G.; Mankani, M.H. A Three-Dimensional Computerized Burn Chart: Stage I: Development of Three-Dimensional Renderings. J. Burn Care Rehabil. 1994, 15, 80–83. [Google Scholar] [CrossRef]
- Mankani, M.H.; Kicska, G.; Lee, R.C. A Three-Dimensional Computerized Burn Chart: Stage II: Assessment of Accuracy. J. Burn Care Rehabil. 1994, 15, 191–192. [Google Scholar] [CrossRef]
- Parvizi, D.; Giretzlehner, M.; Wurzer, P.; Klein, L.D.; Shoham, Y.; Bohanon, F.J.; Haller, H.L.; Tuca, A.; Branski, L.K.; Lumenta, D.B.; et al. BurnCase 3D Software Validation Study: Burn Size Measurement Accuracy and Inter-Rater Reliability. Burns 2016, 42, 329–335. [Google Scholar] [CrossRef]
- Klasen, H.J. Chapter I: Classification of burns. In History of Burns; Erasmus Publishing: Rotterdam, The Netherlands, 2004; pp. 21–66. ISBN 90 5235 168 6. [Google Scholar]
- Törnvall, E.; Wahren, L.K.; Wilhelmsson, S. Advancing Nursing Documentation--an Intervention Study Using Patients with Leg Ulcer as an Example. Int. J. Med. Inf. 2009, 78, 605–617. [Google Scholar] [CrossRef]
- Hübner, U.; Flemming, D.; Schultz-Gödker, A. Software Zur Digitalen Wunddokumentation: Marktübersicht und Bewertungskriterien. Wundmanagement 2009, 3, 16–25. [Google Scholar]
- Törnvall, E.; Wahren, L.K.; Wilhelmsson, S. Advancing Nursing Documentation--an Intervention Study Using Patients with Leg Ulcer as an Example. Int. J. Med. Inf. 2009, 78, 605–617. [Google Scholar] [CrossRef]
- Kinnunen, U.-M.; Saranto, K.; Ensio, A.; Iivanainen, A.; Dykes, P. Developing the Standardized Wound Care Documentation Model: A Delphi Study to Improve the Quality of Patient Care Documentation. J. Wound Ostomy Cont. Nurs. 2012, 39, 397–407. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Lo, Y.-H.; Chiou, W.-K. The 3D Scanner for Measuring Body Surface Area: A Simplified Calculation in the Chinese Adult. Appl. Ergon. 2003, 34, 273–278. [Google Scholar] [CrossRef]




