
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pavel Kindlmann | + 3215 word(s) | 3215 | 2021-04-01 05:32:34 | | | |
| 2 | Vivi Li | + 77 word(s) | 3292 | 2021-04-06 04:18:01 | | |
Video Upload Options
In this entry, we determined the associations of orchid species richness and the degree of their specialization to specific environmental conditions (expressed by species specialization index) with altitude in six floristic areas in the Czech Republic.
1. Introduction
Orchids are disappearing worldwide, mostly due to habitat loss, but other factors like climate change are likely to increase in importance during the 21st century [1][2]. Thus, one of the most worrying issues is that we still do not know the optimal abiotic and biotic requirements for population persistence of many of the ca. 30,000 species of orchids [3]. There are only a few studies in the Czech Republic dealing with the factors that determine orchid presence/absence and distribution in space, and most of them include only one or a few species and/or a limited part of the distribution of the species studied (e.g., [4][5]). We still lack critical information necessary for the conservation of Orchidaceae, especially those that are threatened or endangered.
Understanding the abundance and distribution patterns of species at large spatial scales is one of the key goals of biogeography and macroecology [6][7][8], but effective conservation requires knowledge of species at small spatial scales [9][10]. Thus, the knowledge of orchid ecology including ecological gradients that influence the patterns in orchid abundance, distribution, richness and composition is essential for planning and applying conservation strategies and actions [9][10], as lack of such knowledge negatively affects our ability to identify sites that are worth protecting.
There are two crucially important values when orchid conservation and survival under climate change is considered: number of species per unit area, and the degree to which an orchid species is specialized to specific environmental conditions. The former clearly determines the conservation value of the area, while the latter tells us how much a species may be endangered by changes of environmental conditions, e.g., by climate change.
The need for taking effective conservation measures is urgently required for areas and countries that have been affected by human activities in the past decades, and thus have lost a part of their biodiversity or their species distributions have been largely diminished [11][12]. This is especially true in the case of Central European countries, which have been intensively affected by land use change or agricultural intensification [11]. Among these countries, the Czech Republic was strongly affected by such changes over the last few decades. As a result, many orchids declined and can only be found at a small number of sites [12].
There are many publications on the distribution of orchids in the Czech Republic, which indicates that both professionals and the lay public are interested in orchids (e.g., [13][14][15][16][17][18][19]). However, there is only scattered information on the factors determining orchid distribution and species richness throughout the Czech Republic. Recently, Štípková et al. [20] determined the association between pollination mechanisms (presence/absence of nectar) and orchid species density and mean species specialization index along an altitudinal gradient in the six different phytogeographical regions in the Czech Republic.
In addition to their often specialized pollination strategies, orchids in temperate regions differ in their rooting systems, which are thought to represent particular strategies for underground storage of resources [21]. In this context, Tsiftsis et al. [8] report that the spatial patterns in the distribution of orchids in Greece are associated with their rooting systems, latitude, altitude and climate. They categorized orchids based on the morphology of their rooting system: (i) rhizomatous orchids (considered to be the most primitive), (ii) “intermediate orchids” (in evolutionary history intermediate between rhizomatous and tuberous orchids) and (iii) tuberous orchids (the most derived)—see Dressler [22], Averyanov [23] and Tatarenko [24] for reference. The typical shapes of terrestrial orchid roots in the three groups are depicted in Figure 1.
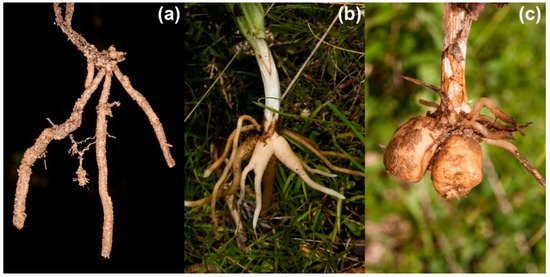
Figure 1. Different types of orchid root system: (a) rhizomatous, (b) intermediate and (c) tuberous.
Tsiftsis et al. [8] report that species richness of orchids with these three rooting systems is significantly associated with altitude, whereas the degree to which an orchid species is specialized to specific environmental conditions (e.g., their mean species specialization index that will be described later here) are largely associated with their evolutionary history, represented by their rooting system.
However, these associations have not been studied in central Europe, where the climate is more continental and less variable than in countries in the south of Europe (e.g., Greece). The expectation is that the more continental climate in Central Europe would affect the distribution of species in a more uniform way. However, there are six different phytogeographical regions in the Czech Republic. They differ in altitude and consequently in their climatic conditions, but also in the spatial distribution of different habitats and their geological substrates [25], as well as in the intensity of human activities in the past. Thus, it is expected that these differences may also affect the distribution of orchids. Therefore, it is very important to analyze each of the phytogeographical regions separately, instead of all of them together.
To fill this gap in our knowledge, here we explore the associations of orchid species richness (adjusted for area considered) and the degree to which an orchid species is specialized to specific environmental conditions (measured as species specialization index—see its definition below) with altitude in the Czech Republic. For the reasons described above, we distinguish six phytogeographical regions and perform the analyses for each of them separately. We distinguish three basic trends in these relationships: linear, parabolic or cubic, and we then look at whether these trends differ between the three rooting systems studied here.
2. Orchid Species Density along Altitudinal Gradients
The fact that species are not uniformly distributed across the globe stimulated Stevens [26] to write and publish his research on latitudinal Rapoport’s rule (LRR), which considers patterns of species distribution along latitudinal gradients, and a few years later he extended this hypothesis to include elevational gradient (elevational Rapoport’s rule, ERR [27]). Since then, a large number of studies have been published on different organisms (e.g., plants, animals) occurring in different areas of the world (e.g., [28][29][30][31]). It is worth mentioning that even for the same group of organisms, the explanations for the distributions differ considerably, so it is possible that the trends are associated with their functional groups (e.g., [8][20][32]).
The results presented support the existence of a hump-shaped curve; however, linear as well as cubic trends were also recorded. The highest species density was recorded between 300 and 900 m, which could be attributed to the fact that the distributions of many species of orchids overlap at these altitudes [32]. Naturally, phytogeographical regions in the Czech Republic do not have clear boundaries and there are many transitional zones between regions. Unlike the high number of species at mid altitudes, there are far fewer species at the highest altitudes, and they are mainly specialists that flourish in extreme conditions in mountains.
3. Patterns in the Distributions of the Three Orchid Groups
The distributions of each of the three orchid groups correspond to their specific ecological requirements, like altitude, type of bedrock or type of habitat, which confirm the patterns identified by Tsiftsis et al. [8] in southern Europe. In the Czech Republic, the most widely distributed are the rhizomatous orchids, closely followed by those with an intermediate root system. The rhizomatous orchids include genera like Corallorhiza, Epipactis, Epipogium and Cephalanthera, which mainly thrive in various kinds of forest habitats. This group has a wider distribution than the other two groups. It is likely that forest habitats, where rhizomatous orchids mostly occur, are more or less uniformly distributed throughout the Czech Republic. Like the rhizomatous orchids, intermediate orchids, such as Dactylorhiza spp., Gymnadenia spp. and Platanthera spp., occur in forests and open habitats. This means that many species occur almost everywhere in the Czech Republic. The least widely distributed group is the tuberous orchids. In general, tuberous orchids (species in the genera Anacamptis, Ophrys and Orchis) mainly grow in open habitats, such as grasslands or meadows. However, our results for this group differ from those reported for Southern Europe [8], where tuberous orchids are the most widely distributed group. Tuberous orchids evolved later than rhizomatous or intermediate orchids and are well adapted to the dry and hot climate around the Mediterranean, where their richness is really high [8][33]. As a result, they are better represented in southern countries, e.g., in the Mediterranean, compared to countries where the climate is continental, as in Central Europe. However, there are some tuberous orchids that are well adapted and occur in areas with a continental climate (e.g., Orchis pallens, Traunsteinera globosa, Anacamptis morio). Although such species can occur throughout the Czech Republic from a climatological point of view, they are restricted because they mostly prefer a calcareous substrate that does not occur everywhere. The large number of species of tuberous orchids recorded in the SE part of the country (Bílé Karpaty), however, could be attributed either to the presence of calcareous substrates, the extensive distribution of grassland communities or the higher temperatures there than in other areas in the Czech Republic. The significance of this area was identified in the 1990s and then subject to huge restoration program (in Bílé Karpaty NCA). About 500 hectares of arable land were converted back into dry or mesic grasslands, where tuberous orchids flourish, using a high-diversity regional seed mixture. Nearly all species sown successfully established and nearly half of unsown target species established spontaneously, including orchids. All of these grasslands are maintained by regular mowing, which favors grassland orchids by reducing the competition with the other herbaceous species of plants [34][35][36].
4. Orchid Species Richness in the Different Phytogeographical Areas
Previously, it was reported that the association of the composition of the orchid flora in the Czech Republic with altitude is much stronger than with biogeography [20], which differs from the results reported for other countries (e.g., Greece, Colombia) where different areas host different orchid taxa [8][37]. This may be attributed to the different distribution and height of mountains in the above-mentioned countries. In countries with high mountain ranges (e.g., Greece, Colombia), natural barriers can exist, which together with other factors can delimit the dispersal of orchid seeds from one place to another. In contrast, in the Czech Republic, the highest mountains are present mainly at the borders with other countries and are not so high (max 1600 m), so orchid seeds are able to be dispersed over long distances within the country.
In general, the trend in the density of species of orchids along altitudinal gradients is hump-shaped, but linear and cubic trends were also recorded. Density of species of orchids in the three groups differed in the six phytogeographical regions but the trends were similar in the Bohemian-Moravian mesophyticum and both oreophyticums, whereas in the other three regions the trends were different. These differences can be attributed to many aspects connected with the life history strategies of orchids. Orchids need vegetation in which they can grow and establish new populations from seeds. Thus, the spatial distribution of suitable vegetation has a big effect on the density of orchids in different regions [1][20][38][39][40]. In addition to vegetation, geological substrate plays an important role in determining the distribution of orchids [39]. Many tuberous orchids show a clear preference for areas with a calcareous substrate, whereas rhizomatous and intermediate orchids occur at sites with siliceous substrates. The same pattern is also recorded in Greece, where numbers of species of tuberous orchids increase with increase in the percentage of calcareous substrates within grid cells [8]. This may account for the difference in tuberous orchids recorded in the Bohemian part of the Czech Republic and in the Pannonian and Carpathian parts of these regions. Granite is frequent and there are only small calcareous areas, the preferred substrate for tuberous orchids, in the Bohemian part of the Czech Republic, whereas calcareous substrates are frequent in the Pannonian and Carpathian regions.
Mycorrhizal fungi and orchid pollinators are other important factors that indirectly affect orchid distribution. Distribution of mycorrhizal fungi in soil and of pollinators at different altitudes is expected to play a significant role. Orchids are strongly dependent on fungi for seedling germination, establishment and growth, so the distribution and diversity of orchids might depend on the associated fungal communities [41]. Although mycorrhizal associations are predominantly generalist, specialized mycorrhizal interactions have repeatedly evolved in orchids, indicating their potential role in limiting the geographical range of orchids [42]. It is mentioned by several authors that mycorrhizal fungi affect local plant distribution [43] and orchids are not an exception [44]. There is evidence that the abundance together with distribution of orchid mycorrhizal fungi are important drivers of orchid density [45]. However, more studies on orchid mycorrhizal fungi are needed for a better understanding of the factors shaping the distribution of mycorrhizal fungi along altitudinal gradients [46].
Distribution of pollinators differs with altitude, with high altitude areas being the poorest [47]. Such differences may play an important role in pollination success, with orchids at low altitudes producing more fruit (and seeds) than those at high altitudes. This is reported for Hungary by Gilián et al. [48], where the reproduction success of the two lowest mountain populations of Cephalanthera rubra was extremely high (83–85% of the flowers produced fruit), whereas populations at higher altitudes produce significantly fewer fruits (about 40% of the flowers produced fruit). Populations at high altitudes might be limited by pollinators as well as by climatic conditions (such as low temperature and irradiance). Thus, temperatures and subsequent climatic conditions in different phytogeographical regions also play a role in the density of the three orchid groups.
5. Relationship between Mean Species Specialization Index and Altitude
The general distribution of each of the three orchid groups is determined by the specific ecological requirements of all species in each group, the spatial distribution of different habitats and the presence of pollinators and mycorrhizal fungi [49][50]. We used the species specialization index (SSI). SSI was calculated using the climatic conditions in all the places in the Czech Republic where an orchid occurred. In general, the breadth of a species niche increases with increase in altitude [51]. However, Tsiftsis et al. [8] in their study on Greek orchids found that these trends are mostly associated with orchid life forms. Similar to Tsiftsis et al. [8], we also recorded different trends in the three species groups and six phytogeographical areas in the Czech Republic.
We assume that the differences in the trends in the phytogeographical regions might be based on the distribution patterns of orchids specific to a given area. In the current study, the majority of the specialized species of orchids were recorded at low to middle altitudes. However, most regression curves were typically hump-shaped, which means that species recorded at high and low altitudes are generalists, as they are recorded at sites with a wide range of environmental conditions. This is in accordance with our previous study in which different pollination strategies of orchids were considered [20]. However, there are some differences in the occurrence of specialist and generalist orchids. In the Bohemian-Moravian mesophyticum, the trend recorded for the intermediate group supports the previous statement, whereas the distribution patterns of rhizomatous and tuberous orchids differed slightly. The most specialized species of rhizomatous orchids (such as Epipactis pontica) were recorded also at the highest altitudes in this region, and in the case of tuberous orchids, the incidence of specialist species decreased with increase in altitude and is not hump-shaped. Another example of a difference are the trends for the species distributed in oreophyticums. In both areas, intermediate orchids were the least specialized and tuberous orchids the most specialized orchid group. This specific pattern may be attributed to the ecological requirements of these species. In both areas, there are tuberous orchids with narrow species specialization index (e.g., Anacamptis pyramidalis, Ophrys insectifera or Neotinea tridentata), which occur more frequently in southern Europe [8] and are rather rare at low and medium altitudes in both oreophyticums. In the case of Carpathian oreophyticum, the trends for all species groups only differ slightly. The most specialized species (the rhizomatous Cephalanthera rubra, intermediate Pseudorchis albida and tuberous Anacamptis morio) occur at low (ca. 600 m) and the highest altitudes in this phytogeographical region. At other altitudes, orchids are generalists, occurring in a wide range of environmental conditions. The narrower niches of tuberous orchids compared to rhizomatous and intermediate orchids could be due to the distribution of forest and grassland habitats at particular altitudes. In the Carpathian oreophyticum, there are more forests at low and high altitudes, which are replaced by grassland at middle altitudes, where tuberous orchids typically occur. These grasslands are currently maintained by traditional management, mainly pastoralism. At the highest altitudes, this area is covered by alpine vegetation that is unsuitable for orchids, with only specialist orchids occurring in these harsh conditions.
In general, the differences in the distribution of specialists and generalists within each group might be due to the very different distributions of open and forested habitats in the phytogeographical regions. In the Czech Republic, the middle and high altitudes are abundantly covered by forests [19], where most rhizomatous and several of the intermediate orchids typically occur. This is in accordance with our results: rhizomatous and intermediate orchids were more widely distributed in floristic areas at high altitudes (mesophyticums and orephyticums). However, natural non-forested areas are also present in the highest altitudinal zone in the Carpathians [19][52], where most specialist tuberous orchids occur. Open habitats are typically inhabited by tuberous and some of the intermediate orchids. Some species in these groups belong to the most endangered orchid taxa in the Czech Republic [53]. We fully agree with Jacquemyn et al. [49] that the extinction of orchids is significantly related to the survival of the habitats where these orchids occur.
Distribution of habitats is closely connected with the distribution of orchid pollinators. Some orchids, mainly those occurring in forests, rely on pollinator being present to produce seeds [54]. The abundance of pollinators is declining all over the world [55] and is influenced by climate (e.g., temperature and seasonality) in a given area, which in turn is strongly determined by altitude [47]. Another factor influencing species specialization index is orchid rarity. Previously, it was reported that a nectar reward affects rarity and the probability of extinction of orchids [20][49][56][57]. Similarly, in south-western Australia, sexually deceptive taxa are more often rare than food-rewarding taxa [58]. Conservation efforts will be most effective if they combine ex situ strategies at locations with high habitat conversion rates and reservation strategies in rarity and richness hotspots, particularly where they overlap [59]. Moreover, in the Australian hotspot, the Southwest Australian Floristic Region, the greatest number of rare taxa occurred in areas of high taxon richness and naturally fragmented edaphic environments [58].
Another important finding of the present study is the mismatch in the number of species and SSI values. Comparing Figure 4 with Figure 5 reveals that in most areas with high species richness (expressed in terms of orchid density) are associated with low values of the mean species specialization index, and vice versa. This could be attributed to the fact that in areas with a high number of species, most species or a considerable number of species are widely distributed with low SSI values, which results in low values for the mean SSI. In contrast, in areas where only a few species were recorded (e.g., tuberous orchids in the Bohemian-Moravian oreophyticum), most of these species are not widespread in the Czech Republic, and as a result their SSI values are high. It could be hypothesized that the ecological conditions in areas with just a few species are extreme (e.g., climatic, soil) and consequently only a small number of species can occur there and nowhere else.
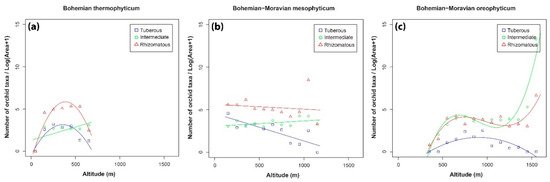

Figure 4. Density of tuberous, intermediate and rhizomatous orchid taxa recorded at different altitudes in the phytogeographical region of: (a) Bohemian thermophyticum, (b) Bohemian-Moravian mesophyticum, (c) Bohemian-Moravian oreophyticum, (d) Pannonian thermophyticum, (e) Carpathian mesophyticum and (f) Carpathian oreophyticum.
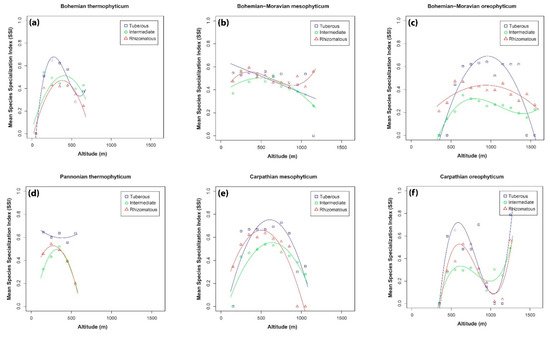
Figure 5. Relationships between mean species specialization index (SSI) and altitude recorded for the three orchid groups in the phytogeographical area of: (a) Bohemian thermophyticum, (b) Bohemian-Moravian mesophyticum, (c) Bohemian-Moravian oreophyticum, (d) Pannonian thermophyticum, (e) Carpathian mesophyticum and (f) Carpathian oreophyticum.
References
- Wotavová, K.; Balounová, Z.; Kindlmann, P. Factors affecting persistence of terrestrial orchids in wet meadows and implications for their conservation in a changing agricultural landscape. Biol. Conserv. 2004, 118, 271–279.
- Pfeifer, M.; Wiegand, K.; Heinrich, W.; Jetschke, G. Long-term demographic fluctuations in an orchid species driven by weather: Implications for conservation planning. J. Appl Ecol. 2006, 43, 313–324.
- Swarts, N.D.; Dixon, K.W. Conservation Methods for Terrestrial Orchids; J. Ross Publishing: Plantation, FL, USA, 2017.
- Štípková, Z.; Romportl, D.; Černocká, V.; Kindlmann, P. Factors associated with the distributions of orchids in the Jeseníky Mountains, Czech Republic. Eur. J. Environ. Sci. 2017, 7, 135–145.
- Štípková, Z.; Kosánová, K.; Romportl, D.; Kindlmann, P. Determinants of Orchid Occurrence: A Czech Example. In Selected Studies in Biodiversity; Şen, B., Grillo, O., Eds.; InTech Open: London, UK, 2018; pp. 1–24.
- Brown, J.H. Macroecology; University of Chicago Press: Chicago, IL, USA, 1995.
- Gaston, K.J.; Blackburn, T.M. Pattern and Process in Macroecology; Blackwell Science Ltd.: Malden, MA, USA, 2000.
- Tsiftsis, S.; Štípková, Z.; Kindlmann, P. Role of way of life, latitude, elevation and climate in the richness and distribution of orchid species. Biodivers Conserv. 2019, 28, 75–96.
- Swarts, N.D.; Dixon, W.D. Terrestrial orchid conservation in the age of extinction. Ann. Bot-Lond. 2009, 104, 543–556.
- Tsiftsis, S.; Tsiripidis, I.; Karagiannakidou, V.; Alifragis, D. Niche analysis and conservation of orchids of east Macedonia (NE Greece). Acta Oecol. 2008, 33, 27–35.
- Štípková, Z.; Tsiftsis, S.; Kindlmann, P. How did the agricultural policy during the communist period affect the decline in orchid biodiversity in Central and Eastern Europe? Glob. Ecol. Conserv. 2021, 26, e01498.
- Štípková, Z.; Kindlmann, P. Orchid extinction over the last 150 years in the Czech Republic. Diversity 2021, 13, 78.
- Dykyjová, D. Ekologie Středoevropských Orchidejí; KOPP: České Budějovice, Česká republika, 2003.
- Jersáková, J.; Kindlmann, P. Zásady Péče o Orchidejová Stanoviště; KOPP: České Budějovice, Česká republika, 2004.
- Průša, D. Orchideje České Republiky; Computer press: Brno, Česká republika, 2005.
- Slavík, S.; Hejný, B. Květena České Republiky 8; Academia: Praha, Česká republika, 2010.
- Danihelka, J.; Chrtek, J., Jr.; Kaplan, Z. Checklist of vascular plants of the Czech Republic. Preslia 2012, 84, 647–811.
- Lepší, P.; Lepší, M.; Boublík, K.; Stech, M.; Hans, V. Červená Kniha Květeny Jižní Části Čech; Jihočeské muzeum v Českých Budějovicích: České Budějovice, Česká republika, 2013.
- Chytrý, M.; Danihelka, J.; Kaplan, Z.; Pyšek, P. Flora and Vegetation of the Czech. Republic; Springer International Publishing: Cham, Switzerland, 2017.
- Štípková, Z.; Tsiftsis, S.; Kindlmann, P. Pollination mechanisms are driving orchid distribution in space. Sci. Rep. 2020, 10, 850.
- Rasmussen, H.N. Terrestrial Orchids from Seed to Mycotrophic Plant; Cambridge University Press: Cambridge, UK, 1995.
- Dressler, R.L. The Orchids: Natural History and Classification; Harvard University Press: Cambridge, MA, USA, 1981.
- Averyanov, L. A review of the genus Dactylorhiza. In Orchid Biology—Reviews and Perspectives; Arditti, J., Ed.; V. Timber Press Inc.: Portland, OR, USA, 1990; pp. 159–206.
- Tatarenko, I. Growth habits of temperate terrestrial orchids. In Orchid Biology—Reviews and Perspectives, IX; Cameron, K.M., Arditti, J., Kull, T., Eds.; The New York Botanical Garden Press: Bronx, NY, USA, 2007; pp. 91–161.
- Kaplan, Z. Flora and phytogeography of the Czech Republic. Preslia 2012, 84, 505–573.
- Stevens, G.C. The latitudinal gradient in geographical range: How so many species coexist in the tropics. Am. Nat. 1989, 133, 240–256.
- Stevens, G.C. The elevational gradient in altitudinal range: An extension of Rapoport’s latitudinal rule to altitude. Am. Nat. 1992, 140, 893–911.
- Bhattarai, K.R.; Vetaas, O.R. Variation in plant species richness of different life forms along a subtropical elevation gradient in the Himalayas, east Nepal. Glob. Ecol. Biogeogr. 2003, 12, 327–340.
- Grytnes, J.A. Species-richness patterns of vascular plants along seven altitudinal transects in Norway. Ecography 2003, 26, 291–300.
- Hrivnák, R.; Gömöry, D.; Slezák, M.; Ujházy, K.; Hédl, R.; Jarčuška, B.; Ujházyová, M. Species richness pattern along altitudinal gradient in central European beech forests. Folia. Geobot. 2014, 49, 425–441.
- Zhou, Y.D.; Ochola, A.C.; Njogu, A.W.; Boru, B.H.; Mwachala, G.; Hu, G.W.; Xin, H.P.; Wang, Q.F. The species richness pattern of vascular plants along a tropical elevational gradient and the test of elevational Rapoport’s rule depend on different life-forms and phytogeographic affinities. Ecol. Evol. 2019, 9, 4495–4503.
- Jacquemyn, H.; Micheneau, C.; Roberts, D.L.; Pailler, T. Elevational gradients of species diversity, breeding system and floral traits of orchid species on Réunion Island. J. Biogeogr. 2005, 32, 1751–1761.
- Del Prete, C.; Mazzola, P. Endemism and speciation in the orchids of Mediterranean Islands. Ecol. Mediterr. 1995, 21, 119–134.
- Prach, K.; Jongepierová, I.; Řehounková, K. Large-scale restoration of dry grasslands on ex-arable land using a regional seed mixture: Establishment of target species. Restor. Ecol. 2004, 21, 33–39.
- Johanidesová, E.; Fajmon, K.; Jongepierová, I.; Prach, K. Spontaneous colonization of restored dry grasslands by target species: Restoration proceeds beyond sowing regional seed mixtures. Grass Forage Sci. 2003, 70, 631–638.
- Kull, T.; Hutchings, M.J. A comparative analysis of decline in the distribution ranges of orchid species in Estonia and the United Kingdom. Biol. Conserv. 2006, 129, 31–39.
- Swenson, J.J.; Young, B.E.; Beck, S.; Comer, P.; Cordova, J.H.; Dyson, J.; Embert, D.; Encarnacion, F.; Ferreira, W.; Franke, I.; et al. Plant and animal endemism in the eastern Andean slope: Challenges to conservation. BMC Ecol. 2012, 12, 1.
- Tsiftsis, S.; Tsiripidis, I.; Karagiannakidou, V. Identifying areas of high importance for orchid conservation in east Macedonia (NE Greece). Biodivers Conserv. 2009, 18, 1765–1780.
- Djordjević, V.; Tsiftsis, S.; Lakušić, D.; Jovanović, S.; Stevanović, V. Factors affecting the distribution and abundance of orchids in grasslands and herbaceous wetlands. Syst. Biodivers 2016, 14, 355–370.
- Djordjević, V.; Tsiftsis, S. The role of ecological factors in distribution and abundance of terrestrial orchids. In Orchids Phytochemistry, Biology and Horticulture; Mérillon, J.M., Kodja, H., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 1–71.
- Pecoraro, K.; Caruso, T.; Cai, L.; Gupta, V.K.; Liu, Z.-J. Fungal networks and orchid distribution: New insight from above- and below-ground analyses of fungal communities. IMA Fungus 2018, 9, 1–11.
- Davis, B.J.; Phillips, R.D.; Wright, M.; Linde, C.C.; Dixon, K.W. Continent-wide distribution in mycorrhizal fungi: Implications for the biogeography of specialized species. Ann. Bot-Lond. 2015, 116, 413–421.
- Carvalho, L.M.; Correia, P.M.; Ryel, R.J.; Martins-Loucao, M.A. Spatial variability of arbuscular mycorrhizal fungal spores in two natural plant communities. Plant. Soil 2003, 251, 227–236.
- McCormick, M.K.; Jacquemyn, H. What constraints the distribution of orchid populations? New Phytol. 2014, 202, 392–400.
- McCormick, M.K.; Whigham, D.F.; Canchani-Viruet, A. Mycorrhizal fungi affect orchid distribution and population dynamics. New Phytol. 2018, 219, 1207–1215.
- Geml, J. Altitudinal gradients in mycorrhizal symbioses. In Biogeography of Mycorrhizal Symbiosis; Tedersoo, L., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 107–123.
- Arroyo, M.T.K.; Primack, R.; Armesto, J. Community studies in pollination ecology in the high temperate Andes of central Chile. I. Pollination mechanisms and altitudinal variation. Am. J. Bot. 1982, 69, 82.
- Gilián, L.D.; Enrédi, A.; Zsinka, B.; Neményi, A.; Nagy, J.G. Morphological and reproductive trait-variability of a food deceptive orchid, Cephalanthera rubra along different altitudes. Appl. Ecol. Environ. Res. 2019, 17, 5619–5639.
- Jacquemyn, H.; Brys, R.; Hermy, M.; Willems, J.H. Does nectar reward affect rarity and extinction probabilities of orchid species? An assessment using historical records from Belgium and the Netherlands. Biol. Conserv. 2005, 121, 257–263.
- Devoto, M.; Medan, D.; Montaldo, N.H. Patterns of interaction between plants and pollinators along an environmental gradient. Oikos 2005, 109, 461–472.
- Rasmann, S.; Alvarez, N.; Pellissier, L. The altitudinal niche-breadth hypothesis in insect-plant interactions. Annu. Plant. Rev. 2014, 47, 339–360.
- Chytrý, M.; Kučera, T.; Kočí, M. Katalog Biotopů České Republiky; AOPK ČR: Praha, Česká republika, 2001.
- Grulich, V. The Red List of vascular plants of the Czech Republic. Příroda 2017, 35, 75–132.
- Tremblay, R.L. Trends in the pollination ecology of the Orchidaceae: Evolution and systematics. Can. J. Bot. 1992, 70, 642–650.
- Tylianakis, J.M. The global plight of pollinators. Science 2013, 339, 1532–1533.
- Darwin, C. The Various Contrivances by Which Orchids Are Fertilized by Orchids; John Murray: London, UK, 1862.
- Neiland, M.R.M.; Wilcock, C.C. Fruit set, nectar reward, and rarity in the Orchidaceae. Am. J. Bot 1998, 85, 1657–1671.
- Phillips, R.D.; Brown, A.P.; Dixon, K.W.; Hopper, S.D. Orchid biogeography and factors associated with rarity in a biodiversity hotspot, the Southwest Australian Floristic Region. J. Biogeogr. 2010, 28, 487–501.
- Crain, B.J.; Tremblay, R.L. Do richness and rarity hotspots really matter for orchid conservation inlight of anticipated habitat loss? Divers. Distrib. 2014, 20, 652–662.




