
Video Upload Options
Carcinogenesis is a multistep process characterized by a progression of molecular changes that ultimately transform a cell to undergo uncontrolled proliferation.
1. An Overview of Carcinogenesis
Carcinogenesis is a multistep process characterized by a progression of molecular changes that ultimately transform a cell to undergo uncontrolled proliferation. Human cancer is a progressive disease that develops in distinct stages by long periods of latency. Cancer development requires 20–40 years or more, and therefore, the initiation stage might occur decades before an individual is clinically diagnosed for cancer. Cells undergo changes with each disruption fundamentally featured by tumor initiation, promotion, and progression.
The occurrence of probable mutations within genes that encode growth factors, transcription factors, protein kinases, apoptotic signaling proteins, or adhesion molecules, predispose a cell to become tumorigenic during initiation. The additional irreversible genetic changes, accompanying with each new cycle of proliferation, were accumulated as “initiated cells”. Subsequently, the second stage, promotion, a relatively lengthy and reversible process of carcinogenesis, ultimately resulted from genetic and epigenetic alterations by repetitive cycles of proliferation, selective clonal expansion. The cells during promotion have acquired the ability to evade programmed apoptosis and immune surveillance, accompanying with sustained angiogenesis. Many cancers are not diagnosed until third stage, progression, in which the tumor becomes malignant and maybe metastasizes to multiple body regions. The tumors in this stage are very difficult to treat, which usually requires surgery in combination with chemotherapeutic drugs and radiotherapy.
Each individual has a unique cancer risk profile which is determined by sequential accumulation genetic and epigenetic factors over a period of many years. According to the carcinogenesis, cancer control strategies can be divided into three stages, including prevent/inhibit one third of cancers caused by tobacco and alcohol, cure/treat another third, and provide good, palliative care to the remaining third [1][2]. As further data accumulated, the transition from one cancer phase to the next can be stimulated by certain carcinogenic factors, such as obesity, alcohol, smoke, biological agents, infections, and ultraviolet radiation as well as an unhealthy dietary habit, and others [3], which ultimately resulted in invasive/metastatic carcinoma. It is predicted that between 30% and 50% of cancer incidences can be prevented with proper medical awareness [4]. Evidence indicated that two thirds of the cancer deaths in the US each year can be attributed to diet, physical activity habits, and cigarette smoking. Therefore, chemoprevention as a rapid evolving field, can perturb a variety of steps in tumor development to reduce or delay the occurrence of malignancy.
2. Scientific Principles of Cancer Chemoprevention
Interest in cancer chemoprevention of research has markedly increased with improved illustration of the biology of carcinogenesis and identification of potential molecular targets involving this process. It has become apparent that chemoprevention should incorporate the concept of ‘delay’, which implies that many years or decades may be added to human lifespan. This interest has been further aroused by successes in the prevention of prostate, breast, and colon cancers, and there are 10 medications that were approved by Food and Drug Administration (FDA) for the risk reduction of cancer [5].
Chemoprophylaxis, the term coined by Lee Wattenberg in 1966, is an effective method focused on inhibition of tumor/cancer through synthetic or naturally occurring agents [6]. Later, in 1976, Michael B. Sporn and other coauthors introduced the term “cancer chemoprevention” which was defined as the prevention of the cancer occurrence by administration of natural compounds [7][8]. Gary Kello, chief of the Chemoprevention Branch, extended the definition of “cancer chemoprevention” as utilization of agents to suppress, prevent, or reverse the carcinogenic process to invasive cancer [9]. Furthermore, other important considerations, “combination chemoprevention”, introduced in 1980 as a new strategy, can not only enhance potential synergistic efficacy of drugs, but also decrease the toxicity of the individual agents, with a lower dose treatment in a combination regimen. This strategy was clinically believed in a definitive investigation, demonstrating that the combination of difluoromethylornithine and sulindac showed more effective in preventing colon cancer [10]. Recently, chemoprevention was considered as an important and hopeful tool for controlling cancer. It plays a role in preventing the development of invasive and metastatic properties in established neoplasms, and this approach is to lower the rate of cancer incidence. Chemoprevention can be organized into three parts: (1) primary prevention, inhibiting the tumor in healthy individuals with high risk, for instance, hepatitis B vaccine; (2) secondary prevention, inhibiting tumor development in individuals with precancerous lesions like invasion; (3) tertiary prevention, inhibiting recurrence or second primary cancers in target patients [11].
3. Agents for Cancer Chemoprevention
3.1. Synthetic Drugs
Until now, an increasing number of agents have been used for preventing cancer in various experimental models, and many of these drugs have even been clinically proven. Among these drugs, the selective estrogen receptor modulators (SERMs), such as tamoxifen, raloxifene, and arzoxifene, are the most important class, which are also beneficial for preventing osteoporosis in addition to breast cancer prevention in women at risk [12][13][14].
Other significant chemopreventive agents in various stages of clinical trials include the androgen analogs (finasteride and dutasteride), aromatase inhibitors, anastrozole and exemestane, as well as aspirin and metformin. More interesting, metformin is recently being evaluated prospectively for many cancers [15][16]. These synthetic drugs in reality have been “repurposed” because they were used for many years in other disease treatments, rather than originally developed. This largely solved many issues of dosage selection and long-term safety before these agents entered into a clinical trial phase [17].
3.2. Natural Products
Besides, natural products derived from food, spices, and herbal medicine have gradually become the major resource for cancer chemoprevention. In contrast to many synthetic drugs, they have been extremely safe for human administration for thousands of years (Table 1).
Table 1. Chemopreventive properties of phytochemicals.
| Common Phytochemicals | |||||||
|---|---|---|---|---|---|---|---|
| Groups | Phytochemicals | Structure | Cancer Type | The Subjects | Chemopreventive Property | Common Source | Reference |
| Phenols | (−)-epicatechin (EC) | 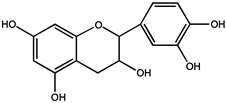 |
Prostate cancer | Human | Oral administration of 600 mg/d for 1 year reduced the incidence of diagnosed cancers in volunteers with high-grade intraepithelial neoplasia of the prostate (H GPIN) | Green tea | [18] |
| (−)-epigalocatachin (EGC) |  |
Breast cancer | MCF10A cells | Completely inhibited Met, AKT and ERK phosphorylation at 0.6 mM | [19] | ||
| (−)-epicatechin-3-gallate (ECG) | 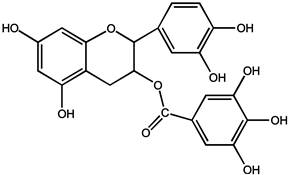 |
Liver cancer | HepG2 cells | Interrupted closures by the disassembly of microtubules | [20] | ||
| Osteosarcoma cancer | U2OS cells | ||||||
| (−)-epigallocatechin-3-gallate (EGCG) |  |
Hepatocellular carcinoma | HuH7 cells | Inhibited the growth of HuH7 xenografts | [21] | ||
| Breast cancer | Tumorigenic breast epithelial cells | Blocked the ability of hepatocyte growth factor (HGF) to induce cell motility and invasion | [19] | ||||
| Prostate cancer | Human | Not produce treatment related adverse effects in men with baseline HGPIN or ASAP | [22] | ||||
| Resveratrol | 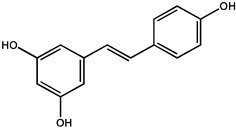 |
Lung cancer | A/J mice | Tumor diversity and volume decreased in mice | Polygonum cuspidatum, red grapes, berries, peanuts, pines etc. | [23] | |
| Colorectal cancer | BALB/c wild-type mice | Marked suppression of dextran sulfate sodium (DSS)-associated tumorigenesis | [24] | ||||
| Human | It can be used as a potential chemoprophylaxis for colorectal cancer tract | [25] | |||||
| Curcumin |  |
Liver cancer | Transgenic mice | Inhibition of hepatocellular carcinoma formation, improvement of liver histopathology, and reduction of total tumor volume in transgenic mice | [26] | ||
| Ovarian cancer | Hens | Reduced the overall ovarian cancer incidence to 31% and 57% | [27] | ||||
| Lung cancer | Lung cancer cells (H1299, A549) | An inhibitory effect in lung carcinogenesis induced by B[a]P, a procarcinogen present in environment and cigarette smoke | Curcuma Longa | [28] | |||
| Curcuma Longa A2 | 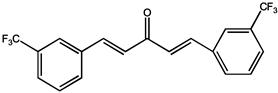 |
ROS-dependent endothelial cell | Human umbilical vein endothelial cells (HUVECs) | Suppresses the migration and tube formation of human umbilical vein endothelial cells (HUVECs) in vitro | [29] | ||
| —————— | Rat | Suppresses newly formed microvessels in chicken chorioallantoic membranes (CAMs) and Matrigel plus in vivo | |||||
| Flavonoids | Quercetin | 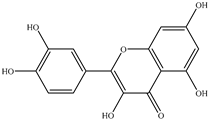 |
Oral squamous cell carcinoma (OSCC) | Hamster | Decreased incidence of oral squamous cell carcinoma (OSCC) and severity of hyperplasia and dysplasia | Apples, onions, tomatoes, broccoli, citrus fruit, etc. | [23] |
| Melanoma | SK-MEL-28 human melanoma cells | Decreased migration rates (26.36% vs. 64.36%) and motility rate by approximately tenfold in SK-MEL-28 cells cultured on collagen I matrices | [30] | ||||
| Lutein |  |
Lung and colon cancers | Human | Reduce the risk of lung and colon cancers by the suppression of k-Ras and β-catenin expression | Papaya, pumpkin, citrus, wolfberry, peach, spinach, leek, corn, Chinese cabbage, etc. | [31] | |
| Zeaxanthin | 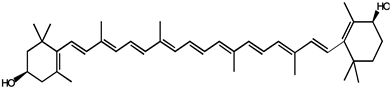 |
||||||
| Lycopene |  |
Benign prostate hyperplasia (BPH) | Human | Inhibited serum prostate-specific antigen (PSA) increase, and further improving clinical diagnostic markers and symptoms of BPH | Tomato, tomato products, watermelon, grapefruit etc. | [32] | |
| Apo-10-lycopenoic | 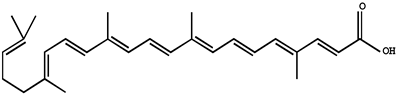 |
Liver tumors | HFD-fed mice | APO10LA can effectively inhibit HFD-promoted hepatic tumorigenesis by stimulating SIRT1 signaling while reducing hepatic inflammation | [33] | ||
| Monoterpene | Thymoquinone | 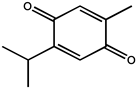 |
Human prostate cancer | PC3 cancer cells | Inhibits tumor angiogenesis and tumor growth and could be used as a potential drug candidate for cancer therapy | The seed oil of Nigella sativa L. | [34] |
| —————— | Human umbilical vein endothelial cell (HUVEC) | Effectively inhibited migration, invasion, and tube formation of human umbilical vein endothelial cell (HUVEC) | [34] | ||||
| Human prostate cancer (PC3) | Male mice | Inhibited human prostate tumor growth in both size and weight in a xenograft human prostate cancer (PC3) model in mice | [34] | ||||
| Triterpenoids | Oleanolic acid | 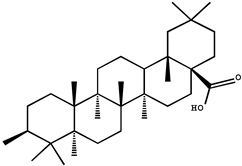 |
Colon cancer | Male mice | Oleanolic acid inhibited, in a dose-dependent manner, the average azomethane (AOM)-induced abnormal colonic cavitation lesions in male F344 rats (36–52%) | Hedyotis Herbaherba, hawthorn, Syzygium Aromaticum, loquat leaf etc. | [35] |
| Colorectal cancer | Mice | Inhibitory tumor growth of xenograft tumor tissue in mice with colorectal cancer | [36] | ||||
| 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid | 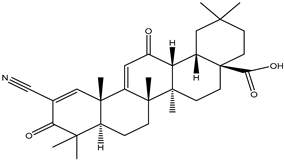 |
Breast cancer | Immunodeficient mice | CDDO (20 mg/kg, i.v.) treatment for 3 weeks abrogated the growth of both MCF7/HER2 and MDA-MB-435/HER2 tumors types in immunodeficient mice, by inhibiting HER2 phosphorylation and decreasing HER2 kinase activity | [37] | ||
| Betulinic acid | 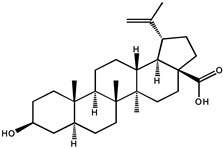 |
Colorectal cancer | Nude mice | Possess antiangiogenic effects by inhibiting aminopeptidase N | White birch bark, Ziziphi Spinosae, semen | [38] | |
| Sulfur compounds | Allicin | 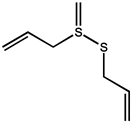 |
—————— | —————— | Inhibited lymphangiogenesis suppressing activation of vascular endothelial growth factor (VEGF) receptor | Garlic, allium, vegetables etc. | [39] |
| Thyroid cancer | SW1736 and HTh-7 cells | Served as an adjunctive therapy for thyroid cancer, as it induces autophagic cell death to alleviate the malignant development of cancer | [40] | ||||
| Diallyl sulfide | 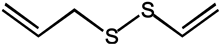 |
—————— | C57BL/6 mice | Compared to the control group, through enhancing the production of antiangiogenic factors such as IL-2 and TIMP | [41] | ||
| Diallyl tetrasulfide |  |
Colon cancer | CoLo 205 cells | Three oil-soluble compounds including DAS, DADS, and DAT at 10 and 25 μM have an inhibitory effect on the migration and invasion of human colon cancer cells with the order of DATS < DADS < DAS | [42] | ||
| Diallyl disulfide |  |
||||||
| Diallyl trisulfide | 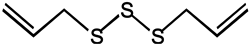 |
||||||
| Sulforaphane | 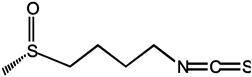 |
Breast cancer | Rats | As a conceptually promising agent in breast cancer prevention. It can be rapidly absorbed and achieved a peak level before 1 h | Broccoli | [43] | |
| Triple negative breast cancer | Orthotopic mouse xenograft model | The addition of sulforaphane can prevent the expansion and clearance of breast CSCs, which will greatly benefit the treatment of TNBC with cytotoxic chemotherapy | [44] | ||||
| Cellulose | Selenium | ———— | Prostate cancer | LNCaP cells | Selenium-induced growth inhibition and apoptosis in PC-3 prostate cancer cells were found to be dose dependent | ———— | [45] |
| Calcium | ———— | Colon cancer | Rats | Inhibited colonic epithelial cell proliferation induced by heme in rats, which suggested that calcium might decrease the colon cancer risk related to high intake of red meat | [46] | ||
| Colon cancer | Human | Intravenous CA/MG can be used as an effective neuroprotectant against the accumulation of SNT in adjuvant colon cancer induced by oxaliplatin | ———— | [47] | |||
| 1, 25-Dihydroxy vitamin D3 |  |
Melanoma tumor | Melanoma cells | Decreased cell proliferation was found in melanoma cells | ———— | [48] | |
| Prostate cancer | Human | Slow the rate of prostate specific antigen (PSA) rise in PCa patients demonstrating proof of concept that 1,25(OH)2D3 exhibits therapeutic activity in men with PCa | [49] | ||||
3.2.1. Polyphenols
Phenolic compounds derived from the main class of secondary metabolites, widely present in food and nutraceuticals, are natural phytochemicals mostly derived from phenylalanine and less from tyrosine [50]. Recently, increasing evidence in the scientific literature indicated that phenolic compounds possess an effective inhibitory effect on cancer invasion and metastasis.
Catechins polyphenols, including (−)-epicatechin (EC), (−)-Epigallocatachin (EGC), (−)-epicatechin-3-gallate (ECG), and (−)-epigallocatechin-3-gallate (EGCG), are the most abundant and representative compounds present in tea (Camellia sinensis, Theaceae) [51][52]. In particular, EGCG accounting for 50–80% of the catechin content, identified as one of the most effective compounds as an epigenetic modifier for cancer treatment and chemoprevention, has received recent attention. Furthermore, related experiments showed that both EGCG (0.01 and 0.1%) could inhibit the growth inhibition of the VEGF-VEGFR axis on hepatocellular carcinoma (HCC) cells [21]. In clinical trials, breast cancer patients treated with long-term EGCG plus radiotherapy for 2–8 weeks showed significantly lower serum levels of vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF), as well as reduced activation of metalloproteinase-9 and metalloproteinase-2 (MMP9/MMP2), which are considered promising chemoprophylaxis targets. In addition, the serum of the patient not only inhibited the proliferation and invasion of the highly metastatic human MDA-MB-231 breast cancer cells in vitro, causing cell cycle arrest in G0/G1 phase, but also decreased the activation of MMP9/MMP2, Bcl-2/Bax, c-Met receptor, NF-κB expression, and Akt phosphorylation level. Apoptosis induced by γ radiation was significantly enhanced in MDA-MB-231 cells treated with 5–10 μm EGCG, accompanied by decreased NF-κB protein level and Akt phosphorylation [53]. These results raise the possibility that tea polyphenols may be a therapeutic adjuvant for human metastatic breast cancer. It is also noted that the polyphenolic compounds isolated from green tea can change the miRNA expression profile associated with angiogenesis in various cancer types [19][20]
In vitro experiments, phenolic compounds (PF) and (NPF), as contributing factors to the chemopreventive effects of green tea, both inhibit the migration of cancer cells by the disassembly of microtubules [54]. In a double-blind placebo-controlled study, oral administration of green tea catechin effectively inhibited cancer growth and reduced lower urinary tract symptoms, helping to treat symptoms of benign prostatic hyperplasia [18]. Moreover, another prior clinical trial experiment showed that daily intake of a standardized, catechin mixture containing 200 mg EGCG taken with food for 1 year was well tolerated and did not produce related side effects or adverse effects in men with HGPIN and/or atypical small acinar proliferation (ASAP) [22].
Resveratrol and trans-resveratrol (3,5,4′-trihydroxy-trans-stilbene), a phytoalexin discovered in more than 70 plant species like Polygonum cuspidatum, red grapes, berries, peanuts, and pines can be produced in response to mechanical injuries, ultraviolet radiation, as well as a defense for fungal infections [55][56]. Increasing studies reported that resveratrol possesses anticancer effects against several different tumor types by affecting multiple stages of tumor initiation and proliferation. Oral intake and intravenous administration are two routine administration methods of resveratrol, and oral intake is the major route. The enterocytes and hepatocytes are major metabolizing cells for resveratrol after oral administration, thus, resveratrol was often used to treat gastrointestinal and liver cancers. Intranasal administration of resveratrol at 105 mM for 25 weeks to A/J mice having 4-[methyl(nitroso)amino]-1-(3-pyridinyl)-1-butanone-induced lung carcinogenesis, showed a 27% decrease in tumor multiplicity and resulting in 45% decrease in tumor volume/mouse. While, oral administration of resveratrol failed to protect mice from chemically induced lung carcinogenesis [23]. Moreover, dietary suppression of dextran sulfate sodium (DSS)-associated tumorigenesis using resveratrol (300 ppm) supplementation for one week before the treatment was markedly exhibited in BALB/c background wild-type mice but not in Mkp-1−/− mice on the C57BL/6 background [24]. Additionally, the mitogen-activated protein kinase phosphatase 1 (Mkp-1) is required in the protective role of the Nrf2 signaling pathway against colitis-associated tumorigenesis. In addition, Kiskova et al. [57] reported that the combination of resveratrol and melatonin showed a reduction in N-methyl-N-nitrosourea (NMU)-induced mammary tumor incidence in SD rats by approximately 17% and decreased the quantity of invasive and in-situ carcinomas [57]. In addition, it is interesting to note that the chemopreventive effect of resveratrol (50, 100, and 300 mg/kg) against rat liver carcinogenesis was devoid of any adverse cardiovascular events. In addition, daily oral doses of resveratrol at 0.5 or 1.0 g tumor doses reduced the level of cell Ki-67 staining (a surrogate marker for cell growth) in the human gastrointestinal tract, by orders of magnitude enough to cause an anticancer effect [25][58]. The major metabolites of resveratrol were identified as sulfate and glucuronic acid conjugates of the phenolic groups and hydrogenation of the aliphatic double bond, such as trans-resveratrol (3,5,4′-trihydroxystilbene), trans-resveratrol 3-O-d-sulfate, and trans-resveratrol 4′-O-d-glucuronide [59][60]. Furthermore, the in vitro experiments also showed that the chemopreventive effect of resveratrol could be mediated by its metabolites [61].
Curcumin (bis-α, β-unsaturated β-diketone) is the most abundant polyphenol isolated from the roots of the perennial Curcuma Longa (Curcuma). Curcumin has been widely used as a remedy for many illnesses in different cultures [62][63]. In recent decades, increasing initial clinical studies focused on identifying whether curcumin plays an essential role in colorectal cancer models, due to its preferential distribution in the colonic mucosa. In a transgenic mouse model, phytosomal curcumin diets (150 mg curcuminoids/kg) exhibited significantly greater effects on inhibition of hepatocellular carcinoma formation, improvement of liver histopathology, and reduction of total tumor volume in transgenic mice, compared with unformulated curcumin [26]. Studies have shown that curcumin can be used as a chemoprophylaxis for ovarian cancer, and if taken daily, it can significantly and dose-dependently reduce the spontaneous incidence and tumor growth of ovarian cancer [27]. In addition, curcumin also showed an inhibitory effect in lung carcinogenesis induced by B[a]P, a procarcinogen present in the environment and cigarette smoke [28].
The study found that curcumin, a similar substance to A2, has the potential to be developed as a novel antiangiogenic drug. Curcumin can inhibit HGF- and promote EMT and angiogenesis by targeting c-Met and blocking PI3K/Akt/mTOR pathway. Curcumin analogs A2 can induce endothelial cell death mainly by enhancing NADH/NADPH oxidase-derived ROS [29][64]. In addition, combined exposure to bile with curcumin (50 and 100 μM) exhibited an inhibitory effect on acidic bile-induced oncogenic mRNA phenotype in a human hypopharyngeal primary cell (HHPC), including NF-ΚB, c-REL, compared to HHPC exposed to acidic bile without curcumin [65]. After treatment with 50 μM of curcumin, the invasion rate of thyroid cells through matrigel was decreased to 44.05 ± 4.64%, and scarcely any cells had migrated into the wound area during 24 h [17].
Following the intravenous injection of 20 mg/kg curcumin to mice, two metabolites of dihydrocurcumin (DHC) and tetrahydrocurcumin (THC) were detected in plasma samples. Additionally, it was distributed into liver, kidney and brain quickly with t1/2z of 32.3 ± 10.8 min and AUC0-∞ of 107.0 ± 18.3 mg·min/L [66]. In previous human and animal studies, curcumin has been reported to undergo metabolic reduction through first-pass hepatic metabolism, which resulted in poor oral bioavailability and limited development of curcumin when orally administered. For this, a curcumin gum formulation alternating chewing and parking gum against buccal mucosa for 30 min, tested in healthy adult volunteers, showed higher release (1.67 g vs. 0.67 g) and absorption than chewing gum for 30 min, indicating a combination of chewing and parking the gum for prolonged mucosal contact could produce better release and absorption of curcumin [67].
3.2.2. Flavones
Quercetin, a dietary bioflavonoid, that naturally occurs either as glycoside or as aglycone, is pharmacologically active and widely distributed in apples, onions, tomatoes, broccoli, and citrus fruits [68]. It can be considered as a potential chemopreventive compound due to its cardinal role in influencing the hallmarks of cancer as well as tumor-associated signaling pathways. A study by Zhang et al. [69] found that quercetin can induce tumor cell apoptosis by regulating the NF-B signaling pathway and its target genes Bcl-2 and Bax, suggesting that quercetin may be a candidate gene for oral squamous cell carcinoma (OSCC) chemoprevention [69]. Additionally, no tumor incidence was observed in SD rats treated with quercetin (200 mg/kg, o.p.), while the prostatic intraepithelial neoplasia (PIN) of 40% in the ventral prostate lobe and 20% adenocarcinoma was found in rats induced by hormone (testosterone) and carcinogen (MNU), respectively. The results showed that dietary quercetin could prevent the occurrence of ventral and dorsolateral prostate cancer induced by MNU + T in Sprague-Dawley rats [70]. Furthermore, an in vitro study showed that quercetin dosing of 50 μM decreased migration rates (26.36% vs. 64.36%) and motility rate by approximately tenfold in SK-MEL-28 cells cultured on collagen I matrices [30]. Additionally, another study suggested that quercetin can also be employed as an analgesic agent for cancer-related pain. They reported that when tested on Ehrlich tumor-induced pain, quercetin treatment (10–100 mg/kg, i.p.) has been shown to reduce mechanical and thermal hyperalgesia, but not affect tumor growth by decreasing hyperalgesia cytokines production, neutrophil recruitment, as well as activating opioid-dependent analgesic pathway [71].
Importantly, quercetin also enhanced the tumor growing inhibitory effect of some chemopreventive drugs, such as green tea, by improving their bioavailability. For instance, a follow-up study demonstrated that combination treatment of quercetin and green tea diet suggested a stronger inhibition of tumor growth in tumor-inoculated mice than green tea alone after 4 weeks of tumor inoculation, and inhibited the tumor growth by 47% compared to control. This cotreatment not only increased the tissue concentrations of total green tea polyphenols by 1.5-fold, but also increased nonmethylated EGCG by 1.8-fold [72][73].
Previous animal and human studies have shown that quercetin has poor bioavailability after a single oral administration because its absorption is affected by macronutrients. However, quercetin has a wide range of intestinal first-pass metabolism, which is absorbed by the intestinal tract and then enters phase II metabolism, and modulates the intestinal microbiota composition and protect the intestinal barrier. [74][75]. When 10 mg/kg of quercetin was orally administrated to rats, Chen et al. [76] found that about 93.3% of quercetin was metabolized in the gut, with only 3.1% metabolized in the liver, and the main forms of quercetin excreted in the rat bile were its glucuronide/sulfate and methylate conjugates [76]. Solid lipid nanoparticles (SLNs), a type of submicron particulate drug delivery system, may be available to enhance the absorption of poorly water-soluble drugs. The Cmax value of quercetin in SLNs in a rat’s intestine was increased from 5.90 to 12.22 μg/mL, while the AUC (0→48h) was increased from 56.73 to 324.18 (μg/mL) × h [77].
Carotenoids belong to the chemical group of isoprenoid polyenes, which are natural fat-soluble pigments that provide bright coloration to plants and animals. Anabolism regulation of carotenoids in plants is a complex process, which is regulated by multiple factors [78]. Nowadays, there are many kinds of carotenoids, more than 750 kinds have been found in nature [79]. Carotenoids are commonly divided into four major classes: (1) vitamin A precursors like α, β-carotene; (2) pigments with partial vitamin A activity like cryptoxanthin; (3) non-vitamin A precursors like violaxanthin and neoxanthin; (4) non-vitamin A precursors like lutein and zeaxanthin. Recently, carotenoids have been found to also play an important role in human health; carotenoids dietary intake and serum and tissue levels of carotenoids are correlated with a lower risk of several types of cancer. Most noticeably, α-carotene had higher activity than β-carotene to suppress the tumorigenesis in the skin, lung, liver, and colon. Further evidence suggests that beta-carotene is more effective than retinoic acid (RA) in preventing hepatocellular carcinoma induced by diethylnitrosamine (DEN) and promoted by phenobarbital (PB) [80].
Another carotenoid compound of lycopene was originally developed as a cancer chemopreventive agent for prostate cancer. One of the studies indicated that lycopene supplementation (at doses of 15 mg/day) for 6 months in elderly men diagnosed with benign prostate hyperplasia (BPH) inhibited serum prostate-specific antigen (PSA) increase, and further improving clinical diagnostic markers and symptoms of BPH [32]. Furthermore, the major metabolite of lycopene, Apo-10′-lycopene acid, in the treatment of human liver The-2 and Huh7 cells, can upregulate sirtuin 1 (SIRT1) by stimulating the SIRT1 signaling pathway, which is a NAD(+)-dependent protein deacetylase and dose-dependent inhibition of cell growth. Dietary supplementation of 10 mg/kg Apo10La for 24 weeks significantly reduced the ability of the DEN-initiated high-fat diet (HFD) to promote liver tumors (tumor diversity decreased by 50%; 65% volume) and the incidence of lung tumors in C57BL/6J mice (reduced by 85%), which also improved glucose intolerance and reduced liver inflammation in HFD mice, and was considered an as an effective chemopreventative agent against hepatic tumorigenesis and inflammation [33]. Similarly, epidemiological studies have shown that dietary supplementation with lutein and zeaxanthin is associated with a lower risk of various cancers. Studies have shown that zeaxanthin can induce apoptosis of human uveal melanoma cells in vitro, or inhibit the growth of various tumor cell lines. Lutein can inhibit the PI3K/Akt signaling pathway, induce the apoptosis of lung cancer cells (A549) and reduce the risk of lung cancer without side effects, all of which may be effective natural anticancer drugs [31][81].
Carotenoids are mostly fat-soluble, following the same intestinal absorption path as dietary fat, and therefore, the absence of bile or any generalized malfunction of the lipid absorption system will interfere with the absorption of carotenoids [82]. The carotenoid bioavailability from foodstuffs may be largely affected by the food matrix and other dietary components. It has been reported that carotenoid absorption may be the greatest when daily recommended vegetables are consumed in one meal compared to smaller doses over multiple meals [83].
3.2.3. Monoterpene and Triterpenoids
Over the last two decades, extensive research on plant-based medicinal compounds has revealed a potential role of triterpenoids in prevention. Thymoquinone (2-methyl-5-isopropyl-1,4-benzoquinone) is a monoterpene derived from the seed oil of Nigella sativa L. (family Ranunculaceae), first isolated from black seed in 1963 by El-Dakhakhany [84]. A large number of studies in vivo and in vitro have indicated that thymoquinone suppressed experimentally carcinogenesis in the colon, breast, and skin, and its mechanism may be associated with suppression on the AKT and ERK signaling pathways. Several studies reported that thymoquinone supplementation in drinking water resulted in significant suppression on forestomach, lung cancer, and hepatic carcinogenesis induced by benzo(α) pyrene, 20-methyclonathrene diethylnitrosamine (DENA), and diethylnitrosamine, respectively [85][34][86]. Additionally, thymoquinone at various concentrations ranging from 50 to 100 nM effectively inhibited migration, invasion, and tube formation of human umbilical vein endothelial cells (HUVEC). This study also suggested when given by subcutaneous injection, thymoquinone (6 mg/kg) inhibited human prostate tumor growth in both size and weight in a xenograft human prostate cancer (PC3) model in mice, and this low dosage almost has no chemotoxic side effects [34]. Oral administration of thymoquinone had a slower absorption characteristic as compared with elimination, but with good bioavailability, with a T1/2 value of 274.61 min and Cmax of 3.48 μg/mL [87]. In addition, geraniol, an acyclic monoterpene, represents a potential chemopreventive agent in colon carcinogenesis, as indicated by the decreased number of total aberrant crypt foci (ACF) and ACF ≥ 4 crypts in the distal colon [88]. In in vivo and in vitro experiments, geraniol can also induce PC-3 prostate cancer cells to inhibit the growth of prostate cancer by targeting cell cycle and apoptosis pathways in cultured cells and tumor grafted mice and regulate the expression of various cell cycle regulators and Bcl-2 family proteins in PC-3 cells [89].
Triterpenoids, such as betulinic acid, lupeol, oleanolic acid, and cucurbitacin, are metabolites of isopentenyl pyrophosphate oligomers and are ubiquitously distributed in the form of free triterpenoids, triterpenic glycosides (saponins), and their precursors with potential cancer prevention properties [90][91].
In a colon cancer model, doses of oleanolic acid (OA, 3b-hydroxyolean-12-en-28-oic acid) ranging from 250 to 1500 ppm inhibited mean colonic aberrant crypt foci (36–52%) induced by azoxymethane (AOM) in a dose-dependent manner in male F344 rats [35]. Daily intraperitoneal injections of oleanolic acid (12.5 mg/kg, i.p.) for 16 days have been found to inhibit tumor growth and reduce intratumoral microvessel density (MVD) in xenograft tumor tissues of colorectal cancer mice [36]. However, the systemic absorption of oleanolic acid was extremely low for oral doses of 25 and 50 mg/kg in rats, that may be due to poor gastrointestinal absorption and subsequent hepatic first-pass metabolism [92]. Therefore, other direct blood delivery methods, such as intraperitoneal injections, are more suitable for ursolic acid on cancer chemoprevention.
The chemoprophylaxis of 2-cyano3, 12-dioxane-1, 9 (11)-diene-28-OIC acid (CDDO) against tumor growth in xenograft mouse models of breast cancer was studied in vivo and in vitro. It not only effectively inhibits the activity of HER2 tyrosine kinase, but also inhibits the growth of HER2 overexpressed breast cancer cells [37]. Moreover, methyl ester or ethyl amide derivatives of CDDO showed more potent for delaying the initial development of tumors, than the individual drug of suberoylanilide hydroxamic acid (SAHA), with a 7-week delay before 50% tumor incidence was reached [93].
Another triterpenoid compound of betulinic acid has also been reported to possess antiangiogenic effects by inhibiting aminopeptidase N, an enzyme that is involved in the regulation of angiogenesis induced by growth factor in endothelial cells, possibly by affecting mitochondrial functions [94][38]. Compared to betulinic acid, 20, 29-dihydro-betulinic acid derivatives were found to exert better antiangiogenic properties than betulinic acid [95]. Betulinic acid is confirmed as an agent with rapid absorption and slow biphasic disappearance from serum. After a single dose of 500 mg/kg intraperitoneal betulinic acid, the serum concentrations reached peaks at 0.23 h, and the elimination half-lives were detected as 11.8 h. The distribution of betulinic acid in tissues at 24 h in descending order was as follows: perirenal fat, ovary, spleen, mammary gland, uterus, and bladder [96].
3.2.4. Sulfur Compounds
As we all know, the anticarcinogenic effect of Allium vegetables including garlic is attributed to organosulfur compounds, such as allicin, which exhibited a high protective effect against cancer in animal models induced by a variety of chemical carcinogens [97]. Allicin is responsible for the characteristic odor of garlic and has been shown to have various health-promoting effects, including cancer chemopreventive actions. It is reported that allicin inhibited lymphangiogenesis suppressing activation of vascular endothelial growth factor (VEGF) receptor, which is a critical cellular process implicated in tumor metastasis [39]. Additionally, allicin also can be served as adjunctive therapy for thyroid cancer, as it induces autophagic cell death to alleviate the malignant development of cancer [40]. Allicin and other thiosulfinates decompose instantly to oil-soluble organosulfur compounds in a short of time, including diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DAT), and diallyl tetrasulfide (DATS). A study by Lai et al. documented that DAS, DADS, and DATS may become the potential of the application of drug resistance, including DATS to colo 205 cells migrating and invading the strongest inhibitory effect [42]. Furthermore, administration of DAS (10 mg/kg, i.p.) also significantly reduced the number of tumor-directed capillaries (16.5 vs. 28.75) in male C57BL/6 mice, compared to the control group, through enhancing the production of antiangiogenic factors such as IL-2 and TIMP [41]. Additionally, the regulating effect on the metabolism of the carcinogen is considered as one of the possible mechanisms for protection against cancers of DAS [98].
Sulforaphane is the most characterized isothiocyanate (ITC) compound, mainly found in high concentrations in broccoli, which is viewed as a conceptually promising agent in breast cancer prevention. It can be rapidly absorbed and achieved a peak level before 1 h [43]. Sulforaphane can inhibit the translocation of the NF-κB p65 subunit, downregulate p52 and its downstream transcriptional activity, and preferentially eliminate breast cancer stem cells (CSC). Sulforaphane combined with docetaxel significantly reduces primary tumor volume and secondary tumor formation compared to treatment alone [44]. However, sulforaphane is superior to glucoraphanin in modulating phase I and phase II enzymes involved in carcinogen metabolizing in vitro [99]. Furthermore, whether sulforaphane exerts a direct chemopreventive action on animal and human mammary tissue was determined. Following with oral dosing of a broccoli sprouts preparation containing 200 μmol of sulforaphane, sulforaphane metabolites of dithiocarbamate were readily measurable in human breast tissue enriched for epithelial cells, providing a strong rationale for evaluating the protective effects of sulforaphane in clinical trials of women at risk for breast cancer [100]. In addition, orally ingested other ITC compounds, such as allyl isothiocyanate (AITC), benzyl isothiocyanate (BITC), erucin (ECN), phenethyl isothiocyanate (PEITC), and sulforaphane (SF), were selectively delivered to the bladder via urinary excretion, and possess promising chemopreventive activities against human bladder cancers [101].
3.2.5. Cellulose
Micronutrients, including vitamin D, vitamin E, folic acid, calcium, and selenium, are currently being evaluated in National Cancer Institute (NCI)-sponsored chemoprevention trials for prostate, colon, and breast cancers.
Selenium is considered as one of the most promising agents currently under laboratory development for prostate cancer chemoprevention. Low selenium levels are associated with a higher risk of prostate, lung, and colon cancer. Selenium may have a protective effect on cancer, and individuals with selenium deficiency may benefit from supplemental selenium, thereby reducing their risk of cancer [102]. In addition, compelling evidence that selenium-induced growth inhibition and apoptosis in PC-3 prostate cancer cells were found to be dose-dependent [45]. However, it is worth noting that Se compounds can be either cytotoxic or possibly carcinogenic at higher concentrations, which is indicated to be associated with oxidative stress [103].
Calcium is not only an essential structural component of the human body but also a key functional element for maintaining cellular structure. Calcium is considered to be an effective chemopreventive agent, and increased intracellular Ca2+ concentration enables cytotoxic T lymphocytes (CTL) and natural killer (NK) cells to achieve effective functional expression, thus killing cancer cells [104]. In an in vivo investigation, calcium inhibited colonic epithelial cell proliferation induced by heme in rats, which suggested that calcium might decrease the colon cancer risk related to a high intake of red meat [46]. There have also been studies supporting the use of intravenous CA/MG as an effective neuroprotective against oxaliplatin-induced cumulative sNT in adjuvant colon cancer [47]. There is also some experimental evidence that calcium can protect against breast cancer development, However, calcium intake was not associated with breast cancer risk in the Singapore Chinese Health Study, comparing highest quartile to lowest quartile of intake [105].
1,25-Dihydroxy vitamin D, a naturally occurring, biologically active form of vitamin D, is an important modulator for the absorption and metabolism of calcium, which can become deficient as a result from inappropriate diet or inadequate exposure to sunlight. Therefore, vitamin D also has a role in preventing the malignant transformation and the progression of various types of human tumors while modulating calcium metabolism. The prophylactic effects of vitamin D on the most common types of cancer have been extensively investigated either in vitro or in vivo. The inhibitory effect of vitamin D on tumor cell growth was first described by Colston et al. The reported that decreased cell proliferation was found in melanoma cells treated with 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) [48]. Subsequently, the growth suppressing the activity of 1,25(OH)2D3 was observed in other tumor cell lines such as colon, lung, and prostate cancer cells [49][106]. These studies also highlighted the presence of a specific vitamin D receptor (VDR) that appeared to be essential for the growth inhibitory activity of 1,25(OH)2D3. In line with these investigations, other in vitro studies revealed that decreased intracellular levels of VDR markedly reduced the sensitivity of 1,25(OH)2D3 towards the antiproliferative effects [107]. The major obstacle of 1,25(OH)2D3 in the clinical development was the reason of induced hypercalcemia at pharmacologically relevant doses [108]. In addition, some major studies also exhibited that non-alpha-tocopherol forms of vitamin E hold considerable promise for the chemoprevention of prostate cancer [109].
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global Cancer Statistics. CA Cancer J. Clin. 2011, 61, 69–90.
- Vincent, T.L.; Gatenby, R.A. An evolutionary model for initiation, promotion, and progression in carcinogenesis. Int. J. Oncol. 2008, 32, 729–737.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Sharma, J.; Goyal, P.K. Chemoprevention of chemical-induced skin cancer by Panax ginseng root extract. J. Ginseng Res. 2015, 39, 265–273.
- Wu, X.; Patterson, S.; Hawk, E. Chemoprevention—History and general principles. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 445–459.
- Wattenberg, L.W. Chemoprophylaxis of carcinogenesis: A review. Cancer Res. 1966, 26, 1520–1526.
- Sporn, M.B.; Dunlop, N.M.; Newton, D.L.; Smith, J.M. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed. Proc. 1976, 35, 1332–1338.
- Sporn, M.B. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 1976, 36, 2699–2702.
- Kelloff, G.J.; Hawk, E.T.; Karp, J.E.; Crowell, J.A.; Boone, C.W.; Steele, V.E.; Lubet, R.A.; Sigman, C.C. Progress in clinical chemoprevention. Semin. Oncol. 1997, 24, 241–252.
- Meyskens, F.L.; McLaren, C.E.; Pelot, D.; Brooks, S.F.; Carpenter, P.M.; Hawk, E.; Kelloff, G.; Lawson, M.J.; Kidao, J.; McCracken, J.; et al. Difluoromethylornithine Plus Sulindac for the Prevention of Sporadic Colorectal Adenomas: A Randomized Placebo Controlled. Double-Blind Trial. Cancer Prev. Res. 2008, 1, 32–38.
- Shukla, Y.; Pal, S.K. Dietary cancer chemoprevention: An overview. Int. J. Hum. Genet. 2004, 4, 265–276.
- LaCroix, A.Z.; Powles, T.; Osborne, C.K.; Wolter, K.; Thompson, J.R.; Thompson, D.D.; Allred, D.C.; Armstrong, R.; Cummings, S.R.; Eastell, R.; et al. Breast Cancer Incidence in the Randomized PEARL Trial of Lasofoxifene in Postmenopausal Osteoporotic Women. J. Natl. Cancer Inst. 2010, 102, 1706–1715.
- Goldstein, S.R.; Bhattoa, H.P.; Neven, P.; Cox, D.A.; Dowsett, S.A.; Alam, J.; Sipos, A.; Muram, D. Gynecologic effects of arzoxifene in postmenopausal women with osteoporosis or low bone mass. Menopause 2012, 19, 41–47.
- Ensrud, K.; Genazzani, A.R.; Geiger, M.J.; McNabb, M.; Dowsett, S.A.; Cox, D.A.; Barrett-Connor, E. Effect of raloxifene on cardiovascular adverse events in postmenopausal women with osteoporosis. Am. J. Cardiol. 2006, 97, 520–527.
- Elgendy, M.; Cirò, M.; Hosseini, A.; Weiszmann, J.; Mazzarella, L.; Ferrari, E.; Cazzoli, R.; Curigliano, G.; DeCensi, A.; Bonanni, B.; et al. Combination of Hypoglycemia and Metformin Impairs Tumor Metabolic Plasticity and Growth by Modulating the PP2A-GSK3b-MCL-1 Axis. Cancer Cell 2019, 35, 1–18.
- Liu, Q.L.; Tong, D.L.; Liu, G.L.; Gao, J.; Wang, L.A.; Xu, J.; Yang, X.X.; Xie, Q.B.; Huang, Y.Q.; Pang, J.; et al. Metformin Inhibits Prostate Cancer Progression by Targeting Tumor-Associated Inflammatory Infiltration. Clin. Cancer Res. 2018, 24, 5622–5634.
- Sporn, M.B.; Liby, K.T. A Mini-Review of Chemoprevention of Cancer—Past, Present, and Future. Prog. Chem. 2013, 25, 1421–1428.
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of Human Prostate Cancer by Oral Administration of Green Tea Catechins in Volunteers with High-Grade Prostate Intraepithelial Neoplasia: A Preliminary Report from a One-Year Proof-of-Principle Study. Cancer. Res. 2006, 66, 1234–1240.
- Rashidi, B.; Malekzadeh, M.; Goodarzi, M.; Masoudifar, A.; Mirzaei, H. Green tea and its anti-angiogenesis effects. Biomed. Pharmacother. 2017, 89, 949–956.
- Bigelow, R.L.H.; Cardelli, J.A. The green tea catechins, (−)-Epigallocatechin-3-gallate (EGCG) and (−)-Epicatechin-3-gallate (ECG), inhibit HGF/Met signaling in immortalized and tumorigenic breast epithelial cells. Oncogene 2006, 25, 1922–1930.
- Shirakami, Y.; Shimizu, M.; Adachi, S.; Sakai, H.; Nakagawa, T.; Yasuda, Y. Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor-vascular endothelial growth factor receptor axis. Cancer Sci. 2009, 100, 1957–1962.
- Kumar1, N.B.; Pow-Sang, J.; Spiess, P.E.; Park, J.; Salup, R.; Williams, C.R.; Parnes, H.; Schell, M.J. Randomized, placebo-controlled trial evaluating the safety of one-year administration of green tea catechins. Oncotarget 2016, 7, 70794–70802.
- Monteillier, A.; Voisin, A.; Furrer, P.; Allémann, E.; Cuendet, M. Intranasal administration of resveratrol successfully prevents lung cancer in A/J mice. Sci. Rep. 2018, 8, 14257.
- Zheng, Z.H.; Chen, Y.; Huang, J.; Deng, H.; Tang, X.W.; Wang, X.J. Mkp-1 is required for chemopreventive activity of butylated hydroxyanisole and resveratrol against colitis-associated colon tumorigenesis. Food Chem. Toxicol. 2019, 127, 72–80.
- Patel, K.R.; Brown, V.A.; Jones, D.J.L.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical Pharmacology of Resveratrol and its Metabolites in Colorectal Cancer Patients. Cancer Res. 2010, 70, 7392–7399.
- Teng, C.F.; Yu, C.H.; Chang, H.Y.; Hsieh, W.C.; Wu, T.H.; Lin, J.H.; Wu, H.C.; Jeng, L.B.; Su, I.J. Chemopreventive effect of phytosomal curcumin on hepatitis B virus-related hepatocellular carcinoma in a transgenic mouse model. Sci. Rep. 2019, 9, 10338.
- Sahin, K.; Orhan, C.; Tuzcu, M.; Sahin, N.; Tastan, H.; Ozercan, I.H.; Guler, O.; Kahraman, N.; Kucuk, O.; Ozpolat, B. Chemopreventive and Antitumor Efficacy of Curcumin in a Spontaneously Developing Hen Ovarian Cancer Model. Cancer Prev. Res. 2017, 11, 59–67.
- Puliyappadamba, V.T.; Thulasidasan, A.K.T.; Vijayakurup, V.; Antony, J.; Bava, S.V.; Anwar, S.; Sundaram, S.; Anto, R.J. Curcumin inhibits B[a]PDE-induced procarcinogenic signals in lung cancer cells, and curbs B[a]P-induced mutagenesis and lung carcinogenesis. Biofactors 2015, 41, 431–442.
- Liu, B.; Cui, L.S.; Zhou, B.; Zhang, L.L.; Liu, Z.H.; Zhang, L. Monocarbonyl curcumin analog A2 potently inhibits angiogenesis by inducing ROS-dependent endothelial cell death. Acta Pharmacol. Sin. 2019, 1412–1423.
- Patel, D.H.; Sharma, N. Inhibitory effect of quercetin on epithelial to mesenchymal transition in SK-MEL -28 human melanoma cells defined by in vitro analysis on 3D collagen gels. OncoTargets Ther. 2016, 9, 6445–6459.
- Goswami, S.; Srivastava, A.; Pushker, N. Abstract 3812: Induction of apoptosis by zeaxanthin in human uveal melanoma cells. Cancer Res. 2015, 75, 3812.
- Schwarz, S.; Obermüller-Jevic, U.C.; Hellmis, E.; Koch, W.; Jacobi, G.; Biesalski, H.K. Lycopene inhibits disease progression in patients with benign prostate hyperplasia. J. Nutr. 2008, 138, 49–53.
- Ip, B.C.; Hu, K.Q.; Liu, C.; Smith, D.E.; Obin, M.S.; Ausman, L.M.; Wang, X.D. Lycopene metabolite, apo-10′-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev. Res. 2013, 6, 1304–1316.
- Yi, T.; Cho, S.G.; Yi, Z.; Pang, X.; Rodriguez, M.; Wang, Y.; Sethi, G.; Aggarwal, B.B.; Liu, M. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and ERK signaling pathways. Mol. Cancer Ther. 2008, 7, 1789–1796.
- Janakiram, N.B.; Indranie, C.; Malisetty, S.V.; Jagan, P.; Steele, V.E.; Rao, C.V. Chemoprevention of Colon Carcinogenesis by Oleanolic Acid and Its Analog in Male F344 Rats and Modulation of COX-2 and Apoptosis in Human Colon HT-29 Cancer Cells. Pharm. Res. 2008, 25, 2151–2157.
- LI, L.; Lin, J.; Sun, G.; Wei, L.; Shen, A.; Zhang, M.; Peng, J. Oleanolic acid inhibits colorectal cancer angiogenesis in vivo and in vitro via suppression of STAT3 and Hedgehog pathways. Mol. Med. Rep. 2016, 13, 5276–5282.
- Konopleva, M.; Zhang, W.; Shi, Y.X.; McQueen, T.; Tsao, T.; Abdelrahim, M.; Munsell, M.F.; Johansen, M.; Yu, D.; Madden, T.; et al. Synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces growth arrest in HER2-overexpressing breast cancer cells. Mol. Cancer Ther. 2006, 5, 317–328.
- Ren, W.; Qin, L.; Xu, Y.; Cheng, N. Inhibition of betulinic acid to growth and angiogenesis of human colorectal cancer cell in nude mice. Chin. Ger. J. Clin. Oncol. 2010, 9, 153–157.
- Wang, W.; Sukamtoh, E.; Xiao, H. Allicin Inhibits Lymphangiogenesis in vitro and in vivo. Mol. Nutr. Food Res. 2015, 59, 2345–2354.
- Xiang, Y.; Zhao, J.; Zhao, M. Allicin activates autophagic cell death to alleviate the malignant development of thyroid cancer. Exp. Ther. Med. 2018, 15, 3537–3543.
- Thejass, P.; Kuttan, G. Immunomodulatory activity of Sulforaphane, a naturally occurring isothiocyanate from broccoli (Brassica oleracea). Phytomedicine 2007, 14, 538–545.
- Lai, K.C.; Hsu, S.C.; Kuo, C.L.; Yang, J.S.; Ma, C.Y.; Lu, H.F.; Tang, N.Y.; Hsia, T.C.; Ho, H.C.; Chung, J.G. Diallyl Sulfide, Diallyl Disulfide, and Diallyl Trisulfide Inhibit Migration and Invasion in Human Colon Cancer Colo 205 Cells Through the Inhibition of Matrix Metalloproteinase-2, -7, and -9 Expressions. Environ. Toxicol. 2013, 28, 479–488.
- Ong, C.; Elbarbry, F. A new validated HPLC method for the determination of sulforaphane: Application to study pharmacokinetics of sulforaphane in rats. Biomed. Chromatogr. 2016, 30, 1016–1021.
- Burnett, J.P.; Lim, G.; Li, Y.; Shah, R.B.; Lim, R.; Paholak, H.J.; McDermott, S.P.; Sun, L.; Tsume, Y.; Bai, S.; et al. Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Lett. 2017, 394, 52–64.
- Kandaş, N.O.; Randolph, C.; Bosland, M.C. Differential effects of selenium on benign and malignant prostate epithelial cells: Stimulation of LNCaP cell growth by noncytotoxic, low selenite concentrations. Nutr. Cancer 2009, 61, 251–264.
- Sesink, A.L.A.; Termont, D.S.M.L.; Kleibeuker, J.H.; Meer, R.V.D. Red meat and colon cancer: Dietary haem-induced colonic cytotoxicity and epithelial hyperproliferation are inhibited by calcium. Carcinogenesis 2001, 22, 1653–1659.
- Grothey, A.; Nikcevich, D.A.; Sloan, J.A.; Kugler, J.W.; Silberstein, P.T.; Dentchev, T.; Wender, D.B.; Novotny, P.J.; Chitaley, U.; Alberts, S.R.; et al. Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J. Clin. Oncol. 2011, 29, 421–427.
- Colston, K.; Colston, M.J.; Feldman, D. 1,25-Dihydroxyvitamin D3 and malignant melanoma: The presence of receptors and inhibition of cell growth in culture. Endocrinology 1981, 108, 1083–1086.
- Krishnan, A.V.; Peehl, D.M.; Feldman, D. Inhibition of Prostate Cancer Growth by Vitamin D: Regulation of Target Gene Expression. J. Cell. Biochem. 2003, 88, 363–371.
- Shankar, S.; Srivastava, R.K. Curcumin: Structure, biology and clinical applications. In Nutrition, Diet and Cancer; Shankar, S., Srivastava, R.K., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 413–457.
- Isemura, M.; Saeki, K.; Kimura, T.; Hayakawa, S.; Minami, T.; Sazuka, M. Tea catechins and related polyphenols as anti-cancer agents. Biofactors 2000, 13, 81–85.
- Shukla, Y. Tea and cancer chemoprevention: A comprehensive review. Asian Pac. J. Cancer Prev. 2007, 8, 155–166.
- Zhang, G. Anti-Cancer Activities of Tea Epigallocatechin-3-Gallate in Breast Cancer Patients under Radiotherapy. Curr. Mol. Med. 2012, 12, 163–176.
- Seo, E.J.; Wu, C.F.; Ali, Z.; Wang, Y.H.; Khan, S.; Walker, L.A.; Khan, I.A.; Efferth, T. Both Phenolic and Non-phenolic Green Tea Fractions Inhibit Migration of Cancer Cells. Front. Pharmacol. 2016, 7, 398.
- Ogas, T.; Kondratyuk, T.P.; Pezzuto, J.M. Resveratrol analogs: Promising chemopreventive agents. Ann. N. Y. Acad. Sci. 2013, 1290, 21–29.
- Leipert, J.; Kässner, F.; Schuster, S.; Händel, N.; Körner, A.; Kiess, W.; Garten, A. Resveratrol potentiates growth inhibitory effects of rapamycin in PTEN-deficient lipoma cells by suppressing p70S6 kinase activity. Nutr. Cancer 2016, 68, 342–349.
- Kiskova, T.; Ekmekcioglu, C.; Garajova, M.; Orenda, S.P.; Bojkova, B.; Bobrov, N.; Jager, W.; Kassayova, M.; Thalhammer, T. A combination of resveratrol and melatonin exerts chemopreventive effects in N-methyl-N-nitrosourea-induced rat mammary carcinogenesis. Eur. J. Cancer Prev. 2012, 21, 163–170.
- Li, R.; Ma, X.Y.; Song, Y.Q.; Zhang, Y.Y.; Xiong, W.B.; Li, L.; Zhou, L.M. Anti-colorectal cancer targets of resveratrol and biological molecular mechanism: Analyses of network pharmacology, human and experimental data. J. Cell Biochem. 2019, 120, 11265–11273.
- Wang, D.; Hang, T.; Wu, C.; Liu, W. Identification of the major metabolites of resveratrol in rat urine by HPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 829, 97–106.
- Aires, V.; Limagne, E.; Cotte, A.K.; Latruffe, N.; Francois, G.; Delmas, D. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol. Nutr. Food Res. 2013, 57, 1–12.
- Storniolo, C.; Moreno, J.J. Resveratrol metabolites have an antiproliferative effect on intestinal epithelial cancer cells. Food Chem. 2012, 134, 1385–1391.
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.H.; Kumar, A.P.; Sethi, G. The Multifaceted Role of Curcumin in Cancer Prevention and Treatment. Molecules 2015, 20, 2728–2769.
- Park, W.; Amin, A.R.M.R.; Chen, Z.G.; Shin, D.M. New Perspectives of Curcumin in Cancer Prevention. Cancer Prev. Res. 2013, 6, 387–400.
- Jiao, D.; Wang, J.; Lu, W.; Tang, X.; Chen, J.; Mou, H.; Chen, Q.Y. Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways in lung cancer. Mol. Ther. Oncolytics 2016, 3, 16018.
- Vageli, D.P.; Doukas, S.G.; Spock, T.; Sasaki, C.T. Curcumin prevents the bile reflux-induced NF-jB-related mRNA oncogenic phenotype, in human hypopharyngeal cells. J. Cell. Mol. Med. 2018, 22, 4209–4220.
- Wang, J.J.; Yu, X.J.; Zhang, L.; Wang, L.; Peng, Z.H.; Chen, Y. The pharmacokinetics and tissue distribution of curcumin and its metabolites in mice. Biomed. Chromatogr. 2018, 32, e4267.
- Boven, L.; Holmes, S.P.; Latimer, B.; McMartin, K.; Ma, X.H.; Moore-Medlin, T.; Khandelwal, A.R.; McLarty, J.; Nathan, C.A.O. Curcumin Gum Formulation for Prevention of Oral Cavity Head and Neck Squamous Cell Carcinoma. Laryngoscope 2019, 129, 1597–1603.
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; Biasi, S.D.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A.A. Quercetin and Cancer Chemoprevention. Evid. Based Complement. Alternat. Med. 2011, 2011, 591356.
- Zhang, W.; Yin, G.; Dai, J.G.; Sun, Y.; Hoffman, R.M.; Yang, Z.J.; Fan, Y. Chemoprevention by Quercetin of Oral Squamous Cell Carcinoma by Suppression of the NF-ĸB Signaling Pathway in DMBA-treated Hamsters. Anticancer Res. 2017, 37, 4041–4050.
- Sharmila, G.; Athirai, T.; Kiruthiga, B.; Senthilkumar, K.; Elumalai, P.; Arunkumar, R.; Arunakaran, J. Chemopreventive Effect of Quercetin in MNU and Testosterone Induced Prostate Cancer of Sprague-Dawley Rats. Nutr. Cancer 2014, 66, 38–46.
- Cassia, C.C.; Corrêa, M.P.; Carvalho, T.T.; Zarpelon, A.C.; Hohmann, M.S.; Rossaneis, A.C.; Coelho-Silva, L.; Pavanelli, W.R.; Pinge-Filho, P.; Crespigio, J. Quercetin reduces Ehrlich tumor-induced cancer pain in mice. Anal. Cell. Pathol. 2015, 2015, 285708.
- Wang, P.; Vadgama, J.V.; Said, J.W.; Magyar, C.E.; Doan, N.; Heber, D.; Henning, S.M. Enhanced inhibition of prostate cancer xenograft tumor growth by combining quercetin and green tea. J. Nutr. Biochem. 2014, 25, 73–80.
- Wang, P.; Heber, D.; Henning, S.M. Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo. Food Funct. 2012, 3, 635–642.
- Rich, G.T.; Buchweitz, M.; Winterbone, M.S.; Kroon, P.A.; Wilde, P.J. Towards an understanding of the low bioavailability of quercetin: A study of its interaction with intestinal lipids. Nutrients 2017, 9, 111.
- Ader, P.; Wessmann, A.; Wolffram, S. Bioavailability and metabolism of the flavonol quercetin in the pig. Free Radic. Biol. Med. 2000, 28, 1056–1067.
- Xiao, C.; Yin, O.Q.P.; Zhong, Z.; Chow, M.S.S. Pharmacokinetics and Modeling of Quercetin and Metabolites. Pharm. Res. 2005, 22, 892–901.
- Li, H.L.; Zhao, X.B.; Ma, Y.K.; Zhai, G.X.; Li, L.B.; Lou, H.X. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control Release 2009, 133, 238–244.
- Wu, Y.Y. Research Progress in Anabolic Control Mechanisms of Plant Carotenoids. Bot. Res. 2020, 9, 217–225.
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer Chemoprevention by Carotenoids. Molecules 2012, 17, 3202–3242.
- Bishayee, A.; Sarkar, A.; Chatterjee, M. Further evidence for chemopreventive potential of beta-carotene against experimental carcinogenesis: Diethylnitrosamine-initiated and phenobarbital-promoted hepatocarcinogenesis is prevented more effectively by beta-carotene than by retinoic acid. Nutr. Cancer 2000, 37, 89–98.
- Zhang, W.L.; Zhao, Y.N.; Shi, Z.Z.; Cong, D.; Bai, Y.S. Lutein Inhibits Cell Growth and Activates Apoptosis via the PI3K/AKT/mTOR Signaling Pathway in A549 Human Non-Small-Cell Lung Cancer Cells. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 341–350.
- Yonekura, L.; Nagao, A. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food Res. 2007, 51, 107–115.
- Brown, M.J.; Ferruzzi, M.G.; Nguyen, M.L.; Cooper, D.A.; Eldridge, A.L.; Schwartz, S.J.; White, W.S. Carotenoid bioavailability is higher from salads ingested with full-fat than fat-reduced salad dressings as measured with electrochemical detection. Am. J. Clin. Nutr. 2004, 80, 396–403.
- Elgendy, M.; Ciro, M.; Hosseini, A.; Weiszmann, J.; Mazzarella, L.; Ferrari, E.; Cazzoli, R.; Curigliano, G.; DeCensi, A.; Bonanni, B.; et al. Studies on the chemical constitution of Egyptian, N. sativaL. Seeds. Planta Med. 1963, 11, 465–470.
- Sayed-Ahmed, M.M.; Aleisa, A.M.; Al-Rejaie, S.S.; Al-Yahya, A.A.; Al-Shabanah, O.A.; Hafez, M.M.; Nagi, M.N. Thymoquinone Attenuates Diethylnitrosamine Induction of Hepatic Carcinogenesis Through Antioxidant Signaling. Oxid. Med. Cell. Longev. 2010, 3, 254–261.
- Aziza, S.; Hussein, S.A.; Khalaf, H.A. Chemopreventive effect of thymoquinone on benzo(a)pyrene-induced lung cancer in male swiss albino mice. Benha Vet. Med. J. 2014, 27, 330–340.
- Alkharfy, K.M.; Ahmad, A.; Khan, R.M.A.; Al-Shagha, W.M. Pharmacokinetic plasma behaviors of intravenous and oral bioavailability of thymoquinone in a rabbit model. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 319–323.
- Vieira, A.; Heidor, R.; Cardozo, M.T.; Scolastici, C.; Purgatto, E.; Shiga, T.M.; Barbisan, L.F.; Ong, T.P.; Moreno, F.S. Efficacy of geraniol but not of β-ionone or their combination for the chemoprevention of rat colon carcinogenesis. Braz. J. Med. Biol. Res. 2011, 44, 538–545.
- Kim, S.H.; Bae, H.C.; Park, E.J.; Lee, C.R.; Kim, B.J.; Lee, S.; Park, H.H.; Kim, S.J.; So, I.; Kim, T.W. Geraniol inhibits prostate cancer growth by targeting cell cycle and apoptosis pathways. Biochem. Biophys. Res. Commun. 2011, 407, 129–134.
- Patlolla, J.M.R.; Rao, C.V. Triterpenoids for Cancer Prevention and Treatment: Current Status and Future Prospects. Curr. Pharm. Biotechnol. 2012, 13, 147–155.
- Mahato, S.B.; Sarkar, S.K.; Poddar, G. Triterpenoid saponins. Phytochemistry 1988, 27, 3037–3067.
- Dong, W.J.; Kim, Y.H.; Hui, H.K.; Ji, H.Y.; Yoo, S.D.; Choi, W.R.; Lee, S.M.; Han, C.K.; Lee, H.S. Dose-Linear Pharmacokinetics of Oleanolic Acid after Intravenous and Oral Administration in Rats. Biopharm. Drug Dispos. 2007, 28, 51–57.
- Tran, K.; Risingsong, R.; Royce, D.B.; Williams, C.R.; Sporn, M.B.; Pioli, P.A.; Gediya, L.K.; Njar, V.C.; Liby, K.T. The combination of the histone deacetylase inhibitor vorinostat and synthetic triterpenoids reduces tumorigenesis in mouse models of cancer. Carcinogenesis 2013, 34, 199–210.
- Kwon, H.J.; Shim, J.S.; Kim, J.H.; Cho, H.Y.; Yum, Y.N.; Kim, S.H.; Yu, J. Betulinic acid inhibits growth factor-induced in vitro angiogenesis via the modulation of mitochondrial function in endothelial cells. Jpn. J. Cancer Res. 2002, 93, 417–425.
- Ribeiro, C.M.S.; Savelkoul, H.F.J.; Wiegertjes, G.F. Immune responses of carp against parasites. Cheminform 2009, 35, 3169–3172.
- Udeani, G.O.; Zhao, G.M.; Geun, S.Y.; Cooke, B.P.; Graham, J.; Beecher, C.W.; Kinghorn, A.D.; Pezzuto, J.M. Pharmacokinetics and tissue distribution of betulinic acid in CD-1 mice. Biopharm. Drug Dispos. 1999, 20, 379–383.
- Herman-Antosiewicz, A.; Powolny, A.A.; Singh, S.V. Molecular targets of cancer chemoprevention by garlic-derived organosulfides. Acta Pharmacol. Sin. 2007, 28, 1355–1364.
- Jakubikova, J.; Sedlak, J. Garlic-derived organosulfides induce cytotoxicity, apoptosis, cell cycle arrest and oxidative stress in human colon carcinoma cell lines. Neoplasma 2006, 53, 191–199.
- Razis, A.F.A.; Noor, N.M. Sulforaphane is superior to glucoraphanin in modulating carcinogen-metabolising enzymes in Hep G2 cells. Asian Pac. J. Cancer Prev. 2013, 14, 4235–4238.
- Cornblatt, B.S.; Ye, L.; Dinkova-Kostova, A.T.; Erb, M.; Fahey, J.W.; Singh, N.K.; Chen, M.S.A.; Stierer, T.; Garrett-Meyer, E.; Argani, P.; et al. Preclinical and Clinical Evaluation of Sulforaphane for Chemoprevention in the Breast. Carcinogenesis 2007, 28, 1485–1490.
- Veeranki, O.L.; Bhattacharya, A.; Tang, L.; Marshall, J.R.; Zhang, Y. Cruciferous Vegetables, Isothiocyanates, and Prevention of Bladder Cancer. Curr. Pharmacol. Rep. 2015, 1, 272–282.
- Davis, C.D. Selenium Supplementation and Cancer Prevention. Curr. Nutr. Rep. 2012, 1, 16–23.
- Letavayová, L.; Vlcková, V.; Brozmanová, J. Selenium: From cancer prevention to DNA damage. Toxicology 2006, 227, 1–14.
- Schwarz, E.C.; Qu, B.; Hoth, M. Calcium, cancer and killing: The role of calcium in killing cancer cells by cytotoxic T lymphocytes and natural killer cells. Biochim. Biophys. Acta 2013, 1833, 1603–1611.
- Li, J.; Koh, W.P.; Jin, A.Z.; Yuan, J.M.; Yu, M.C.; Butler, L.M. Calcium intake is not related to breast cancer risk among Singapore Chinese women. Int. J. Cancer 2013, 133, 680–686.
- Pommergaard, H.C.; Raskov, H.; Rosenberg, B.J. Chemoprevention with Acetylsalicylic Acid, Vitamin D and Calcium Reduces Risk of Carcinogen-induced Lung Tumors. Anticancer Res. 2013, 33, 4767.
- Welsh, J. Cellular and molecular effects of vitamin D on carcinogenesis. Arch. Biochem. Biophys. 2012, 523, 107–114.
- Khan, Q.J.; Kimler, B.F.; Fabian, C.J. The relationship between vitamin D and breast cancer incidence and natural history. Curr. Oncol. Rep. 2010, 12, 136–142.
- Stone, W.L.; Campbell, S.E.; Krishnan, K. The Role of Vitamin E in Prostate Cancer. In Oxidative Stress in Cancer Biology and Therapy; Basu, S., Wiklund, L., Eds.; Human Press: Totowa, NJ, USA, 2012; pp. 333–354.




