| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Blair Steele | + 3136 word(s) | 3136 | 2021-03-04 07:04:16 | | | |
| 2 | Karina Chen | -3 word(s) | 3133 | 2021-04-01 07:58:30 | | |
Video Upload Options
Cannabinoid and non-cannabinoid phytochemicals possess bioactive and protective properties that are beneficial to human health. In addition to cannabinoids, the cannabis plant also produces hundreds of non-cannabinoid secondary metabolites including approximately 120 terpenoids (61 monoterpenes, 52 sesquiterpenoids, and 5 triterpenoids, essential oils, over 26 flavonoids lignans, stilbenoid derivatives, alkaloids, amino acids, spiroindans, polyphenols, 20 steroids ), dihydrophenanthrenes, glycoproteins (such as galactose, glucose, mannose and xylose), and dibenzyls. This list also includes a-cannabispiranol, chrysoeriol, 6-prenylapigenin, cannflavin A and b-acetyl cannabispiranol.
1. Terpenes and Their Derivatives, Terpenoids
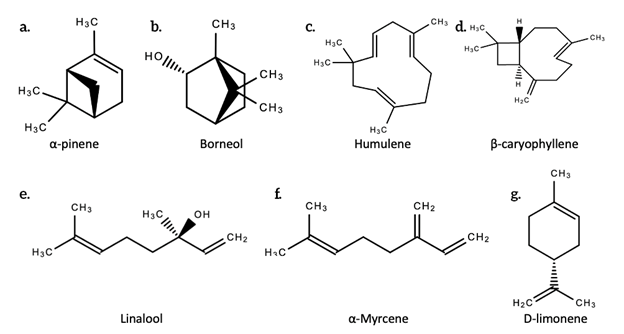
The word “terpene” was devised in 1866 by August Kekulé, a German organic chemist. Terpenes are hydrocarbons and are made up of isoprene units (5-carbon building blocks) [1], while terpenoids are an oxidized and denatured form of terpenes that differ in that they contain an additional functional group with oxygen [1]. They are not the same, despite being used synonymously. This oxidation occurs during the drying and curing processes when the plant is exposed to open air. Terpenes are typically classified by the number of isoprene units in the molecule. Isoterpene is the only hemiterpenes. Hemiterpenes and hemiterpenoids such as prenol and isovaleric acid only have a single isoprene unit. Monoterpenes and monoterpenoids such as pinene (most common terpene produced across plant species), limonene, myrcene, geraniol, and terpineol have two isoprene units. Sesquiterpenes and sesquiterpenoids such as humulene and farnesol have three isoprene units. Triterpenes, such as squalene—the precursors to all steroids [1]—have six isoprene units. Sesquiterpenes and tetraterpenes have seven and eight isoprene units, respectively. Polyterpenes and norisoprenoids have multiple isoprene units in their molecule. Figure 1 below shows the structures of some terpenoids.
Figure 1. The chemical structures of some terpenoids produced by Cannabis sativa L. a. α-pinene; b. Borneol; c. Humulene; d. β-caryophyllene; e. Linalool; f. α-Myrcene; g. D-linonene.
Figure 1 above shows the biosynthetic pathway of terpenes and terpenoids, which are synthesized from an isoprenoid precursor Isopentenyl pyrophosphate (IPP). This is achieved via the plastidial deoxyxylulose phosphate/methyl-erythritol phosphate (DOXP/MEP) pathway (monoterpenoids), and the cytoplasmic mevalonate (MVA) pathway (sesquiterpenoids, triterpenoids, and sterols) [2]. Along with cannabinoids, terpenes are considered a main physiological marker of secondary metabolites [3][4].
Terpenes are a large and diverse class of aromatic compounds responsible for the unique flavors and scents of many herbs and plants and phenotypic variation across plant species. Over 20,000 terpenes exist across plant species [5], and over 150 alone in the cannabis plant [6]. This makes terpenes the largest classification of phytochemicals (Andre, Hausman, Guerriero, 2016). Their primary role in plants vary but, in some plants, may attract pollinators, and in others, deter herbivores and inhibit microbial growth.
The most common terpenes in cannabis include limonene, α-pinene, β-pinene, humulene, β-caryophyllene, linalool and myrcene [2]. Other common terpenes in cannabis include bisabolol, borneol, camphene, geraniol, ocimene, terpineol, and valencene. These fall into the monoterpenoids and sesquiterpenoids categories. Refer to Table 1 below for examples of mono- and sesquiterpenoids profiled in a 2020 study by Jin and colleagues that profiled secondary metabolites in cannabis inflorescences, leaves, stem bars, and roots for medicinal purposes [2].
Table 1. Examples of mono- and sesquiterpenoids profiled in cannabis inflorescences, leaves, stem bars, and roots for medicinal purposes[2].
|
|
Monoterpenoids |
|
Sesquiterpenoids |
|
|
α-Pinene |
Eucalyptol |
Borneol |
(-)-β-Elemene |
Viridiflorol |
|
Camphene |
Ocimene |
Terpinen-4-ol |
β-Caryophyllene |
(-)-Guaiol |
|
Sabinene |
γ-Terpinene |
-Terpineol |
Aromadendrene |
(+)-Cedrol |
|
(-)-β-Pinene |
Sabinene Hydrate |
(+)-Dihydrocarvone |
Trans-β-Farnesene |
β-Eudesmol |
|
β-Myrcene |
Terpinolene |
Nerol |
α-Humulene |
α-Bisabolol |
|
α-Phellandrene |
Frenchone |
Pulegone |
Valencene |
|
|
Δ 3-Carene |
Linalool |
Carvone |
Ledene |
|
|
α-Terpinene |
Frenchol |
Geraniol |
Trans-Nerolidol |
|
|
p-Cymene |
(-)-Isopulegol |
Geranyl Acetate |
Caryophyllene Oxide |
|
|
Limonene |
Camphor |
|
Globulol |
|
Terpenes provide aromatherapeutic benefits to humans including stress, anxiety, and depression relief, decongestion, and a general pharmacologically synergistic effect in combination with cannabinoids and flavonoids. As a result, terpenes are frequently used in cosmeceuticals, perfumes and beverage flavoring.
Terpenes and terpenoids are highly concentrated in the essential oils extracted from the cannabis plant, and are also responsible for the therapeutic benefits that essential oils provide. A study by Piccaglia et al., 2016 explored the biological properties of some terpenes making up said essential oils extracted from the cannabis plant (Table 2) by distillation and characterized by gas chromatography–mass spectrometry (GC–MS). Table 2 also shows the biological properties of other common terpenes found in cannabis.
Table 2. The biological properties of some common terpenes in cannabis.
|
Terpene |
Biological Property |
|
Myrcene |
Potent analgesic |
|
Antioxidant; neuroprotective; anti-inflammatory |
|
|
Anticonvulsant |
|
|
1,8-cineole |
Increases cerebral blood flow and enhances cortical activity |
|
Limonene |
inhibits many species of bacteria and fungi—repellant |
|
Anti-inflammatory; antioxidant; antiviral; antidiabetic; anticancer |
|
|
Antidepressant; anticonvulsant |
|
|
α-Pinene |
Antimicrobial; repellant |
|
Bronchodilator; anti-inflammatory, |
|
|
Memory improvement/enhancement; acetylcholinesterase inhibitor |
|
|
Linalool (Lavender scent) |
Anxiolytic; anti-inflammatory; antimicrobial; anticancer; neuroprotective; antidepressant |
|
Anti-influenza |
|
|
Sedative; induces apoptosis in cancer cells |
|
|
α-terpineol |
Antimicrobial; repellant |
|
Anti-inflammatory |
|
|
Analgesic |
|
|
Nociception inhibition |
|
|
Anticonvulsant |
|
|
Antimicrobial |
|
|
Gastroprotective |
|
|
Borneol |
Antimicrobial; repellant |
|
β- caryophyllene |
Anti-inflammatory Analgesic |
|
Antispasmic in gut muscles |
|
|
Humulene |
Antiallergy; anticancer |
|
Ocimene |
Antifungal; antibacterial; antioxidant; antiviral; anti-inflammatory |
The Medical Cannabis Network also reports that a current study is being undertaken by researchers at the Israel Institute of Technology investigating the therapeutic efficacy of a cannabis terpene inhalant formulation in suppressing the immune system response against COVID-19.
2. Phenolic Compounds in Cannabis sativa
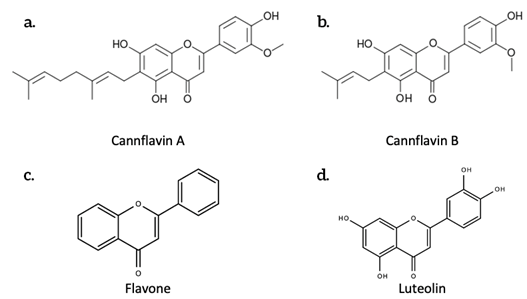
Polyphenols, also known as phenylpropanoids, are a class of over 10,000 chemical compounds that have phenyl groups/rings (C6H5). A phenyl ring consists of a 6-carbon structure formed on a hexagonal plane. Five of these carbon atoms are each individually bonded to hydrogen atoms (Figure 2a).
Figure 2. The chemical structures of some flavonoids produced by Cannabis sativa L. a. Cannflavin A; b. Cannflavin B; c. Flavone; d. Luteolin.
A 2020 study by Izzo and colleagues analyzed some phenolic compounds in Cannabis sativa L. inflorescences using ultra-high-performance liquid chromatography-quadruple-orbitrap high-resolution mass spectrometry (UHPLC-Q-Orbitrap HRMS). Table 3 below lists some of the phenolic compounds investigated. Cannabisin A, B and C were the predominant lignanamides identified [7].
Table 3. List of phenolic compounds in commercial Cannabis sativa L. [7].
|
Flavonols |
Phenolic Amides |
Flavones |
Phenolic acids |
|
Catechin |
N-trans-caffeoyltyramine |
Cannflavin A |
Hydroxycinnamic acids |
|
Epicatechin |
Flavonoids |
Cannflavin B |
Chlorogenic acid |
|
Flavanone |
Flavonol |
Luteolin-7-O-glucoside |
Caffeic acid |
|
Naringenin |
Rutin |
Apigenin-7-O-glucoside |
p-Coumaric acid |
|
Lignanamides |
Quercetin-3-glucoside |
Luteolin |
Ferulic acid |
|
Cannabisin A |
Kaempferol-3-O-glucoside |
Apigenin |
|
|
Cannabisin B |
Quercetin |
|
|
|
Cannabisin C |
Kaempferol |
|
|
3. Flavonoids
Flavonoids are a large family of polyphenolic plant compounds that naturally occur in fruits, vegetables, chocolate, and beverages such as wine and tea.
There are six classes of flavonoids including anthocyanidins, flavan-3-ols, flavonols, flavanones, flavones, and isoflavones. Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B), and a heterocyclic ring. This carbon structure can be abbreviated C6-C3-C6. Figure 3 below shows the structures of some flavonoids.
Flavonoids are found in a wide range of plants and may act as physiological regulators, cell-cycle inhibitors and/or chemical messengers. They are also responsible for plant pigmentation (flower coloration to attract pollinator animals/insects), UV filtration/protection, and symbiotic nitrogen fixation.
Flavonoids provide color, flavor and aroma. These class of compounds also have anticancer, antioxidant, antithrombogenic, antidiabetic and neuroprotective activities via modulation of a number of cell-signaling cascades.
A 2016 study by Bertoia and colleagues explored the relationship between dietary flavonoid intake (of flavonols, flavones, flavanones, flavan-3-ols, anthocyanins, and flavonoid polymers) and weight change at 4-year intervals between 1986 and 2011 of 124,086 men and women. In conclusion of this study, intake of foods rich in flavonols, flavan-3-ols, anthocyanins, and flavonoid polymers, inversely related to weight gain. Anthocyanidins had the greatest negative correlation with weight maintenance [8]. See Tables 4 and 5 below for some biological properties of some common classes of flavonoids.
Table 4. The biological properties of some common classes of flavonoids.
|
Flavonoid |
Biological Property |
|
Flavonols (e.g., quercetin and kaempferol) |
Antioxidant; cardioprotective |
|
Flavanones |
Antioxidant; anticancer; anti-inflammatory |
|
Isoflavonoids |
Phytoestrogenic (mimic the hormone estrogen); hormone balance and metabolism |
|
Anthocyanins (responsible for a plant’s unique colour) |
Antioxidant and anti-inflammatory |
Over 20 different flavonoids have been identified in cannabis, most of which fall into the flavonol and flavone group [9]. One such flavonoid is cannflavin A, which is considered to be 30 times more effective than Aspirin at inhibiting prostaglandin E2, a significant modulator of inflammation [10]. Cannflavin B is also an inhibitor of prostaglandin E2 [11].
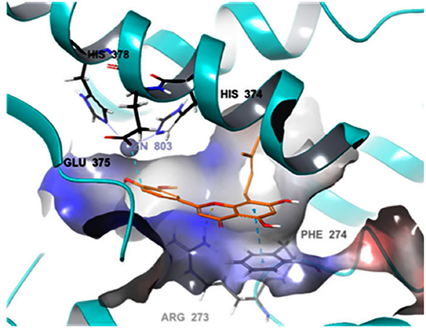
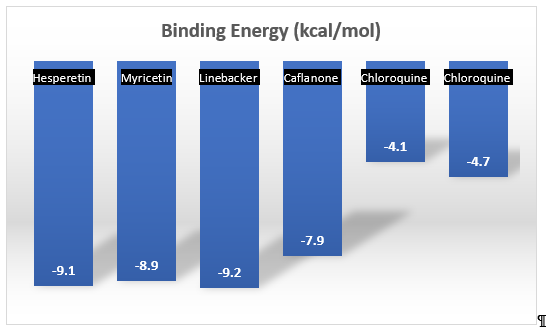
Another major flavonoid derived from cannabis is caflanone. In recent studies, caflanone demonstrated activity against the human coronavirus OC43 (HCoV-OC43) also known as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). HCoV-OC43 belongs to clade b of the genus Betacoronavirus[12]. In vitro, caflanone inhibited HCoV-OC43 with an EC50 of 0.42 µM [12]. In silico studies showed that caflanone may act by inhibiting the angiotensin-converting enzyme 2 (ACE2) receptor found in the lung and respiratory tract, and used by the virus during cell entry and infection. Caflanone was also shown to have strong binding affinity to two of the proteases (PLpro and 3CLpro) are vital to the replication of SARS-CoV-2 in humans, thereby inhibiting viral entry to and/or replication within human cell [12]. Figure 3 shows the main interactions of caflanone with the proteins ACE2 (catalytic site/zinc metallopeptidase domain), Glu375, Glu402, ZN803, HIS374, Phe274 and Arg 273 [12]. The docking/binding studies results below show that the phytoantiviral flavonoids (Hesperetin, myricetin, linebacker, and caflanone) could bind equally or more effectively than chloroquine (CLQ), another investigated drug for SARS-CoV-2 [12] (Figure 4).
Table 5. More therapeutic benefits of flavonoids.
|
Therapeutic Window/Benefits of Flavonoids |
|
Flavonoid derivatives targeting kinases, sirtuins and oncogenic agents for the treatment of cancers. |
|
Agent containing flavonoid derivatives for treating cancer and inflammation. |
|
Therapeutic agents containing cannabis flavonoid derivative for ocular disorders. |
|
Therapeutic agents containing cannabis flavonoid derivatives for the prevention and treatment of neurodegenerative disorders. |
|
Pi 4-kinase inhibitor as a therapeutic for viral hepatitis, cancer, malaria. autoimmune disorders and inflammation, and a radiosensitizer and immunosuppressant. |
|
Therapeutic antiviral agents containing cannabis cannabinoid derivatives. |
Figure 3. The main protein interactions of caflanone [12]. Reproduced with permission from Wilfred Ngwa, Potential of Flavonoid-Inspired Phytomedicines against COVID-19, published by Molecules Open-Access Journal, 11 June 2020 [12].
Figure 4. A comparison of the docking/binding studies results between the phytoantiviral flavonoids hesperetin, myricetin, linebacker, and caflanone [12].
4. Fatty Acids of Cannabis Seeds
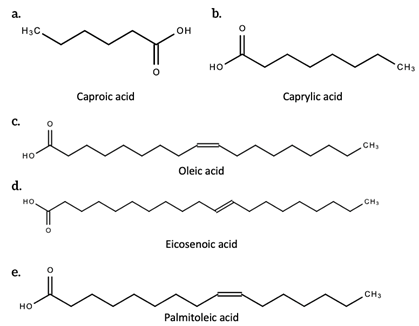
Fatty acids have high nutritional value. They are a class of molecules made up of a chain of carbon atoms bonded with hydrogen atoms, with a function carboxyl group (-COOH) attached to a terminal end. It is this functional group that participates in chemical reactions that allow fatty acids to perform their physiological roles. Figure 5 below shows the structure of some fatty acids produced by C. sativa L. These roles include providing insulation, storing and providing energy for cells in the absence of glucose, providing the precursor (cholesterol) for the production of hormones and intracellular membranes (estrogen, testosterone, vitamin D hormone, steroids, and prostaglandins), forming the building blocks of glycolipids and phospholipids which form the cell membrane and subcellular membrane, transporting fat soluble vitamins (A, D, E and K), and modifying proteins. Many of these fatty acids have nutritional and pharmaceutical potential [13].
Figure 5. The structure of some fatty acids found in C. sativa L. [14]. a. Caproic acid; b. Caprylic acid; c. Oleic acid; d. Eicosenoic acid; e. Palmitoleic acid.
A 1996 study by Ross and colleagues explored the composition of fatty acids present in the lipid matter of [commercial] cannabis seeds from different countries. Some fatty acids in commercial Cannabis sativa (seeds) include caproic acid, caprylic acid, myristic acid, palmitoleic acid, palmitic acid, margaric acid, oleic acid, linolenic acid, isolinolenic acid, linoleic acid, stearic acid, eicosenoic acid, arachidic acid, isoarachidic acid, and behenic acid [14].
It should also be noted that C. sativa L. seeds, particularly those of the hemp variety, typically have nutritional value. The seed is made up of approximately 25% high-quality protein and 35% fat [15]. Table 6 below lists some vitamins, minerals and macronutrients found in C. sativa L. seeds. In addition to significant concentrations of fatty acids (particularly omega-6 fatty acids), hemp oil extracted from C. sativa L. seeds also contain vitamin C [16][17]; thiamine [16][17]; riboflavin [16][17]; vitamin E [16][17]; minerals such as calcium [16], magnesium [16][17], potassium [16][17], phosphorus [16][17], iron[16][17], zinc [16][17], sodium [16][17], and copper [17]; and macronutrients such as fat [16], carbohydrates [16], fiber [16], and protein [16].
Table 6. Examples of vitamins, minerals and macronutrients produced by C. sativa L. seeds [16].
|
Vitamins |
Minerals |
Macronutrients |
|
C |
Calcium |
Fat |
|
Thiamine |
Iron |
Carbohydrate |
|
Riboflavin |
Magnesium |
Fiber |
|
Niacin |
Phosphorus |
Protein |
|
B-6 |
Potassium |
kJ:2313 |
|
Folate |
Sodium |
|
|
A |
Zinc |
|
|
E |
|
|
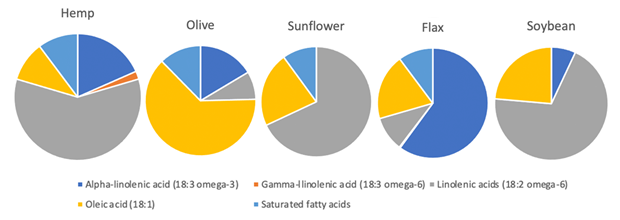
In comparison to other popular and nutritionally-rich edible oils such as canola, sunflower, pumpkin and soybean oils, only hemp oil provides the optimal ratio (3:1) of omega-6 fatty acid (linolic acid) and omega-3-fatty acids (alpha-linolenic acid), along with gamma-linolenic acid (GLA) and stearidonic acid (SDA) [15]. See Figure 6 for a comparison of the composition of edible plant oils.
Figure 6. Comparison of the fatty acid composition of edible plant oils[15].
5. Alkaloids in Cannabis
In 1876, Preobrajensky claimed to find nicotine in cannabis resin (hashish) from Uzbekistan in 1876 [18]. This was later rejected on the basis that cannabis users from that part of the world tend to mix the resin with tobacco before smoking. This made it likely that the presence of nicotine was due to the tobacco. Later in 1881, at the British Pharmaceutical Conference, Siebold and Bradbury reported on the isolation of the alkaloid cannabinine [19]. Two years later, in 1883, Hay isolated tetanocannabin, another biologically active alkaloid. It was so called because it produced strychnine-like convulsions in frogs [20]. In 1986, even Merck (of Darmstadt) began marketing and advertising a “cannabine alkaloid” product [21].
Alkaloids are a class of heterocyclical organic compounds that contain one or more nitrogen atoms. They may also contain an oxygen, sulfur, chlorine, bromine, or phosphorus atom attached to the molecule. They are most associated with plants, but are also produced by microorganisms and animals [22]. In plants, alkaloids are a form of chemical defense against herbivores. Many alkaloids are pharmacologically active. In fact, alkaloids make up about 60% of plant-derived drugs [23]. On the same tangent, dndogenous indole alkaloids have been confirmed in hemp [24].
Alkaloids have a variety of therapeutic applications as analgesics, antibacterial, anticancer, antiarrhythmic, antiasthma agents, antimalarials, anticholinergics, bronchodilatory, laxative, miotic, oxytocic, vasodilatory, psychotropic, and stimulating agents. This class of compounds include morphine, cocaine, nicotine, caffeine, quinine, ephedrine, among others.
Cannabinaceous alkaloids are alkaloids produced by Cannabis sativa. These include, but are not limited to, cannabisativine and anhydrocannabisativine [25].
A 1971 study by Klein and colleagues investigated the constituents of alkaloids mixtures extracted from cannabis plants and reported the isolated of four alkaloids, namely cannabimines A–D [26]. It was also noted that both cannabimine A and anhydrocannabisativine (isolated in 1976) share the same molecular formular (C21 H37 N3 O2) [27].
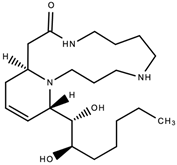
Cannabisativine was the first cannabinaceous alkaloid to be fully identified. It was isolated in 1975 in Mississippi from the roots of a Mexican variant of Cannabis sativa [28]. See Figure 7 below. These alkaloids demonstrated antiparasitic, antipyretic, antiemetic, antitumor, diuretic and analgesic properties [29]. In a 2004 study, Kuethe and Comins were able to achieve total asymmetric synthesis of cannabisativine with a high degree of stereo control [30].
Figure 7. The chemical structure of cannabisativine, the first cannabinaceous alkaloid to be fully identified.
6. Lignanamides and Phenolic Acids
Lignanamides are another example of naturally occurring, non-cannabinoid secondary metabolites with bioactivity. This class of phytochemicals produced in cannabis include Cannabisin A–G, and Grossamide [31].
These phytochemicals are powerful anticancer, antitumor, antiviral, antioxidant, antidiabetic, cardiovascular, cytotoxic, antineoplastic, anti-inflammatory, anti-obesity, analgesic, antihyperlipidemic and antihyperlipidemic agents [31], and they also inhibit acetylcholinesterase [32]. In this 2015 study by Yan and colleagues, four new lignanamides were also isolated from the hemp seed. These were Cannabisin M, Cannabisin N, Cannabisin O, and 3,3′-demethyl-heliotropamide [32]. Cannabisins are unique to the cannabis plant. It should also be noted that phenolic amides share these bioactive properties [32].
7. Amino Acids
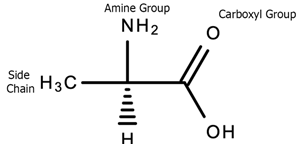
Amino acids are the building blocks of proteins which are involved in multiple biological functions in the body including energy production, fat metabolism, muscle metabolism, immune system function, response and maintenance, the synthesis of enzymes, hormones and neurotransmitters, tissue growth, nutrient absorption, sleep–wake cycles, regulation of blood sugar levels, hemoglobin production, digestion, and sexual function [33]. The amino acid molecule consists of a central carbon atom, an amino group (-NH2), a function R group side chain that defines the chemical properties of that specific amino acid, and a carboxyl group (COOH). Refer to Figure 8 below for the chemical structure of an amino acid.
Figure 8. The basic structure of alanine, an amino acid.
Cannabis sativa (hemp) seeds contain nine essential amino acids, namely include histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, and valine [34]. Essential amino acids are defined as those that cannot be synthesized in our bodies, but instead have to be obtained through food. In this same 2014 study by Audu, and colleagues, it is suggested that amino acids might be most concentrated within the leaves of the Cannabis sativa plant [34]. This is understandable because trichomes (the cell’s factory) are most concentrated in the leaves of plant. In one study aspartic acid, glutamic acid and arginine were the most prominent amino acids produced in hempseed [35]. In another study, hemp (C. sativa L.) isolates produced significantly higher levels of essential amino acid to total produced amino acids (with the exception of lysine), in comparison to a soy protein isolate [36].
Hempseed protein is unique within the plant kingdom because it contains the highest composition (65%) of the globulin edistin which make up many enzymes, antibodies, and hormones in the body [37].
8. Stilbenes and Stilbenoids
Stilbenes (and their derivatives) are naturally occurring polyphenolic phytochemicals that, aside from being a form of herbivore and disease resistance mechanisms for many lower and higher plants in the plant kingdom [38], have a wide range of biological activity and medicinal value to humans [39]. Stilbenoids are stilbene derivates that have been hydroxylated [40].
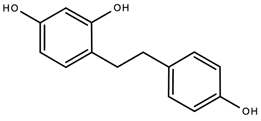
A 2004 study by El-Feraly isolated, characterized and synthesized dihydro-resveratrol (3,5,4′-trihydroxybibenzyl), a metabolite derivative of the antioxidant, resveratrol, a dihydrostilbenoid from Cannabis sativa (Figure 9 above) [41]. A 2019 study by Giménez-Bastida and colleagues reported that dihydro-resveratrol and other metabolite derivatives of resveratrol induced senescence in breast cancer cells [42]. Studies on the antioxidant activity of dihydro-resveratrol are limited. A 2016 study by Tsang and colleagues report that dihydro-resveratrol demonstrated the ability to attenuate pancreatic oxidative damage and could possibly have therapeutic potential in the management of acute pancreatitis [43].
Figure 9. The chemical structure of dihydroresveratrol, a hydroxylated stilbene derivative.
Nineteen stilbenoids unique to the cannabis plant were identified in a 1995 study by Ross and ElSohly. These can be divided into three main structural classes: eleven spiroindans, eight dihydrostilbenes/bibenzyls, five phenanthrenes and prenylated, geranylated and glycosylated derivates [44]. Table 7 below is a list of these compounds.
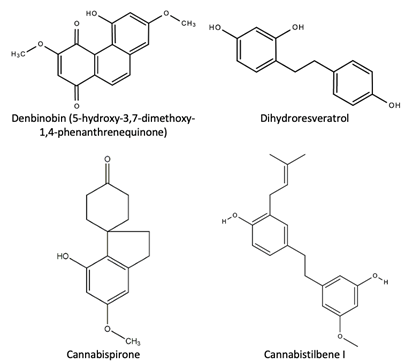
Denbinobin, first isolated in a 2008 study by Sànchez-Duffhues, is reported to have beneficial bioactive properties to human health as an anti-HIV, antioxidant, antitumor, and inhibitor of platelet aggregation [45]. The structure of denbinobin is shown in Figure 10 below.
Figure 10. The chemical structures of some stilbenes and their derivates found in C. sativa L.
Table 7. Some stilbenes and their derivatives found in C. sativa L.
|
Spiroindans |
Eight Dihydrostilbenes/Bibenzyls |
Phenanthrenes and Derivates |
|
1. Cannabispirone 2. Cannabispirenone-A 3. Isocannabispirenone 4. Isocannabispiradienone 5. Cannabispirenone-B 6. β-cannabispiranol 7. α-cannabispiranol 8. Acetyl cannabispirol Cyclohexane Spirans 9. 5,7-dihydroxyindan-1-spiro-cyclohexane 10. 7-hydroxy-5-methoxyindan-1-spiro-ciclohexane 11. 5-hydroxy-7-methoxyindan-1-spiro-ciclohexane |
a. Prenylated i. 3,4′-dihydroxy-5,3′-dimethoxy-5′ isoprenyl ii. Canniprene iii. Cannabistilbene I, iv. Cannabistilbene IIa v. Cannabistilbene IIb b. Non-Prenylated i. 3,4′-dihydroxy-5-methoxy bibenzyl ii. 3,3′-dihydroxy-5,4′-dimethoxy bibenzyl iii. Dihydroresveratrol |
1. 9,10-dihydrophenanthrene i. cannithrene-1 ii. cannithrene-2 2. 4,7-dimethoxy-1,2,5-trihydroxyphenanthrene 3. 4,5-dihydroxy-2,3,6-trimethoxy-9,10-dihydrophenanthrene 4. 4-hydroxy-2,3,6,7-tetramethoxy-9,10-dihydrophenanthrene 5. Denbinobin (5-hydroxy-3,7-dimethoxy-1,4-phenanthrenequinone) |
References
- Al-Taweel, A.; Perveen, S. Terpenes and Terpenoids; IntechOpen: London, UK, 2018; Chapter 1.
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary metabolites profiled in Cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020, 10.
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 2010, 71.
- Elzinga, S.; Fischedick, J.; Podkolinski, R.; Raber, J.C. Cannabinoids and terpenes as chemotaxonomic markers in cannabis. Nat. Prod. Chem. Res. 2015, 3, 81.
- Jacobs, M. The Difference between Cannabinoids and Terpenes (Analytical Cannabis). 27 July 2020. Available online: https://www.analyticalcannabis.com/articles/the-difference-between-cannabinoids-and-terpenes-311502 (accessed on 28 July 2020).
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa—From plant genome to humans. Plant Sci. 2019, 284, 67–72.
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of phenolic compounds in commercial Cannabis sativa L. Inflorescences using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631.
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124,086 US men and women followed for up to 24 years. BMJ 2016, 352, 1–53.
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary metabolites profiled in Cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020, 10.
- Barrett, M.L.; Gordon, D.; Evans, F.J. Isolation from Cannabis sativa L. Of cannflavin—A novel inhibitor of prostaglandin production. Biochem. Pharm. 1985, 34, 2019–2024.
- Werz, O.; Seegers, J.; Schaible, A.M.; Weinigel, C.; Barz, D.; Koeberle, A.; Appendino, G. Cannflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. Pharma Nutr. 2014, 2, 53–60.
- Ngwa, W.; Kumar, R.; Thompson, D.; Lyerly, W.; Moore, R.; Reid, T.; Toyang, N. Potential of flavonoid-inspired phytomedicines against COVID-19. Molecules 2020, 25, 2707.
- Mölleken, H.; Theimer, R.R. Survey of minor fatty acids in Cannabis sativa L. fruits of various origins. J. Int. Hemp Assoc. 1997, 4, 13–17. Available online: http://www.internationalhempassociation.org/jiha/jiha4107.html (accessed on 15 November 2020).
- Ross, S.A.; ElSohly, H.N.; ElKashoury, E.A.; ElSohly, M.A. Fatty acids of Cannabis seeds. Phytochem. Anal. 1996, 7, 279–283.
- Hazekamp, A.; Fischedick, J.; Llano-Diez, M.; Lubbe, A.; Ruhaak, L. Chemistry of Cannabis. In Comprehensive Natural Products Chemistry II, 2nd ed.; Elsevier Ltd.: Oxford, UK, 2010.
- Cooke, J. Does Cannabis Have Nutritional Benefits? Available online: https://thesunlightexperiment.com/blog/cannabis-nutritional-benefits (accessed on 28 July 2020).
- Pontassuglia, L.; Dongo, D. Hemp, the Italian Superfood–Hemp. Available online: https://www.greatitalianfoodtrade.it/en/markets/hemp-the-italian-superfood (accessed on 28 July 2020).
- Kennedy, G.W. The Asserted Presence of Nicotine in Cannabis. In Proceedings of the American Pharmaceutical Association at the Annual Meeting; The Association: Philadelphia, PA, USA, 1886; Volume 34, pp. 119–121.
- Siebold, L.; Bradbury, T. Note on the alleged presence of Nicotine in Indian hemp. Pharm. J. Trans. 1881, 12, 326–327.
- Hay, M. A new alkaloid in Cannabis indica. Pharm. J. Trans. 1883, 13, 996–997.
- Merck, E. Merck's 1896 Index: An Encyclopedia for the Physician and the Pharmacist: Stating the Names and Synonyms, Source of Origin, Chemical Nature and Formulas, Physical Form, Appearance, and Properties, Melting and Boiling Points, Solubilities, Gravities and Percentage Strengths, Physiological Effects, Therapeutic Uses, Modes of Administration and Application, Regular and Maximum Dosage, Incompatibles, Antidotes: Special Cautions, Hints on Keeping and Handling, Methods of Testing, Market Values, etc., etc. of the Chemicals and Drugs Used in Medicine, in Chemistry, and in the Arts; Merck & Co.: New York, NY, USA, 1895.
- Roberts, M.F. Alkaloids: Biochemistry, Ecology, and Medicinal Applications; Wink, M., Ed.; Springer: Boston, MA, USA, 1998; ISBN 9781475729054. OCLC 851770197.
- Gowda, L.; Venkategowda, A. Isolation & characterization of antimicrobial alklaoids from Plumeria albaflowers against food borne pathogens. Am. J. Life Sci. 2014, 2, 1–6.
- Yan, X.; Zhou, Y.; Tang, J.; Ji, M.; Lou, H.; Fan, P. Diketopiperazine indole alkaloids from hemp seed. Phytochem. Lett. 2016, 18, 77–82.
- Mechoulam, R.; Hanuš, L. A historical overview of chemical research on cannabinoids. Chem. Phys. Lipids 2000, 108, 1–13.
- Klein, F.K.; Rapoport, H.; Elliott, H.W. Cannabis alkaloids. Nature 1971, 232, 258–259.
- Mechoulam, R.; Hanuš, L. A historical overview of chemical research on cannabinoids. Chem. Phys. Lipids 2000, 108, 1–13.
- Lotter, H.L.; Abraham, D.J.; Turner, C.E.; Knapp, J.E.; Schiff, P.L.; Slatkin, D.J., Jr. Cannabisativine. A new alkaloid from Cannabis sati6a L. Root. Tetrahedron Lett. 1975, 33, 2815–2818.
- Wink, M. Potential of DNA intercalating alkaloids and other plant secondary metabolites against SARS-CoV-2 causing COVID-19. Diversity 2020, 12, 175.
- Kuethe, J.T.; Comins, D.L. Asymmetric total synthesis of (+)-cannabisativine (I). Chem. Inform. 2004, 35.
- Flores-Sánchez, I.J.; Verpoorte, R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639.
- Yan, X.; Tang, J.; Passos, C.; Nurisso, A.; Simões-Pires, C.; Ji, M.; Lou, H.; Fan, P. Characterization of lignanamides from hemp (Cannabis sativa L.) seed and their antioxidant and acetylcholinesterase inhibitory activities. J. Agric. Food Chem. 2015, 63.
- Kubala, J. Essential Amino Acids: Definition, Benefits and Food Sources. 12 June 2018. Available online: https://www.healthline.com/nutrition/essential-amino-acids (accessed on 28 July 2020).
- Audu, B.S.; Ofojekwu, P.C.; Ujah, A.; Ajima, M.N.O. Phytochemical, proximate composition, amino acid profile and characterization of Marijuana (Cannabis sativa L.). J. Phytopharm. 2014, 3, 35–43, Scientific Figure on ResearchGate. Available online: https://www.researchgate.net/figure/Amino-acid-composition-of-the-leaf-stem-and-seeds-of-Cannabis-sativa-obtained-from_tbl3_273692476 (accessed on 26 July 2020).
- Jang, H.; Park, S.; Nam, J. The Effects of Heat Treatment on the Nutritional Composition and Antioxidant Properties of Hempseed (Cannabis sativa L.). J. Korean Soc. Food Sci. Nutr. 2018, 47, 885–894.
- Wang, X.; Tang, C.; Yang, X.; Gao, W. Characterization, amino acid composition and in vitro digestibility of hemp (Cannabis sativa L.) proteins. Food Chem. 2008, 107, 11–18.
- Osburn, L. Hemp seed: The most nutritionally complete food source in the world. Hemp. Line J. 1992, 1, 14–15.
- Orsini, F.; Verotta, L. Stilbenes and bibenzyls with potential anticancer or chemopreventive activity. In Advances in Nutrition and Cancer 2. Advances in Experimental Medicine and Biology; Zappia, V., Della Ragione, F., Barbarisi, A., Russo, G.L., Iacovo, R.D., Eds.; Springer: Boston, MA, USA, 1999; Volume 472.
- Baur, J.; Sinclair, D. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506.
- Montané, X.; Kowalczyk, O.; Reig-Vano, B.; Bajek, A.; Roszkowski, K.; Tomczyk, R.; Tylkowski, B. Current perspectives of the applications of polyphenols and flavonoids in cancer therapy. Molecules 2020, 25, 3342.
- El-Feraly, F. Isolation, characterization, and synthesis of 3,5,4’–Trihydroxybibenzyl from cannabis sativa. J. Nat. Prod. 2004, 47.
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. Conjugated physiological resveratrol metabolites induce senescence in breast cancer cells: Role of p53/p21 and p16/Rb pathways, and ABC transporters. Mol. Nutr. Food Res. 2019, 63.
- Tsang, S.W.; Guan, Y.; Wang, J.; Bian, Z.; Zhang, H. Inhibition of pancreatic oxidative damage by stilbene derivative dihydro-resveratrol: Implication for treatment of acute pancreatitis. Sci. Rep. 2016, 6.
- Pollastro, F.; Minassi, A.; Fresu, L. Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018, 24.
- Sànchez-Duffhues, G.; Calzado, M.A.; de Vinuesa, A.G.; Caballero, F.J.; Ech-Chahad, A.; Appendino, G.; Krohn, K.; Fiebich, B.L.; Muñoz, E. Denbinobin, a naturally occurring 1,4-phenanthrenequinone, inhibits HIV-1 replication through an NF-kB-dependent pathway. Biochem. Pharmacol. 2008, 76, 1240–1250.