
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Knaislová | + 4101 word(s) | 4101 | 2021-03-31 06:10:43 | | | |
| 2 | Peter Tang | Meta information modification | 4101 | 2021-04-01 07:19:36 | | |
Video Upload Options
The experimental generation of TiAl–Si alloys is composed of titanium aluminide (TiAl, Ti3Al or TiAl3) matrix reinforced by hard and heat-resistant titanium silicides (especially Ti5Si3). The alloys are characterized by wear resistance comparable with tool steels, high hardness, and very good resistance to oxidation at high temperatures (up to 1000 °C), but also low room-temperature ductility, as is typical also for other intermetallic materials.
1. Introduction
There is currently extensive research on intermetallics for high temperature applications, especially for the automotive, aviation, and cosmic applications [1][2][3]. Titanium aluminides are of the greatest importance for the automotive industry, when turbochargers made of TiAl intermetallic alloys have already started to be used in passenger cars [4]. The use of these alloys in aircraft engines (for low-pressure turbine blades) is very interesting [4][5]. Today’s titanium alloys and titanium aluminides account for a third of the weight of aircraft engines and are the second most widely used material for engine components after nickel superalloys [6].
Cold extreme tool steels or high speed steels are most commonly used today for extreme adhesive and abrasive wear [7][8][9]. High temperature applications use nickel superalloys in aviation-related applications, such as engine turbines. Another widely used material is heat-resistant steels, the great advantage of which is the relatively simple production [10]. However, the problem is their high density [11]. In aviation, there is an effort to replace nickel [12] and heat-resistant steels with lighter materials to decrease the weight of the aircraft structure and to improve engine performance [10][11]. The strength-to-weight ratio could be significantly improved by significantly reducing weight by the means of a substitution of nickel-based superalloys with intermetallic alloys, such as titanium aluminides [6].
Titanium alloys with other light elements (aluminum, silicon) are therefore very promising materials for applications at higher temperatures [13][14], especially for use in structural applications operating under static load and at the same time high temperatures [15]. Their biggest advantage is the low density, which ranges around 4 g·cm−3 [16][17].
2. History of Using TiAl Alloys
In the second half of the last century, TiAl alloys began to be used as technical materials. Rolls-Royce, P&W, and GE tested automotive engines made from TiAl intermetallic alloy. Advantageous properties of TiAl motors include better specific tensile strength compared to other titanium alloys and higher strength at temperatures up to 800 °C compared to steels or nickel superalloys. However, a major disadvantage is the low room-temperature strain, usually up to 1% (1% is generally an acceptable minimum ductility value, cast samples seldom reach this value). Another problem is the difficult production of the component [18].
In the 1970s, extensive research was carried out on Ti3Al-based systems for the production of aircraft engines, but this production failed due to the high difficulty and the limit temperature of use of the component (600 °C) [5]. In the 1980s, research was shifted to γ–TiAl alloys, which had better creep and oxidation resistance, and the maximum application temperature was increased to 750 °C. In addition to aviation, alloys have potential use in the automotive and nuclear industries [5]. The US Air Force then stated that TiAl intermetallics provide unique weight savings as a replacement for nickel superalloys in the hot parts of low-pressure turbine jet engines [19]. However, problems with the use of TiAl alloys in aviation include large deviation of mechanical properties, difficult production and high costs [5]. On the other hand, the use of an aircraft engine made of TiAl alloy reduces the weight from 20% to 50% compared to conventional materials, but production costs increase by 10% to 100% [5].
In this millennium, research has focused on TiAl alloys with 44–48 at. % aluminum, but for some applications, a maximum of 42 at. % aluminum is required. The β-phase appears at aluminum concentrations lower than 42 at. %, which significantly improves the heat treatment [20]. However, this phase has lower mechanical properties, so most applications requiring high heat strength must contain about 46 at. % aluminum [18]. If the TiAl46 alloy (at. %) is cooled sufficiently quickly from the α-phase (the rates are similar to thin castings), a lamellar structure is formed, and γ-phases precipitate on the crystallographic plane (0001). If the sample is heat-treated in the region of a two-phase structure, a duplex structure formed by γ-phase with lamellar grains is formed during cooling [18][21].
TiAl alloys have already begun to be used as turbochargers in commercial vehicles [4]. The development of TiAl alloy turbochargers began in Japan, when a relatively small number (about 1000) of TiAl turbochargers were installed in 1998 on Lancer cars [18]. In 1999, turbochargers were shown to Mitsubishi Motors Inc. for the model Lancer 6 [19] and great success in the following years led to the addition of more than 20,000 cars in 2003 with a TiAl alloy turbocharger [18]. Today, some companies now manufacture turbochargers based on TiAl, and it seems possible to successfully use a TiAl alloy in this relatively simple application, where very low toughness is acceptable to designers [18].
3. Properties of TiAl Alloys and Effect of Silicon on Them
As already mentioned, TiAl alloys excel in low density. Their other properties include good resistance to oxidation at temperatures of 600 to 800 °C [5][11], high specific strength at higher temperatures [22][23], and a favorable ratio between density and mechanical properties [12]. However, TiAl alloys have low ductility at room temperature (1–2%) [10][19][24] and fracture toughness [5][25]. Other properties of TiAl alloys are low thermal expansion and thermal conductivity comparable to nickel superalloys and refractory steels. The electrical conductivity is lower than that of commonly used alloys [26].
TiAl (50:50) alloy cannot be used above 827 °C, although it contains 50% aluminum, because the aluminum content is not enough to form a layer of alumina with protective effect. Approximately 75% aluminum is required to form the oxide layer [27]. Improvement of oxidation resistance can be achieved, for example, by surface modification or by adding silicon to TiAl powder in mechanical alloying [28]. By modifying the surface, silicon diffuses into the TiAl alloy, demonstrating the high affinity of silicon and titanium. The resistance to oxidation of TiAl alloys was also increased by the method of liquid phase siliconization with Al–Si alloy [28]. Silicon surface treatments are described, for example, in articles [29][30][31][32][33][34][35][36][37][38][39]. Siliconizing is a treatment to improve the chemical and mechanical properties of intermetallics based on TiAl system. Siliconizing methods can be used to obtain silicide layers on the surface of titanium-based alloys. These methods [30][32][34][35][36] combine in a siliconization process in the vapor phase and subsequent heat treatment [37], siliconization in the liquid phase combined with aluminizing in an Al–Si melt [19][38][40], heat treatment in quartz glass ampoules under low partial pressure of oxygen [39], or sol–gel coating in combination with heat treatment [30]. The silicide layers are very hard and prevent oxidation up to 950 °C.
The addition of silicon into the TiAl alloys improves oxidation and corrosion resistance at elevated temperatures, as well as creep resistance [41][42]. Silicon dissolves very little in TiAl alloys and therefore forms hard and brittle titanium silicides in the structure [17], mainly Ti5Si3 silicides, which have a strengthening effect [22][43]. A major problem of TiAl–Si alloys is the brittleness of titanium silicides, so they need to be as fine and fibrous as possible, which would increase the fracture toughness of the material [14]. Silicon is a light element, and the addition of more silicon to TiAl alloys improves the creep resistance and oxidation resistance due to the refinement of titanium dioxide particles formed on the surface of TiAl alloys. Silicon promotes the diffusion of aluminum in the oxidation layer and binds titanium to a stable silicide, then reduces the activity of TiIV and prevents the diffusion of TiIV outwards. The formation of titanium dioxide is thus limited [6]. The addition of silicon to TiAl alloys improves the oxidation resistance due to the formation of titanium nitride, which is formed in the form of a layer or isolated particles between the oxide layer and the base material and thus protects the base material from further oxidation [7].
High oxygen solubility in α-Ti (about 14.5 wt. %) leads to the formation of a brittle and hard subsurface layer enriched with oxygen, which cracks and causes deterioration of fatigue resistance and ductility [44][45][46][47]. Alloying by silicon improves oxidation resistance, oxide layer adhesion, and creep behavior at elevated temperatures [48][49]. High concentrations of silicon are more advantageous for improving resistance to oxidation than concentrations in the solubility range of silicon in α-Ti [50]. Kitashima et al. [51] showed similar oxidation behavior of an near-alpha alloy Ti-5Al-2Sn-2Zr-2Mo with 0.2 and 0.9 wt. % silicon [51]. However, commercial titanium alloys do not contain silicon in concentrations exceeding its solubility range in α-Ti (0.3 wt. %), which is mostly due to the negative effect of silicon on their ductility, especially when the silicon contents exceed 2 wt. % [52]. In addition, some researchers speculate that precipitation of silicides in aluminum-containing alloys may limit the formation of the heat-resistant phase Ti3Al, thereby reducing high temperature strength. Mechanisms that increase oxidation resistance include reducing the depth of oxygen penetration into the metal substrate and dissolving the silicon in the oxide surface layer. Silicon dissolved in the oxide layer reduces the rate of oxygen diffusion through the layer and modulates the stress relaxation that leads to a more compact and less porous oxide layer [49][50]. In addition, the formation of silica (SiO2) in the oxide surface layer inhibits the recrystallization of rutile (TiO2) crystals. In the work of Novák et al. [53], the addition of silicon and aluminum has been found to reduce the oxidation rate of titanium to approximately 850 °C. Silica is formed in the oxide layer on titanium. In silica, oxygen diffuses more slowly than in rutile (titanium dioxide). In addition, the almost continuous sub-layer of silicides is formed beneath the oxide layer as a result of the depletion by aluminum, which diffuses to the oxidized surface [53]. This layer acts as the secondary protection and suppresses the diffusion of both oxygen and nitrogen towards the core of the material. As a result, titanium nitride TiN is formed below the oxide layer. It has been proved that TiN is due to the presence of silicon in the material, because TiN formation was not observed in the binary alloy TiAl [7].
4. TiAl–Si Alloys as a “CRM-Free” Material
TiAl–Si alloys have been developed as a replacement for materials containing critical metals (especially cobalt, tungsten, chromium, or niobium) [54][55]. In 2010, the European Union (EU) published a study on critical raw materials. A list of 14 critical raw materials (CRM) was created based on the economic importance and the risk of supply disruptions to the EU; in 2014, it already contained 20 critical raw materials, and in 2017 already 27 [54]. In 2017, silicon (metal) also appeared on the list; however, silicon obtained from recycling (e.g., of electronics or Al–Si castings from the combustion engines of passenger cars) is sufficient for TiAl–Si alloys [56]. In 2020, four more elements appeared on the list: bauxite, lithium, strontium, and titanium [56][57].
There are three solutions to the CRM situation: (a) improving raw material production processes (increasing sustainable extraction, reducing extraction costs, increasing material efficiency, etc.), (b) finding suitable substitutes to partially or completely replace critical materials, and (c) increasing recycling. The replacement of CRM should lead to innovative materials with comparable or better properties; materials should be easily and quickly integrated into production processes, with lower risks to the human health, environment, and lower prices [54]. Substitution of critical raw materials for use in extreme conditions is an important request, as extreme temperatures, wear, and corrosion often occur in many applications [54][58]. On the other hand, when some material is used as a substitute of a particular CRM, it increases its economic importance and it might become critical, which is probably the case of titanium. Oppositely, the substituted raw material could be potentially shifted out of the CRM list, so the CRM list will be always dynamic.
5. Preparation of TiAl–Si Alloys by Melting Metallurgy (MM)
The synthesis of the TiAl intermetallic compounds is one of the important directions in the development of new materials with heat resistance and high thermal stability [59]. Methods for producing TiAl alloys (and intermetallics in general) include conventional casting techniques, arc melting in an argon atmosphere, powder metallurgy, etc. [59].
The preparation of TiAl–Si alloy is very difficult using MM. This is due to the very high melting point of intermetallic compounds (e.g., titanium silicide Ti5Si3 melts at temperatures above 2100 °C [12][60]), high melt reactivity with crucibles [43], damage to crucibles (crucibles made of Y2O3 and ZrO2 are reported to be required [40], which is more expensive than graphite or corundum crucibles), and melt contamination. Other disadvantages of the preparation of TiAl–Si alloys by MM include very poor casting properties of intermetallics (very frequent occurrence of casting defects, such as pores or microcracks) [11]. In order to eliminate defects, the castings are post-processed by hot isostatic pressing (HIP). This technology is used by GE for GEnx engines for Boeing airliners [61].
The preparation of TiAl alloys with addition of silicon by MM is limited to hypoeutectic and eutectic alloys based on α2-Ti3Al, as hypereutectic alloys are extremely brittle due to the formation of coarse primary silicides during solidification [22]. It is necessary to design the material so that the composition of the alloys is around the eutectic point, so that the eutectic phases are fine. The eutectic composition is advantageous, because the meltability and fluidity are improved during casting [14].
Melting and casting of TiAl–Si alloys produce brittle and hard titanium silicides Ti5Si3, and these coarse and randomly oriented sharp-edged silicide particles are undesirable in terms of deterioration of some mechanical properties (especially fracture toughness) [7][24]. Previous work has sought to eliminate it and to form an in-situ composite of elongated Ti5Si3 silicide particles in a tough matrix of TiAl or Ti3Al titanium aluminides. However, there were cracks in the structure perpendicular to the solidification direction (due to the different thermal expansion coefficient of the Ti5Si3 titanium silicide in different crystal directions, therefore tensile stresses and crack initiation occurred). This results in a limited applicability of TiAl–Si alloys prepared by directional crystallization [7]. The stress in the material can generally be reduced by gradual slow heating and cooling, but for a polycrystalline material with high anisotropy of the coefficient of thermal expansion, it is not possible to reduce the residual stress [62].
TiAl–Si alloys prepared by MM have a very coarse microstructure with concentrated pores in the center of the material. The particles of titanium silicide, which are coarse, are oriented depending on the local direction of heat dissipation, which causes the elongated silicides, which was described in the article [17][62]. These silicides are cracked due to the high rate of cooling after melting process (Figure 1) [62]. The porosity in the center of sample is given by the production technology. The gases are trapped inside the material as a result of the elements evaporation (especially aluminum) during the exothermic reaction of intermetallic phases and the relatively fast cooling of the reacted sample. Gases cannot escape from the material due to the rapid solidification of the surface. Therefore, the porosity is concentrated in the center of the sample. The same applies to samples prepared by reactive sintering described, for example, in [14][63].
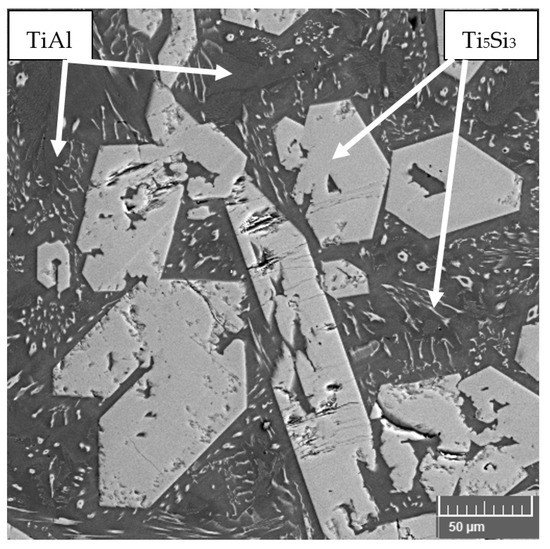
Figure 1. Microstructure of TiAl15Si15 prepared by vacuum induction melting.
6. Preparation of TiAl–Si Alloys by Powder Metallurgy (PM)
The advantages of powder metallurgy include the acquisition of a finer and more homogeneous microstructure, which could cause an improvement in mechanical properties.
6.1. Reactive Sintering (RS)
Reactive sintering is a thermally activated process that produces compounds from elemental components, usually powders [43][64]. Reactive sintering usually results in very exothermic reactions, due to which there is no need to supply additional energy after initiation and the reaction spreads spontaneously, so it is sometimes referred to as self-propagating high-temperature synthesis [12][64][65]. The reaction, which changes the reactants to products, is exothermic, and the reaction rates may be high enough for adiabatic conditions to occur. All the heat of reaction raises the temperature of the material to an adiabatic temperature with nearly zero heat loss. The maximum experimental temperature reached during the reaction is called the combustion temperature [66].
Reactive sintering is used for the preparation of ceramics, intermetallic compounds, and their composites [11][64], but also for the preparation of borides, carbides, or nitrides [67]. The advantages of reactive sintering include energy savings [66], low temperatures and reaction time [66], higher purity of products [67], or evaporation of impurities with a low boiling point [65][66]. The disadvantage of reactive sintering is the porosity of the resulting material in many cases or the limited possibilities of process control (fast reaction, maximum temperature sustainable only for a short time, impossibility to change time) [66].
Self-Propagating High Temperature Synthesis (SHS) is divided according to the method of initiating to the “Thermal Explosion” (TE–SHS) and Plane Wave Propagation (PWP–SHS) reactions. These processes are characterized by high reaction temperatures (up to 4000 °C) and short reaction times (several seconds for TE–SHS and wave speed for PWP–SHS up to 250 mm/s) [68]. In the case of PWP–SHS, the compact reactant ignited at one end creates a reaction wave that consumes the reactants and produces products. If the reaction is initiated using an external heat source, the reaction is self-sustaining, using the heat of reaction to heat the adjacent reactant layer to the ignition temperature, and the wave propagates without the need for an external heat source until the entire material has reacted [66]. The second method of ignition is called thermal explosion (TE–SHS), in which the entire volume of the material is heated to the ignition temperature and all the reaction powder is spontaneously converted into a product. SHS converts powders to the desired compound [64]. The use of SHS in combination with the subsequent compaction and shaping of the product makes it possible to prepare various materials based on intermetallic compounds [59]. Both methods have been used in the past to produce nickel aluminides [66].
The RS of titanium aluminides is very well described. The initiation temperature for the formation of aluminides is given around the melting point of aluminum (660 °C). Aluminum is melted, filling the pores through capillary forces, thereby reducing the porosity of the product [7][64]. However, the product of reactive sintering of titanium and aluminum is still very porous. The reason for porosity may be Kirkendall porosity due to unbalanced diffusion (aluminum diffuses faster in titanium than titanium in aluminum), gas evolution due to evaporation of impurities on the powder particles surface (especially chlorides in titanium [69]), and the difference in density of the reactants and the product (TiAl has a density of 5.2% higher than the starting mixture of titanium and aluminum powders [70]) [66].
6.2. Mechanical Alloying (MA)
Mechanical alloying is an energy-intensive process in which a mixture of powders of different metals or alloys is ground together in a grinding vessel to form a homogeneous alloy [71]. It is an effective process of achieving a very fine grain [72]. By mechanical alloying it is possible to synthesize a number of equilibrium and nonequilibrium phases of the alloy and to obtain powders with grain size in the order of nanometers and homogeneous dispersion of individual particles [73], supersaturated solid solutions, metastable crystalline and quasicrystalline phases, amorphous powders [72], nanocrystalline structures [15][71][73][74][75], or to form an alloy of elements that are immiscible at equilibrium [23]. Recently, emphasis has also been placed on the preparation of intermetallic alloys based on titanium and aluminum [76][77][78], ternary systems based on TiAl [22] and TiAl/Ti5Si3 composites [75].
The starting materials (powders) for MA are usually 1–200 μm in size and must be smaller than the grinding medium [71]. MA begins by mixing the powders in a selected ratio and placing them in a mill together with the grinding medium (usually steel balls); this mixture is then ground for the required time until the composition of each powder particle is the same as the proportion of elements in the powder base mixture. The mechanically alloyed powder is then heat treated to obtain the suitable microstructure and mechanical properties [71].
At the beginning of MA, metallic powder particles are relatively soft and tend to flatten and cold weld to form large particles (some are up to three times larger than the original particles [71]). Thus, the particle size of the powder increases in the initial phase and subsequently refracts. The reason for the reduction in particle size is strain hardening, which causes an increase in strength and a decrease in plasticity, which significantly increases the brittleness [79][80]. As a result of the intense high-energy interaction between the grinding balls and the powder, the ductile phase undergoes a continuous cycle of plastic deformation, fracture, and welding [72]. At this stage, fracturing predominates over the cold weld. This creates fresh surfaces, which helps further cold welding of powder particles. These processes are repeated several thousand times during MA in a high-energy ball mill [23][42][71][72][75]. The grinding time must be chosen so as to reach a steady state between cold welding and fracturing of the powder particle [71]. After grinding, the steady-state is reached when the welding speed is equalized, which tends to increase the average particle size and the crushing speed, which in turn leads to a decrease in particle size. Smaller particles are able to withstand deformation without breaking and are welded into larger particles. At this stage, each particle contains essentially all the starting components in the ratio in which they were mixed [71]. Fragmentation of powder particles leading to the formation of fresh surfaces, reduced distance between particles, increased defect concentration, and a slight rise in temperature contribute to MA [23]. During the mechanical alloying, large deformation is brought into the particles, which is exhibited by the presence of crystallographic disturbances, such as dislocations, vacancies, or a large amount of grain boundaries. The presence of this defective structure increases the solubility of the elements in the matrix [71].
From the previous results of the investigation of the MA process at the University of Chemistry and Technology in Prague (UCT), it can be concluded that the temperature reached at the point of contact between the powder, the sphere and the wall exceeds 650 °C. The temperature of 650 °C is required for the formation of some intermetallic compounds, such as the binary phases Fe-Al of Ni-Ti prepared in [81][82]. The required temperature for the formation of these phases was determined by the DTA method on pure metal powder mixtures [83]. Therefore, the energy including friction and the use of a high ball to powder ratio can be expected to be more than twice as high (approximately 400–500 W). Current high-speed mills reach an estimated power supply of about 400 W [84].
6.3. Spark Plasma Sintering (SPS)
Spark Plasma Sintering (SPS) is a modern method of powder compaction using compression while passing an electric current [7][85][86][87]. It is a technique using unidirectional pressure and direct pulsed electric current under low atmospheric pressure [88]. The SPS method is used to produce amorphous materials, intermetallic compounds, nanostructured materials, highly refractory metals and ceramics, or composites with a metal and ceramic matrix, which are difficult to prepare by conventional methods [85]. In the SPS process, heating rates of up to 1000 °C per minute can be achieved [85][88]. The heating rate depends on the geometry of the mold, the sample, and its electrical and thermal properties [88]. The sintering time usually reaches several minutes (usually a maximum of 10 min) depending on the sample, its dimensions, and the capacity of the equipment at low sintering temperature (sintering temperature is about 200 to 500 °C lower than most conventional sintering techniques [85][88]. With SPS, it is possible to consolidate a large amount of powder materials to a very high density in a short time [89]. One of the advantages of spark plasma sintering is rapid heating of the material and high thermal efficiency of the process [7][74]. Under pressure and pulse current flow, the temperature rises rapidly to 1000 to 2500 °C and the ambient temperature, leading to the production of a high quality sintered compact within a few minutes [88]. The high sintering rate at relatively low temperatures limits grain growth for most materials and the sintering efficiency is significantly increased compared to commonly used hot pressing (HP) and isostatic hot pressing (HIP) [74][85][90]. Other advantages over conventional sintering are, in addition to high sintering rate, high reproducibility, safety, and reliability [88].
6.4. High Pressure Spark Plasma Sintering (HP SPS)
High pressure plasma sintering (HP SPS) was also tested. This innovative spark plasma sintering technology is used for difficult-to-sinter materials, such as polycrystalline diamond, refractory materials, cubic boron nitride, and ceramic composites [91][92][93][94][95], and has already been used for sintering ZrC-based composites [96]. A pulsed electric current heats the material from the outside as well as inside, which guarantees good powder compaction [94]. It is performed at very high pressure (up to 8 GPa) and very short time (tens of seconds) [91]. The short sintering time (maximum 3 min) eliminates the main problem of high-temperature processes, i.e., the tendency of grain growth in sintered material with increasing temperature and time [96][97][98]. Due to the high pressure, the process can also be applied to intermetallic compounds or minerals [99]. Brittle materials can acquire better mechanical properties after HP SPS due to the extended plastic deformation range [91].
References
- Clemens, H.; Mayer, S. Intermetallic titanium aluminides in aerospace applications—Processing, microstructure and properties. Mater. High Temp. 2016, 33, 560–570.
- Cinca, N.; Lima, C.R.C.; Guilemany, J.M. An overview of intermetallics research and application: Status of thermal spray coatings. J. Mater. Res. Technol. 2013, 2, 75–86.
- Tewari, R.; Sarkar, N.K.; Harish, D.; Vishwanadh, B.; Dey, G.K.; Banerjee, S. Chapter 9—Intermetallics and Alloys for High Temperature Applications. In Materials under Extreme Conditions; Tyagi, A.K., Banerjee, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 293–335.
- Yamaguchi, M.; Inui, H.; Ito, K. High-temperature structural intermetallics. Acta Mater. 2000, 48, 307–322.
- Lasalmonie, A. Intermetallics: Why is it so difficult to introduce them in gas turbine engines? Intermetallics 2006, 14, 1123–1129.
- Dai, J.; Zhu, J.; Chen, C.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloys Compd. 2016, 685, 784–798.
- Novák, P. Příprava, vlastnosti a použití intermetalických sloučenin. Chem. Listy 2012, 106, 884–889.
- Bourithis, L.; Papadimitriou, G.D.; Sideris, J. Comparison of wear properties of tool steels AISI D2 and O1 with the same hardness. Tribol. Int. 2006, 39, 479–489.
- Toboła, D.; Brostow, W.; Czechowski, K.; Rusek, P. Improvement of wear resistance of some cold working tool steels. Wear 2017, 382–383, 29–39.
- Kumaran, S.; Sasikumar, T.; Arockiakumar, R.; Srinivasa Rao, T. Nanostructured titanium aluminides prepared by mechanical alloying and subsequent thermal treatment. Powder Technol. 2008, 185, 124–130.
- Novák, P.; Průša, F.; Šerák, J.; Vojtěch, D.; Michalcová, A. Oxidation resistance and thermal stability of Ti-Al-Si alloys produced by reactive sintering. In Proceedings of the Metal 2009—18th International Conference on Metallurgy and Materials, Hradec nad Moravicí, Czech Republic, 19–21 May 2009.
- Novák, P.; Vojtěch, D.; Šerák, J.; Kubásek, J.; Průša, F.; Knotek, V.; Michalcová, A.; Novák, M. Synthesis of Intermediary Phases in Ti-Al-Si System by Reactive Sintering. Chem. Listy 2009, 103, 1022–1026.
- McKamey, C.G.; DeVan, J.H.; Tortorelli, P.F.; Sikka, V.K. A review of recent developments in Fe3Al-based alloys. J. Mater. Res. 1991, 6, 1779–1805.
- Novák, P.; Michalcová, A.; Šerák, J.; Vojtěch, D.; Fabián, T.; Randáková, S.; Průša, F.; Knotek, V.; Novák, M. Preparation of Ti–Al–Si alloys by reactive sintering. J. Alloys Compd. 2009, 470, 123–126.
- Li, X.-W.; Sun, H.-F.; Fang, W.-B.; Ding, Y.-F. Structure and morphology of Ti-Al composite powders treated by mechanical alloying. Trans. Nonferrous Met. Soc. China 2011, 21, s338–s341.
- Stoloff, N.S. Iron aluminides: Present status and future prospects. Mater. Sci. Eng. A 1998, 258, 1–14.
- Vojtěch, D.; Lejček, P.; Kopeček, J.; Bialasová, K. Směrová krystalizace eutektik systému Ti-Al-Si. In Proceedings of the Metal 2009-18th International Conference on Metallurgy and Materials, Hradec nad Moravicí, Czech Republic, 19–21 May 2009.
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2006, 14, 1114–1122.
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559.
- Tetsui, T.; Shindo, K.; Kaji, S.; Kobayashi, S.; Takeyama, M. Fabrication of TiAl components by means of hot forging and machining. Intermetallics 2005, 13, 971–978.
- Okamoto, H.; Massalski, T.B. Binary alloy phase diagrams requiring further studies. J. Phase Equilibria 1994, 15, 500–521.
- Guan, Z.Q.; Pfullmann, T.; Oehring, M.; Bormann, R. Phase formation during ball milling and subsequent thermal decomposition of Ti–Al–Si powder blends. J. Alloys Compd. 1997, 252, 245–251.
- Suryanarayana, C. Synthesis of nanocomposites by mechanical alloying. J. Alloys Compd. 2011, 509 (Suppl. 1), S229–S234.
- Kimura, Y.; Pope, D.P. Ductility and toughness in intermetallics. Intermetallics 1998, 6, 567–571.
- Stoloff, N.S.; Liu, C.T.; Deevi, S.C. Emerging applications of intermetallics. Intermetallics 2000, 8, 1313–1320.
- Zhang, W.J.; Reddy, B.V.; Deevi, S.C. Physical properties of TiAl-base alloys. Scr. Mater. 2001, 45, 645–651.
- Shida, Y.; Anada, H. The influence of ternary element addition on the oxidation behaviour of TiAl intermetallic compound in high temperature air. Corros. Sci. 1993, 35, 945–953.
- Xiong, H.-P.; Mao, W.; Xie, Y.-H.; Cheng, Y.-Y.; Li, X.-H. Formation of silicide coatings on the surface of a TiAl-based alloy and improvement in oxidation resistance. Mater. Sci. Eng. A 2005, 391, 10–18.
- Goral, M.; Swadzba, L.; Moskal, G.; Hetmanczyk, M.; Tetsui, T. Si-modified aluminide coatings deposited on Ti46Al7Nb alloy by slurry method. Intermetallics 2009, 17, 965–967.
- Teng, S.; Liang, W.; Li, Z.; Ma, X. Improvement of high-temperature oxidation resistance of TiAl-based alloy by sol–gel method. J. Alloys Compd. 2008, 464, 452–456.
- Popela, T.; Vojtěch, D. Characterization of pack-borided last-generation TiAl intermetallics. Surf. Coat. Technol. 2012, 209, 90–96.
- Li, X.Y.; Taniguchi, S.; Matsunaga, Y.; Nakagawa, K.; Fujita, K. Influence of siliconizing on the oxidation behavior of a γ-TiAl based alloy. Intermetallics 2003, 11, 143–150.
- Xiong, H.P.; Xie, Y.H.; Mao, W.; Ma, W.L.; Chen, Y.F.; Li, X.H.; Cheng, Y.Y. Improvement in the oxidation resistance of the TiAl-based alloy by liquid-phase siliconizing. Scr. Mater. 2003, 49, 1117–1122.
- Munro, T.C.; Gleeson, B. The deposition of aluminide and silicide coatings on γ-TiAl using the halide-activated pack cementation method. Metall. Mater. Trans. A 1996, 27, 3761–3772.
- Liang, W.; Ma, X.X.; Zhao, X.G.; Zhang, F.; Shi, J.Y.; Zhang, J. Oxidation kinetics of the pack siliconized TiAl-based alloy and microstructure evolution of the coating. Intermetallics 2007, 15, 1–8.
- Xiang, Z.D.; Rose, S.R.; Datta, P.K. Codeposition of Al and Si to form oxidation-resistant coatings on γ-TiAl by the pack cementation process. Mater. Chem. Phys. 2003, 80, 482–489.
- Vojtěch, D.; Novák, P.; Macháč, P.; Morťaniková, M.; Jurek, K. Surface protection of titanium by Ti5Si3 silicide layer prepared by combination of vapour phase siliconizing and heat treatment. J. Alloys Compd. 2008, 464, 179–184.
- Xiong, H.-P.; Mao, W.; Ma, W.-L.; Xie, Y.-H.; Chen, Y.-F.; Yuan, H.; Li, X.-H. Liquid-phase aluminizing and siliconizing at the surface of a Ti60 alloy and improvement in oxidation resistance. Mater. Sci. Eng. A 2006, 433, 108–113.
- Gray, S.; Jacobs, M.H.; Ponton, C.B.; Voice, W.; Evans, H.E. A method of heat-treatment of near γ-TiAl to enhance oxidation resistance by the formation of a Ti5Si3 layer. Mater. Sci. Eng. A 2004, 384, 77–82.
- Zemčík, L.; Dlouhý, A.; Król, S.; Prażmowskic, M. Vacuum Metallurgy of TiAl Intermetallics. In Proceedings of the Metal 2005—14th International Conference on Metallurgy and Materials, Hradec nad Moravicí, Czech Republic, 24–26 May 2005.
- de Farias Azevedo, C.R.; Flower, H.M. Microstructure and phase relationships in Ti–Al–Si system. Mater. Sci. Technol. 1999, 15, 869–877.
- Wu, J.S.; Beaven, P.A.; Wagner, R. The Ti3(Al, Si) + Ti5(Si, Al)3 Eutectic Reaction in the Ti-Al-Si system. Scr. Metall. Mater. 1990, 24, 207–212.
- Novák, P.; Kříž, J.; Průša, F.; Kubásek, J.; Marek, I.; Michalcová, A.; Voděrová, M.; Vojtěch, D. Structure and properties of Ti–Al–Si-X alloys produced by SHS method. Intermetallics 2013, 39, 11–19.
- Tkachenko, S.; Datskevich, O.; Dvořák, K.; Spotz, Z.; Kulak, L.; Čelko, L. Isothermal oxidation behavior of experimental Ti−Al−Si alloys at 700 °C in air. J. Alloys Compd. 2017, 694, 1098–1108.
- Gurrappa, I. An oxidation model for predicting the life of titanium alloy components in gas turbine engines. J. Alloys Compd. 2005, 389, 190–197.
- Gaddam, R.; Antti, M.L.; Pederson, R. Influence of alpha-case layer on the low cycle fatigue properties of Ti-6Al-2Sn-4Zr-2Mo alloy. Mater. Sci. Eng. A 2014, 599, 51–56.
- Montanari, R.; Costanza, G.; Tata, M.E.; Testani, C. Lattice expansion of Ti–6Al–4V by nitrogen and oxygen absorption. Mater. Charact. 2008, 59, 334–337.
- Woodfield, A.P.; Postans, P.J.; Loretto, M.H.; Smallman, R.E. The effect of long-term high temperature exposure on the structure and properties of the titanium alloy Ti 5331S. Acta Metall. 1988, 36, 507–515.
- Vojtěch, D.; Čížová, H.; Jurek, K.; Maixner, J. Influence of silicon on high-temperature cyclic oxidation behaviour of titanium. J. Alloys Compd. 2005, 394, 240–249.
- Vojtěch, D.; Bártová, B.; Kubatík, T. High temperature oxidation of titanium–silicon alloys. Mater. Sci. Eng. A 2003, 361, 50–57.
- Kitashima, T.; Yamabe-Mitarai, Y. Oxidation Behavior of Germanium- and/or Silicon-Bearing Near-α Titanium Alloys in Air. Metall. Mater. Trans. A 2015, 46, 2758–2767.
- Saha, R.; Nandy, T.; Misra, R.; Jacob, K.T. Microstructural changes induced by ternary additions in a hypo-eutectic titanium-silicon alloy. J. Mater. Sci. 1991, 26, 2637–2644.
- Novák, P.; Kříž, J.; Michalcová, A.; Vojtěch, D. Microstructure Evolution of Fe-Al-Si and Ti-Al-Si Alloys during High-Temperature Oxidation. Mater. Sci. Forum 2014, 782, 353–358.
- Grilli, M.; Bellezze, T.; Gamsjäger, E.; Rinaldi, A.; Novak, P.; Balos, S.; Piticescu, R.; Ruello, M. Solutions for Critical Raw Materials under Extreme Conditions: A Review. Materials 2017, 10, 285.
- Bartl, A.; Tkaczyk, A.; Amato, A.; Beolchini, F.; Lapkovskis, V.; Petranik, M. Supply and Substitution Options for Selected Critical Raw Materials: Cobalt, Niobium, Tungsten, Yttrium and Rare Earths Elements. Detritus 2018, 3, 37–42.
- Novák, P.; Jaworska, L.; Cabibbo, M. Intermetallics as innovative CRM-free materials. IOP Conf. Ser. Mater. Sci. Eng. 2018, 329, 12013.
- Critical Raw Materials. Available online: (accessed on 23 January 2021).
- Urbina, M.; Rinaldi, A.; Cuesta-Lopez, S.; Sobetkii, A.; Slobozeanu, A.E.; Szakalos, P.; Qin, Y.; Prakasam, M.; Piticescu, R.-R.; Ducros, C.; et al. The methodologies and strategies for the development of novel material systems and coatings for applications in extreme environments—A critical review. Manuf. Rev. 2018, 5, 9.
- Kvanin, V.L.; Balikhina, N.T.; Vadchenko, S.G.; Borovinskaya, I.P.; Sychev, A.E. Preparation of γ-TiAl intermetallic compounds through self-propagating high-temperature synthesis and compaction. Inorg. Mater. 2008, 44, 1194–1198.
- Sauthoff, G. Intermetallics; VCH: Weinheim, Germany; New York, NY, USA; Basel, Switzerland; Cambridge, UK; Tokyo, Japan, 1995.
- Lapin, J. TiAl-based alloys: Present status and future perspectives. In Proceedings of the Metal 2009, Hradec nad Moravicí, Czech Republic, 19–21 May 2009.
- Stoloff, N.S.; Sikka, V.K. Physical Metallurgy and Processing of Intermetallic Compounds; Springer: Boston, MA USA, 1996.
- Novák, P.; Kříž, J.; Michalcová, A.; Vojtěch, D. Effect of alloying elements on properties of PM Ti-Al-Si alloys. Acta Metall. Slovaca 2013, 19, 240–246.
- Alman, D.E. Reactive sintering of TiAl–Ti5Si3 in situ composites. Intermetallics 2005, 13, 572–579.
- Subrahmanyam, J.; Vijayakumar, M. Self-propagating high-temperature synthesis. J. Mater. Sci. 1992, 27, 6249–6273.
- Morsi, K. Review: Reaction synthesis processing of Ni–Al intermetallic materials. Mater. Sci. Eng. A 2001, 299, 1–15.
- Ma, Y.; Fan, Q.; Zhang, J.; Shi, J.; Xiao, G.; Gu, M. Microstructural evolution during self-propagating high-temperature synthesis of Ti-Al system. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2008, 23, 381–385.
- Wang, T.; Liu, R.Y.; Zhu, M.L.; Zhang, J.S. Activation energy of self-heating process Studied by DSC. J. Therm. Anal. Calorim. 2002, 70, 507–519.
- Ferguson, H.; Whychell, D.T.; Federation, M.P.I.; International, A. Advances in Powder Metallurgy and Particulate Materials 2000. In 2000 International Conference on Powder Metallurgy & Particulate Materials, Tyoto, Japan, 12–16 November 2000; Metal Powder Industries Federation: University Park, PA, USA, 2000.
- Rice, R.W.; McDonough, W.J. Intrinsic Volume Changes of Self-propagating Synthesis. J. Am. Ceram. Soc. 1985, 68, C-122–C-123.
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184.
- Skotnicová, K.; Kursa, M. Prášková Metalurgie; Vysoká škola báňská—Technická univerzita Ostrava: Ostrava, Czech Republic, 2013.
- Rao, K.P.; Zhou, J.B. Characterization of mechanically alloyed Ti–Al–Si powder blends and their subsequent thermal stability. Mater. Sci. Eng. A 2002, 338, 282–298.
- Gu, J.; Gu, S.; Xue, L.; Wu, S.; Yan, Y. Microstructure and mechanical properties of in-situ Al13Fe4/Al composites prepared by mechanical alloying and spark plasma sintering. Mater. Sci. Eng. A 2012, 558, 684–691.
- Vyas, A.; Rao, K.P.; Prasad, Y.V.R.K. Mechanical alloying characteristics and thermal stability of Ti–Al–Si and Ti–Al–Si–C powders. J. Alloys Compd. 2009, 475, 252–260.
- Guo, W.; Iasonna, A.; Magini, M.; Martelli, S.; Padella, F. Synthesis of amorphous and metastable Ti40Al60 alloys by mechanical alloying of elemental powders. J. Mater. Sci. 1994, 29, 2436–2444.
- Szewczak, E.; Presz, A.; Witek, A.; Wyrzykowski, J.W.; Matyja, H. Microstructure and phase composition of mechanically alloyed and hot pressed Ti-Al alloys. Nanostructured Mater. 1999, 12, 167–170.
- Benhaddad, S.; Bhan, S.; Rahmat, A. Effect of ball milling time on Ti-Al and Ni-Al powder mixtures. J. Mater. Sci. Lett. 1997, 16, 855–857.
- Krasnowski, M.; Grabias, A.; Kulik, T. Phase transformations during mechanical alloying of Fe–50% Al and subsequent heating of the milling product. J. Alloys Compd. 2006, 424, 119–127.
- Haghighi, S.E.; Janghorban, K.; Izadi, S. Structural evolution of Fe–50at.% Al powders during mechanical alloying and subsequent annealing processes. J. Alloys Compd. 2010, 495, 260–264.
- Novák, P.; Průša, F.; Nová, K.; Bernatiková, A.; Salvetr, P.; Kopeček, J.; Haušild, P. Application of Mechanical Alloying in Synthesis of Intermetallics. Acta Phys. Pol. A 2018, 134, 720–723.
- Nová, K.; Novák, P.; Průša, F.; Kopeček, J.; Čech, J. Synthesis of Intermetallics in Fe-Al-Si System by Mechanical Alloying. Metals 2018, 9, 20.
- Novák, P.; Moravec, H.; Vojtěch, V.; Knaislová, A.; Školáková, A.; Kubatík, T.F.; Kopeček, J. Powder-metallurgy preparation of NiTi shape-memory alloy using mechanical alloying and Spark Plasma Sintering. Mater. Tehnol. 2017, 51, 141–144.
- Zoz, H.; Reichardt, R.; Ren, H. Energy Balance during Mechanical Alloying, Measurement and Calculation Method supported by the MALTOZ®-software. Adv. Powder Metall. 1999, 1, 1–109.
- Zhang, Z.-H.; Liu, Z.-F.; Lu, J.-F.; Shen, X.-B.; Wang, F.-C.; Wang, Y.-D. The sintering mechanism in spark plasma sintering—Proof of the occurrence of spark discharge. Scr. Mater. 2014, 81, 56–59.
- Balima, F.; Bellin, F.; Michau, D.; Viraphong, O.; Poulon-Quintin, A.; Chung, U.C.; Dourfaye, A.; Largeteau, A. High pressure pulsed electric current activated equipment (HP-SPS) for material processing. Mater. Des. 2018, 139, 541–548.
- Prakasam, M.; Balima, F.; Cygan, S.; Klimczyk, P.; Jaworska, L.; Largeteau, A. Chapter 9—Ultrahigh pressure SPS (HP-SPS) as new syntheses and exploration tool in materials science. In Spark Plasma Sintering; Cao, G., Estournès, C., Garay, J., Orrù, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 201–218.
- Suárez, M.; Fernández, A.; Menéndez, J.L.; Torrecillas, R.; Kessel, H.U.; Hennicke, J.; Kirchner, R.; Kessel, T. Challenges and Opportunities for Spark Plasma Sintering: A Key Technology for a New Generation of Materials; InTechOpen: London, UK, 2013.
- Groza, J.R.; Zavaliangos, A. Sintering activation by external electrical field. Mater. Sci. Eng. A 2000, 287, 171–177.
- Liu, Y.; Liu, W. Mechanical alloying and spark plasma sintering of the intermetallic compound Ti50Al50. J. Alloys Compd. 2007, 440, 154–157.
- Knaislová, A.; Novák, P.; Cygan, S.; Jaworska, L.; Cabibbo, M. High-Pressure Spark Plasma Sintering (HP SPS): A Promising and Reliable Method for Preparing Ti–Al–Si Alloys. Materials 2017, 10, 465.
- Kermani, M.; Razavi, M.; Rahimipour, M.R.; Zakeri, M. The effect of temperature on the in situ synthesis–sintering and mechanical properties of MoSi2 prepared by spark plasma sintering. J. Alloys Compd. 2014, 585, 229–233.
- Licheri, R.; Orrù, R.; Musa, C.; Locci, A.M.; Cao, G. Consolidation via spark plasma sintering of HfB2/SiC and HfB2/HfC/SiC composite powders obtained by self-propagating high-temperature synthesis. J. Alloys Compd. 2009, 478, 572–578.
- Licheri, R.; Musa, C.; Orrù, R.; Cao, G. Influence of the heating rate on the in situ synthesis and consolidation of ZrB2 by reactive Spark Plasma Sintering. J. Eur. Ceram. Soc. 2015, 35, 1129–1137.
- Lagos, M.A.; Agote, I.; Atxaga, G.; Adarraga, O.; Pambaguian, L. Fabrication and characterisation of Titanium Matrix Composites obtained using a combination of Self propagating High temperature Synthesis and Spark Plasma Sintering. Mater. Sci. Eng. A 2016, 655, 44–49.
- Yung, D.-L.; Cygan, S.; Antonov, M.; Jaworska, L.; Hussainova, I. Ultra high-pressure spark plasma sintered ZrC-Mo and ZrC-TiC composites. Int. J. Refract. Met. Hard Mater. 2016, 61, 201–206.
- Vallauri, D.; DeBenedetti, B.; Jaworska, L.; Klimczyk, P.; Rodriguez, M.A. Wear-resistant ceramic and metal–ceramic ultrafine composites fabricated from combustion synthesised metastable powders. Int. J. Refract. Met. Hard Mater. 2009, 27, 996–1003.
- Klimczyk, P.; Figiel, P.; Jaworska, L.; Bućko, M.M. Wysokociśnieniowe spiekanie nanoproszków w układzie Si3N4-SiC. Ceramics 2008, 103, 459–466.
- Dadlez, R.; Jaroszewski, W. Tektonika; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1994; p. 744.




