
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sonia Sentellas | + 3424 word(s) | 3424 | 2021-03-22 09:34:27 | | | |
| 2 | Vivi Li | Meta information modification | 3424 | 2021-03-31 05:55:12 | | |
Video Upload Options
This study aims to cover the main strategies based on ion mobility spectrometry (IMS) for the analysis of biological samples. The determination of endogenous and exogenous compounds in such samples is important for the understanding of the health status of individuals. For this reason, the development of new approaches that can be complementary to the ones already established (mainly based on liquid chromatography coupled to mass spectrometry) is welcomed. In this regard, ion mobility spectrometry has appeared in the analytical scenario as a powerful technique for the separation and characterization of compounds based on their mobility. IMS has been used in several areas taking advantage of its orthogonality with other analytical separation techniques, such as liquid chromatography, gas chromatography, capillary electrophoresis, or supercritical fluid chromatography. Bioanalysis is not one of the areas where IMS has been more extensively applied. However, over the last years, the interest in using this approach for the analysis of biological samples has clearly increased.
1. Introduction
Methods and technologies for the determination of endogenous and exogenous compounds in biological matrices such as plasma, urine, saliva, sweat, infected tissues, infected exudates, feces, breath, and breast milk [1][2][3][4][5][6][7][8][9][10][11][12] are under continuous development as a consequence of society’s growing interest in improving the knowledge of individuals’ health conditions. In this regard, the research on proper biomarkers or biological indicators of a medical state observed from outside the patient, which can be accurately and reproducibly measured, has gained special interest in the pharmaceutical and biomedical fields [13]. In addition, in the bioanalysis area, the determination of drugs and related compounds in biological samples is essential not only for correlating drug exposure to efficacy but also for predicting adverse effects related to the drug or its metabolites. In bioanalysis, samples are analyzed for qualitative or quantitative purposes, i.e., for the identification and structural elucidation or the quantitation of exogenous or endogenous molecules [14][15][16]. Due to the inherent complexity of biological matrices, samples are usually subjected to clean-up or preconcentration procedures to enrich the sample and to eliminate possible interferences that can hinder the determination of the compound(s) of interest. The complexity of such procedures ranges from simple dilution of the sample (dilute and shoot (DAS)) [17][18][19] or protein precipitation (PPT) [20][21] to intricate procedures dealing with solid-phase (SPE) [22][23][24] or liquid–liquid (LLE) [25] extractions [26][27]. It is clear that the sample treatment procedure is determined by the information available regarding the structural and the physicochemical properties of the analytes, the matrix, and the purpose of the analysis. Thus, for metabolomic fingerprinting, nonspecific methods (such as DAS or PPT) are preferred to prevent the loss of compounds that can result in important features [28]. On the contrary, the quantitative determination of drugs and/or known metabolites often requires high sensitivity, which means that specific methods developed considering the physicochemical properties of the analyte(s) should be used. Extracts obtained after sample treatment are then analyzed using several instrumental platforms. In metabolomics, analytical techniques, such as NMR, which can provide a huge amount of data that can be analyzed using mathematical models are commonly used for the analysis of the biological matrices [29][30][31][32]. However, in bioanalysis in general, high-performance separation techniques are usually welcomed to improve the efficiency of the analysis. In this regard, liquid chromatography (LC) has been the most extensively exploited technique. While first LC was used mostly with UV detection (LC-UV), nowadays, LC coupled with mass spectrometry has become the gold standard in bioanalysis combining high (compound) resolution, sensitivity, and specificity with a high sample throughput [33][34][35]. In addition, mass spectrometry provides structural information that is essential for identification purposes. Other separation techniques such as gas chromatography (GC), capillary electrophoresis (CE), or supercritical fluid chromatography (SFC) have also been considered as an alternative because of their complementary selectivity to LC [36][37][38][39][40][41]. However, the application of these techniques is by far less extended because of instrumental or methodological drawbacks.
In the last decades, ion mobility spectrometry (IMS), which separates gas ions according to their size-to-charge ratio, has gained interest as a powerful separation method. Ion mobility studies were already conducted in the early 20th century [42][43][44][45]; however, it was not until the 1970s that IMS was introduced as an analytical tool by Cohen and Karasek [46][47]. Since then, IMS has been extensively used in a wide range of research areas from environmental and security fields to biomedical and pharmaceutical applications [48][49][50][51][52][53][54][55][56][57]. For instance, IMS has been used for the detection of illegal drugs and their precursors (such as acetic anhydride or pyridine) [58][59], environmental analysis [60], the diagnosis of bacterial infections [61], forensic examination [62], military and chemical weapons monitoring [63][64], and aerospace applications [65]. The use of ion mobility has also gained relevance in bioanalysis in recent years owing to the potential improvement of the sensitivity and the capacity of the technique to separate strongly related compounds based on their conformational differences. In addition, ion mobility can provide some structural information by means of CCS determination (see below), which can be considerably helpful for the identification of unknown compounds. Initially, IMS was mainly used as a standalone technique; however, in recent years the coupling of IMS with mass spectrometry has spectacularly gained in importance [66]. This fact responds to the improvement in the analysis of complex samples that can be obtained taking advantage of (1) the separation based on the different mobility of the ions and (2) the structural information that mass spectrometry provides. Besides, the addition of a third separation dimension that is obtained with the coupling of an orthogonal technique, mainly liquid chromatography, has also shown a high potential for the analysis of complex samples [67][68]. In this case, the separation of the compounds of interest from other matrix components is driven by their lipophilicity (LC), shape (IMS), mass (MS), and charge (IMS and MS).
Bioanalysis has remarkable relevance in the analytical field owing to the necessity of linking human health conditions with objective indicators or descriptors. In this regard, the development of new analytical approaches for the characterization of biological samples is fundamental to increase the understanding of human health status. Hence, this paper is an overview of the use of ion mobility mainly coupled with mass spectrometry in the bioanalytical field. A brief introduction to the theoretical background of the technique is given, followed by a description of the different ion mobility techniques used in bioanalysis. Recent applications are described to provide insights into the current landscape of this field. However, an exhaustive coverage of applications is beyond the scope of the present contribution, and therefore, this paper focuses on some relevant applications published over the last few years. The main search was done using SciFinder, considering publications from 2015 to the present time and using concepts such as ion mobility, bioanalysis, metabolism, and drugs as the general search inputs. However, to cover other more specific points, keywords such as different ion mobility types, mass spectrometry, and liquid chromatography and a more extended period were also considered. Besides, relevant papers from a historical perspective that deal with fundamentals and background are also included.
2. Ion Mobility Techniques
As mentioned before, different ion mobility technologies have been developed, with the mobility of gas ions being the common driving force for their separation. However, the design and thus, the specific principle of separation is characteristic of each of the different technologies. Note that new variants are being developed, but the lack of commercial instrumentation makes them unfeasible from a practical point of view. In the following paragraphs, the specificities of ion mobility modes mostly used in bioanalysis are described, illustrating their applicability by reviewing some examples.
2.1. Drift Tube Ion Mobility Spectrometry (DTIMS)
In this case, the ion mobility analyzer is a tube (drift tube) consisting of a series of stacked-ring electrodes with a current of an inert gas flowing through the tube (mostly nitrogen or helium) (Figure 1A). The application of a uniform electric field along the drift tube makes the ions move towards the detection region. The collisions of the ions with the drift gas, which moves in the opposite direction than the ions do, determine their mobility; more compact ions, which suffer fewer collisions, move faster than extended ones. Thus, the time that ions need to reach the detector (drift time) depends on their shape, which is related to the CCS value [69][70]. In fact, DTIMS is the only analyzer that, if conditions are well controlled, allows one to directly determine CCS values with high accuracy without the need to use calibrators. For instance, CCS values in combination with mass spectrometry information were used for the identification of antiepileptic drugs in human serum [71]. DTIMS provides an orthogonal dimension to the LC separation, and furthermore, CCS measurements increase the confidence in the identification [71]. A clear example of the utility of CCS values for differentiating isobaric compounds is the case of carbamazepine epoxy, oxacarbazepine, and phenytoin with CCS values of 154.0, 155.8, and 166.6 Å2, respectively [71]. The relevance of measuring CCS is also pointed out by Nichols et al., who created a CCS library based on the analytical standards in the Mass Spectrometry Metabolite Library of Standards [72]. The addition of these values in the analysis of human serum extracts has been shown to be advantageous as a molecular descriptor [72]. Apart from the use of DTIMS for obtaining structural information, the technique has been exploited for the separation of isomers. This is the case for bile acids, for which separation by liquid chromatography is long and offers poor resolution for some isomeric compounds. After the application of ion mobility, good resolution outcomes were obtained for small bile acids (BAs), but BAs with high m/z values still remained unresolved. The addition of metal ions such as copper and zinc to the sample resulted in complexes that had differentiated mobilities and, thus, were separable by DTIMS [73]. Ion mobility has also shown to be an interesting option for the analysis of metabolites in biological samples for metabolite profiling, the so-called metabolomics [74][75][76][77]. As an example, Zhang and coworkers compared three different operation modes, namely FIA/IM-MS, LC-MS, and LC-IM-MS for metabolomics analyses of human plasma and HaCaT cells using DTIMS [75]. A clear benefit was observed by the addition of the third dimension (ion mobility) to the LC-MS analyses. In this regard, a reduction of chemical noise, an accurate measurement of isotope ratios, an increased peak capacity, and additional structural information were achieved by LC-IM-MS [75]. CCS determination has also been demonstrated to be useful in metabolomics since it can aid in the small molecule identification for both targeted and untargeted metabolite screening [77]. Reisdorph and coworkers described a typical DTIMS metabolomics workflow as a proposal to investigators who are interested in using IM-MS in their metabolomic studies [74].
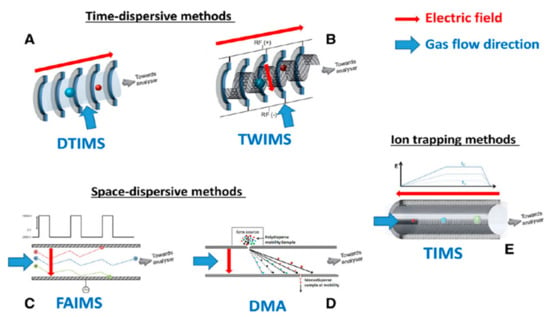
Figure 1. Schematic representation of the commercially available IMS technology: DTIMS (A), TWIMS (B), FAIMS (C), DMA (D) and TIMS (E) Reproduced from [70] with permission.
2.2. Travelling Wave Ion Mobility Spectrometry (TWIMS)
Traveling wave ion mobility spectrometry (TWIMS), like DTIMS, is a time-dispersive technique. The separation cell also consists of a series of stacked-ring electrodes. However, in TWIMS, the separation is obtained by a dynamic application of the electric field rather than a linear one. This is obtained through the alternative application of positive and negative radio frequency voltages to adjacent electrodes. In this way, a traveling wave is created, in which magnitude and speed govern the ion separation. As in the case of DTIMS, more compact ions suffer less friction with the buffer gas, and as a result, they move faster than extended ones [69][70]. Figure 1B shows a scheme of the technique.
In general, TWIMS has been extensively applied as a separation technique; however, the possibility of determining CCS offers the potential of using it for characterization purposes. Nevertheless, unlike in DTIMS, in TWIMS, a calibration with reference standards is needed for CCS calculation. In the bioanalytical field, one can find CCS libraries that are generated for a specific set of compounds, such as steroids [78] or rat metabolites [79]. More specifically, CCS values were also used for the structural elucidation of small molecules. In this regard, several publications using TWIMS have been found to deal with the identification of metabolites of drugs or specific compounds [80][81][82]. For instance, Ross et al. developed a workflow for the structural elucidation of drug metabolites using TWIMS [81]. After in vitro metabolite generation, the samples were analyzed by FIA-IM-MS. The analysis of the obtained data (CCS values and MS information) allowed them to characterize drug metabolites from a diverse panel of drugs. They found that CCS changes depend not only on the type and position of the modification but also on the structural characteristics of the parent drug. As mentioned before, a previous calibration was needed for CCS determination. They used a mixture of polyalanines and drug-like compounds with known DTIMCCS values to calibrate TWIMCCS values (Figure 2). Regarding the use of this technique for separation purposes in bioanalysis, some applications can be found in combination with LC as an orthogonal separation dimension [83][84][85] or as a standalone separation technique [86]. In the latter case, a rapid and sensitive method was developed for the quantification of drugs in tissue sections using matrix-assisted laser desorption ionization (MALDI) as an ionization source. Isomer separations can also be accomplished with TWIMS. As an example, the separation of bile acid isomers was successfully achieved [87]. As has been pointed out before, the small differences in the mobility of some of these isomeric compounds are not enough for a baseline separation. In this case, Chouinard and coworkers used cyclodextrin adduct formation to increase ion mobility resolution [87]. Finally, it is worth mentioning the use of TWIMS in metabolomic studies, which has been reviewed elsewhere [88]. Following a metabolomic approach, Poland and coworkers were able to profile changes in the gut metabolome after biliary diversion surgery. For such a purpose, an untargeted UPLC-IM-MS method was optimized for fecal samples obtained from mice that have undergone bile diversion surgery [89]. Urinary metabolic phenotyping can also be mentioned as an example of metabolomics studies performed using TWIMS [90][91].
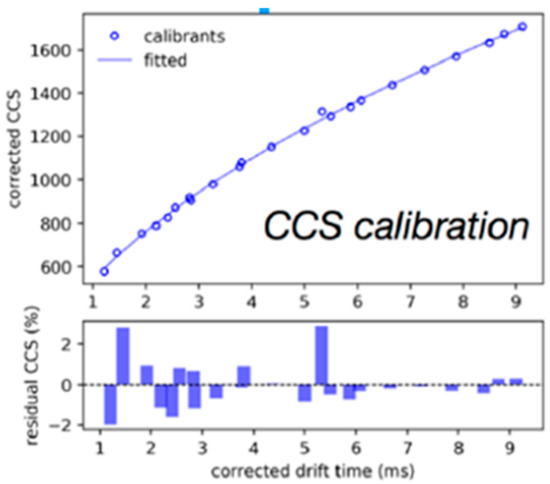
Figure 2. Calibration of TWIMCCS values using known DTIMCCS values from a mixture of polyalanines and drug-like compounds. This figure was adapted from [81] with the permission of the publisher.
2.3. Differential Mobility Analysis (DMA)
Differential mobility analysis (DMA) is a space-dispersive technique in which the ions are separated based on their capacity to reach the ion mobility cell exit so that a scan is needed for the detection of different ions. In this approach, a constant electric field is applied between two cylindrical and concentric metal electrodes. Ions are introduced between the two electrodes where they are pushed towards the exit by means of an orthogonal flow of sheath gas. Only those ions with the appropriate mobility will reach the cell exit. The other ions will collide with the electrode, thus preventing their detection. By scanning the electric field, an ion spectrum based on the different ion mobilities can be recorded (Figure 1D). In general, DMA is mainly used for the determination of large analytes such as aerosol particles or macromolecules and is less applied for small molecules [92]. In fact, very few bioanalytical applications for DMA have been found. Even so, the potential of the technique has been proven in several cases. As an example, the isomers sarcosine and L-alanine were partially resolved in urine, and for such a purpose, a minimal sample preparation consisting of a simple dilution step was required [93]. It is also worth mentioning that the technique has also been applied in metabolomic studies. Thus, the urinary metabolic fingerprint obtained by DMA–quadrupole time-of-flight (QTOF) allowed Martinez-Lozano and coworkers to differentiate between prostate cancer patients and healthy individuals [94].
2.4. Field Asymmetric Waveform Ion Mobility Spectrometry (FAIMS) and Differential Mobility Spectrometry (DMS)
Field asymmetric waveform ion mobility spectrometry (FAIMS), like DMA, is a space-dispersive technique in which separation occurs on a spatial scale rather than in a temporal one (Figure 1C). This technique is often equally named differential mobility spectrometry (DMS); however, although the principle of operation is the same, some small differences exist between both techniques due to the geometry of their electrodes. Devices with cylindrical electrodes are referred to as field asymmetric waveform ion mobility spectrometers, whereas planar electrodes are used in DMS [95]. Here, we will use the term FAIMS when describing the principles of operation. In FAIMS, a high asymmetric electric field is applied between two electrodes. The motion of ions is then driven not only by the changing electric field but also because of the carrier gas, which is injected in the same direction as the ions. Under these conditions, only those ions with the proper mobility will reach the detector. However, a compensation voltage is superposed to the dispersion field, which corrects the trajectory of the ions of interest. Again, those ions whose trajectory is not properly corrected will migrate away. The compensation voltage (CV) can be scanned to generate a CV spectrum. In FAIMS, the ions are separated based not directly on their mobility, but on changes in mobility. This implies that the mechanism of separation in FAIMS is more different from MS when compared to the mechanisms of the other ion mobility techniques [70][95].
Among all the ion mobility techniques, DMS-MS is the most widely used in the bioanalytical field. DMS coupled with MS is mainly used as a filtering process both in combination with liquid chromatography (LC) [96][97][98][99][100] or as a stand-alone separation [101][102]. The filtering capacity of the technique provides clear benefits in terms of eliminating interferences and reducing background noise, which can entail an increase in sensitivity. In Figure 3, the improvement in sensitivity obtained in the determination of eptifibatide in rat plasma with LC-DMS–multiple ion monitoring (LC-DMS-MIM) with respect to LC-MIM or LC–multiple reaction monitoring (LC-MRM) is shown. The results suggest that LC-DMS-MIM can be considered as a proper bioanalytical alternative for compounds with poor CID efficiency [99]. Kayleigh and coworkers reported also an increase in signal-to-noise ratios by eliminating interferences for the determination of anabolic–androgenic steroid metabolites in urine using LC-FAIMS-MS. Furthermore, the separation capacity of FAIMS added to the LC separation allows a substantial reduction in chromatographic run time [97]. Going further, LC can be totally avoided in some cases by using the separation obtained by DMS. This is the case of the determination of cocaine and its metabolites in human serum. The DMS-MS/MS method demonstrated the potential of the technique for high-throughput analysis of these compounds [101]. The separation of isomers was also achieved with DMS [103][104]. However, the scarce structural differences between isomers, above all for small molecules, make the separation challenging. In these cases, but also as a general strategy for improving the separation, gas modifiers can be added. Hence, Ruskic and Hopfgartner studied the effect of using different modifiers on the separation of several isomeric drugs in human plasma [103]. DMS has also been combined with gas chromatography (GC), as proposed by Criado-García and coworkers, who developed a rapid noninvasive method for the determination of toxic levels of alcohols in saliva [105].
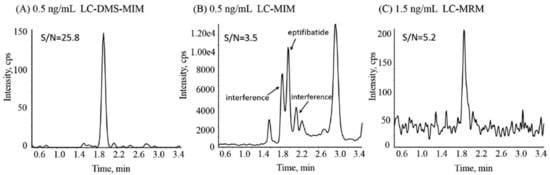
Figure 3. Comparison of the performance of the LC-DMS-MIM assay for eptifibatide in rat plasma with that of similar assays without DMS. Reproduced from [99] with permission.
As stated above, CCS values could not be determined by FAIMS; however, in some cases, compensation voltage, which is characteristic of each analyte at given conditions, could be used for application in metabolite identification [106]. Finally, it is worth pointing out that, although FAIMS (or DMS) has normally been used for targeted analysis, several applications can be found using this technique for metabolomic analysis. As an example, an LC-FAIMS-MS method, which has been demonstrated to be able to resolve co-eluting isomeric species, was applied for the untargeted metabolomic analysis of human urine, allowing the differentiation between fresh and aged urine [107]. Regarding DMS, a novel untargeted metabolomics method, based solely on DMS, was applied in a clinically relevant chronic kidney disease patient population [108].
2.5. Trapped Ion Mobility Spectrometry (TIMS)
Trapped ion mobility spectrometry (TIMS) is a relatively recent technique. Briefly, ions are trapped into the drift cell by means of an electric field that keeps ions static against a circulating gas. Under these conditions, following the same separation principles as those in DTIMS (ions are pushed through a gas using an electric field), the ions are separated based on their size-to-charge ratio. After the separation step, the electric field is decreased gradually, allowing ions to be eluted from high to low size-to-charge ratios [69][70][109]. Few applications in the bioanalytical area have been found using TIMS; among them, publications by Adams and coworkers can be highlighted [110][111]. Their works involving LC-TIMS-MS include the determination of isomeric drugs of abuse and their metabolites in human urine [110] or the targeted monitoring of polychlorinated biphenyl metabolites in human plasma [111]. TIMS has also been used, combined with MALDI, for spatial metabolomics [112][113].
References
- Monedeiro, F.; Milanowski, M.; Ratiu, I.-A.; Zmysłowski, H.; Ligor, T.; Buszewski, B. VOC Profiles of Saliva in Assessment of Halitosis and Submandibular Abscesses Using HS-SPME-GC/MS Technique. Molecules 2019, 24, 2977.
- Löfgren, L.; Pehrsson, S.; Hägglund, G.; Tjellström, H.; Nylander, S. Accurate measurement of endogenous adenosine in human blood. PLoS ONE 2018, 13, e0205707.
- Coulter, C.A.; Moore, C.M. Analysis of drugs in oral fluid using LC-MS/MS. Methods Mol. Biol. 2019, 1872, 237–259.
- Avataneo, V.; D’Avolio, A.; Cusato, J.; Cantu, M.; De Nicolo, A. LC-MS application for therapeutic drug monitoring in alternative matrices. J. Pharm. Biomed. Anal. 2019, 166, 40–51.
- Kim, H.; Lee, D.-H.; Go, A.; Park, M.; Choe, S.; In, S.; Kim, E.; Lee, H.; Shin, K.-H.; Han, E. Differentiation of endogenous and exogenous γ-Hydroxybutyrate in rat and human urine by GC/C/IRMS. Int. J. Leg. Med. 2019, 133, 1785–1794.
- Ratiu, I.-A.; Ligor, T.; Bocos-Bintintan, V.; Szeliga, J.; Machała, K.; Jackowski, M.; Buszewski, B. GC-MS application in determination of volatile profiles emitted by infected and uninfected human tissue. J. Breath Res. 2019, 13, 026003.
- Sarbach, C.; Stevens, P.; Whiting, J.; Puget, P.; Humbert, M.; Cohen-Kaminsky, S.; Postaire, E. Evidence of endogenous volatile organic compounds as biomarkers of diseases in alveolar breath. Ann. Pharm. Fr. 2013, 71, 203–215.
- Garner, C.E.; Smith, S.; Lacy Costello, B.; White, P.; Spencer, R.; Probert, C.S.J.; Ratcliffem, N.M. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J. 2007, 21, 1675–1688.
- Dingess, K.A.; van den Toorn, H.W.P.; Mank, M.; Stahl, B.; Heck, A.J.R. Toward an efficient workflow for the analysis of the human milk peptidome. Anal. Bioanal. Chem. 2019, 411, 1351–1363.
- Tamama, K. Advances in drugs of abuse testing. Clin. Chim. Acta 2021, 514, 40–47.
- Magar, N.R.; Shakil, P.M.S. A review on bioanalytical method development and validation. World J. Pharm. Res. 2020, 9, 1060–1070.
- Dosedelova, V.; Itterheimova, P.; Kuban, P. Analysis of bile acids in human biological samples by microcolumn separation techniques. Electrophoresis 2021, 42, 68–85.
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466.
- Saurina, J.; Sentellas, S. Liquid chromatography coupled to mass spectrometry for metabolite profiling in the field of drug discovery. Expert Opin. Drug Discov. 2019, 14, 469–483.
- Saurina, J.; Sentellas, S. Strategies for metabolite profiling based on liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1044–1045, 103–111.
- Jemal, M.; Xia, Y.-Q. LC-MS Development strategies for quantitative bioanalysis. Curr. Drug Metab. 2006, 7, 491–502.
- Deventer, K.; Pozo, O.J.; Verstraete, A.G.; Van Eenoo, P. Dilute-and-shoot-liquid chromatography-mass spectrometry for urine analysis in doping control and analytical toxicology. TrAC Trends Anal. Chem. 2014, 55, 1–13.
- Agostini, M.; Renzoni, C.; Pierini, E.; Piergiovanni, M.; Termopoli, V.; Famiglini, G.; Palma, P.; Cappiello, A. Rapid, hydrolysis-free, dilute-and-shoot method for the determination of buprenorphine, norbuprenorphine and their glucuronides in urine samples using UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2019, 166, 236–243.
- Yan, J.; Kuzhiumparambil, U.; Bandodkar, S.; Solowij, N.; Fu, S. Development and validation of a simple, rapid and sensitive LC-MS/MS method for the measurement of urinary neurotransmitters and their metabolites. Anal. Bioanal. Chem. 2017, 409, 7191–7199.
- Taevernier, L.; Wynendaele, E.; D’Hondt, M.; De Spiegeleer, B. Analytical quality-by-design approach for sample treatment of BSA-containing solutions. J. Pharm. Anal. 2015, 5, 27–32.
- Polson, C.; Sarkar, P.; Incledon, B.; Raguvaran, V.; Grant, R. Optimization of protein precipitation based upon effectiveness of protein removal and ionization effect in liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2003, 785, 263–275.
- Imam Pasha, S.; Varanasi, M.B.; Mohammed, I. Bioanalysis of monomethyl fumarate in human plasma by a sensitive and rapid LC-MS/MS method and its pharmacokinetic application LC-MS/MS determination of monomethyl fumarate in human plasma. J. Pharm. Biomed. Anal. 2017, 146, 109–116.
- He, J.; Yuan, J.; Du, J.; Chen, X.; Zhang, X.; Ma, A.; Pan, J. Automated on-line SPE determination of amisulpride in human plasma using LC coupled with restricted-access media column. Microchem. J. 2019, 145, 154–161.
- Piotrowski, P.; Bocian, S.; Śliwka, K.; Buszewski, B. Simultaneous analysis of zolpidem and its metabolite in whole blood and oral fluid samples by SPE-LC/MS for clinical and forensic purposes. Adv. Med. Sci. 2015, 60, 167–172.
- Ashri, N.Y.; Abdel-Rehim, M. Sample treatment based on extraction techniques in biological matrices. Bioanalysis 2011, 3, 2003–2018.
- Sentellas, S.; Saurina, J.; Núñez, O. Solid-Phase Extraction in Bioanalytical Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128169063.
- Matysik, F.-M. Steen Honoré Hansen and Stig Pedersen-Bjergaard (Eds.): Bioanalysis of pharmaceuticals: Sample preparation, separation techniques and mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 3369–3370.
- Gonzalez-Dominguez, R.; Gonzalez-Dominguez, A.; Segundo, C.; Schwarz, M.; Sayago, A.; Mateos, R.M.; Duran-Guerrero, E.; Lechuga-Sancho, A.M.; Fernandez-Recamales, A. High-throughput metabolomics based on direct mass spectrometry analysis in biomedical research. Methods Mol. Biol. 2019, 1978, 27–38.
- Pathmasiri, W.; Kay, K.; McRitchie, S.; Sumner, S. Analysis of NMR Metabolomics Data. Methods Mol. Biol. 2020, 2104, 61–97.
- Segers, K.; Declerck, S.; Mangelings, D.; Van der Heyden, Y.; Eeckhaut, A.V. Analytical techniques for metabolomic studies: A review. Bioanalysis 2019, 11, 2297–2318.
- Wishart, D.S. NMR metabolomics: A look ahead. J. Magn. Reson. 2019, 306, 155–161.
- Song, Z.; Wang, H.; Yin, X.; Jiang, W.; Song, Z.; Wang, H.; Yin, X.; Deng, P. Application of NMR metabolomics to search for human disease biomarkers in blood. Clin. Chem. Lab. Med. 2019, 57, 417–441.
- Rajan, M.A.; Raghavendhar, J. Bioanalytical method development and validation—An overview. Int. J. Pharm. Pharm. Res. 2019, 16, 398–411.
- Patil, T.S.; Khadse, S.C.; Chalikwar, S.S. Review on development and validation of bioanalytical method based on LC-MS/MS technique. Eur. J. Biomed. Pharm. Sci. 2019, 6, 222–231.
- Kapri, A.; Gupta, N.; Raj, G. Bioanalytical method development: An updated review. Int. Res. J. Pharm. 2017, 8, 6–9.
- Kavanagh, P.; Grigoryev, A.; Melnik, A.; Savchuk, S.; Simonov, A.; Rozhanets, V. Detection and tentative identification of urinary phase I metabolites of phenylacetylindole cannabimimetics JWH-203 and JWH-251, by GC-MS and LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 934, 102–108.
- Yang, S.; Lu, J.; Xu, Y.; Wang, X. New oxymesterone metabolites in human by gas chromatography-tandem mass spectrometry and their application for doping control. Drug Test. Anal. 2016, 8, 633–643.
- Matabosch, X.; Pozo, O.J.; Pérez-Mañá, C.; Farré, M.; Marcos, J.; Segura, J.; Ventura, R. Identification of budesonide metabolites in human urine after oral administration. Anal. Bioanal. Chem. 2012, 404, 325–340.
- Wang, X.; Li, K.; Adams, E.; Van Schepdael, A. Capillary electrophoresis-mass spectrometry in metabolomics: The potential for driving drug discovery and development. Curr. Drug Metab. 2013, 14, 807–813.
- Bytzek, A.K.; Hartinger, C.G. Capillary electrophoretic methods in the development of metal-based therapeutics and diagnostics: New methodology and applications. Electrophoresis 2012, 33, 622–634.
- Taguchi, K.; Fukusaki, E.; Bamba, T. Supercritical fluid chromatography/mass spectrometry in metabolite analysis. Bioanalysis 2014, 6, 1679–1689.
- Rutherford, E. Velocity and rate of recombination of the ions of gases exposed to rontgen radiation. Philos. Mag. 1897, 44, 422–440.
- Rontgen, W.C. On a new kind of rays. Science 1896, 3, 227–231.
- Langevin, P. Ionization of Gas. Ann. Chim. Phys. 1903, 28, 289–384.
- Langevin, P. A fundamental formula of kinetic theory. Ann. Chim. Phys. 1905, 5, 245–288.
- Cohen, M.J.; Karasek, F.W. Plasma chromatography TM—New dimension for gas chromatography and mass spectrometry. J. Chromatogr. Sci. 1970, 8, 330–337.
- Karasek, F.W. Plasma chromatography. Anal. Chem. 1974, 46, 710A–712A, 714A, 716A, 718A–720A.
- To, K.C.; Ben-Jaber, S.; Parkin, I.P. Recent Developments in the Field of Explosive Trace Detection. ACS Nano 2020, 14, 10804–10833.
- Odenkirk, M.T.; Baker, E.S. Utilizing Drift Tube Ion Mobility Spectrometry for the Evaluation of Metabolites and Xenobiotics. Methods Mol. Biol. 2020, 2084, 35–54.
- Armenta, S.; Esteve-Turrillas, F.A.; Alcala, M. Analysis of hazardous chemicals by “stand alone” drift tube ion mobility spectrometry: A review. Anal. Methods 2020, 12, 1163–1181.
- Ferre, S.; Gonzalez-Ruiz, V.; Guillarme, D.; Rudaz, S. Analytical strategies for the determination of amino acids: Past, present and future trends. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1132, 121819.
- Hernandez-Mesa, M.; Escourrou, A.; Monteau, F.; Le Bizec, B.; Dervilly-Pinel, G. Current applications and perspectives of ion mobility spectrometry to answer chemical food safety issues. TrAC Trends Anal. Chem. 2017, 94, 39–53.
- Burnum-Johnson, K.E.; Zheng, X.; Dodds, J.N.; Ash, J.; Fourches, D.; Nicora, C.D.; Wendler, J.P.; Metz, T.O.; Waters, K.M.; Jansson, J.K.; et al. Ion mobility spectrometry and the omics: Distinguishing isomers, molecular classes and contaminant ions in complex samples. TrAC Trends Anal. Chem. 2019, 116, 292–299.
- Kathirvel, S.; Gayatri Ramya, M.; Rajesh, A. An overview on the benefits and applications of high performance ion mobility spectrometer in pharmaceutical arena- focus on current research. World J. Pharm. Pharm. Sci. 2017, 6, 402–406.
- Tadjimukhamedov, F.K.; Forbes, P.B.C. Mass spectrometry and ion mobility spectrometry [for air pollutant monitoring]. In Comprehensive Anaytical Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2015; Volume 70, pp. 311–329.
- Chouinard, C.D.; Wei, M.S.; Beekman, C.R.; Kemperman, R.H.J.; Yost, R.A. Ion mobility in clinical analysis: Current progress and future perspectives. Clin. Chem. 2016, 62, 124–133.
- Campuzano, I.D.G.; Lippens, J.L. Ion mobility in the pharmaceutical industry: An established biophysical technique or still niche? Curr. Opin. Chem. Biol. 2018, 42, 147–159.
- Bocos-Bintintan, V.; Ghira, G.-B.; Anton, M.; Martiniuc, A.-V.; Ratiu, I.-A.; Ratiu, I.-A. Sensing Precursors of Illegal Drugs-Rapid Detection of Acetic Anhydride Vapors at Trace Levels Using Photoionization Detection and Ion Mobility Spectrometry. Molecules 2020, 25, 1852.
- Ghira, G.-B.; Ratiu, I.-A.; Bocos-Bintintan, V. Fast characterization of pyridine using ion mobility spectrometry and photoionization detection. Environ. Eng. Manag. J. 2013, 12, 251–256.
- Marquez-Sillero, I.; Aguilera-Herrador, E.; Cardenas, S.; Valcarcel, M. Ion-mobility spectrometry for environmental analysis. TrAC Trends Anal. Chem. 2011, 30, 677–690.
- Karpas, Z.; Chaim, W.; Gdalevsky, R.; Tilman, B.; Lorber, A. Diagnosis of vaginal infections by ion mobility spectrometry. Int. J. Ion Mobil. Spectrom. 2002, 5, 49–54.
- Keller, T.; Schneider, A.; Tutsch-Bauer, E.; Jaspers, J.; Aderjan, R.; Skopp, G. Ion mobility spectrometry for the detection of drugs in cases of forensic and criminalistic relevance. Int. J. Ion Mobil. Spectrom. 1999, 2, 22–34.
- Sohn, H.; Steinhanses, J. Use of ion mobility spectrometry for the preliminary evaluation of hazardous military waste sites-opportunities and limitations. Int. J. Ion Mobil. Spectrom. 1998, 1, 1–14.
- Mäkinen, M.A.; Anttalainen, O.A.; Sillanpää, M.E.T. Ion mobility spectrometry and its applications in detection of chemical warfare agents. Anal. Chem. 2010, 82, 9594–9600.
- Cossoul, E.; Hubert-Roux, M.; Sebban, M.; Churlaud, F.; Oulyadi, H.; Afonso, C. Evaluation of atmospheric solid analysis probe ionization coupled to ion mobility mass spectrometry for characterization of poly(ether ether ketone) polymers. Anal. Chim. Acta 2015, 856, 46–53.
- Eldrid, C.; Thalassinos, K. Developments in tandem ion mobility mass spectrometry. Biochem. Soc. Trans. 2020, 48, 2457–2466.
- Kaufmann, A. The use of UHPLC, IMS, and HRMS in multiresidue analytical methods: A critical review. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1158, 122369.
- Purves, R.W. Enhancing biological LC-MS analyses using ion mobility spectrometry. In Comprehensive Anaytical Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 79, pp. 205–240.
- Cumeras, R.; Figueras, E.; Davis, C.E.; Baumbach, J.I.; Gràcia, I. Review on Ion Mobility Spectrometry. Part 2: Hyphenated methods and effects of experimental parameters. Analyst 2015, 140, 1391–1410.
- D’Atri, V.; Causon, T.; Hernandez-Alba, O.; Mutabazi, A.; Veuthey, J.-L.L.; Cianferani, S.; Guillarme, D. Adding a new separation dimension to MS and LC-MS: What is the utility of ion mobility spectrometry? J. Sep. Sci. 2018, 41, 20–67.
- Davis, D.E.J.; Sherrod, S.D.; Gant-Branum, R.L.; McLean, J.A.; Colby, J.M.; Davis, D.E., Jr.; Sherrod, S.D.; Gant-Branum, R.L.; Colby, J.M.; McLean, J.A.; et al. Targeted Strategy to Analyze Antiepileptic Drugs in Human Serum by LC-MS/MS and LC-Ion Mobility-MS. Anal. Chem. 2020, 92, 14648–14656.
- Nichols, C.M.; Dodds, J.N.; Rose, B.S.; Picache, J.A.; Morris, C.B.; Codreanu, S.G.; May, J.C.; Sherrod, S.D.; McLean, J.A. Untargeted Molecular Discovery in Primary Metabolism: Collision Cross Section as a Molecular Descriptor in Ion Mobility-Mass Spectrometry. Anal. Chem. 2018, 90, 14484–14492.
- Zheng, X.; Smith, F.B.; Aly, N.A.; Cai, J.; Smith, R.D.; Patterson, A.D.; Baker, E.S. Evaluating the structural complexity of isomeric bile acids with ion mobility spectrometry. Anal. Bioanal. Chem. 2019, 411, 4673–4682.
- Reisdorph, R.; Michel, C.; Quinn, K.; Doenges, K.; Reisdorph, N. Untargeted Differential Metabolomics Analysis Using Drift Tube Ion Mobility-Mass Spectrometry. Methods Mol. Biol. 2020, 2084, 55–78.
- Zhang, X.; Kew, K.; Reisdorph, R.; Sartain, M.; Powell, R.; Armstrong, M.; Quinn, K.; Cruickshank-Quinn, C.; Walmsley, S.; Bokatzian, S.; et al. Performance of a High-Pressure Liquid Chromatography-Ion Mobility-Mass Spectrometry System for Metabolic Profiling. Anal. Chem. 2017, 89, 6384–6391.
- Causon, T.J.; Kurulugama, R.T.; Hann, S. Drift-Tube Ion Mobility-Mass Spectrometry for Nontargeted Omics. Methods Mol. Biol. 2020, 2084, 79–94.
- Sinclair, E.; Hollywood, K.A.; Yan, C.; Blankley, R.; Breitling, R.; Barran, P. Mobilizing ion mobility mass spectrometry for metabolomics. Analyst 2018, 143, 4783–4788.
- Hernandez-Mesa, M.; Le Bizec, B.; Monteau, F.; Garcia-Campana, A.M.; Dervilly-Pinel, G.; Hernández-Mesa, M.; Le Bizec, B.; Monteau, F.; García-Campaña, A.M.; Dervilly-Pinel, G. Collision cross section (CCS) database: An additional measure to characterize steroids. Anal. Chem. 2018, 90, 4616–4625.
- Nye, L.C.; Williams, J.P.; Munjoma, N.C.; Letertre, M.P.M.; Coen, M.; Bouwmeester, R.; Martens, L.; Swann, J.R.; Nicholson, J.K.; Plumb, R.S.; et al. A comparison of collision cross section values obtained via travelling wave ion mobility-mass spectrometry and ultra high performance liquid chromatography-ion mobility-mass spectrometry: Application to the characterisation of metabolites in rat urine. J. Chromatogr. A 2019, 1602, 386–396.
- Mardal, M.; Dalsgaard, P.W.; Qi, B.; Mollerup, C.B.; Annaert, P.; Linnet, K. Metabolism of the synthetic cannabinoids AMB-CHMICA and 5C-AKB48 in pooled human hepatocytes and rat hepatocytes analyzed by UHPLC-(IMS)-HR-MSE. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1083, 189–197.
- Ross, D.H.; Seguin, R.P.; Xu, L. Characterization of the Impact of Drug Metabolism on the Gas-Phase Structures of Drugs Using Ion Mobility-Mass Spectrometry. Anal. Chem. 2019, 91, 14498–14507.
- Chalet, C.C.; Hollebrands, B.; Duchateau, G.S.; Augustijns, P. Intestinal phase-II metabolism of quercetin in HT29 cells, 3D human intestinal tissues and in healthy volunteers: A qualitative comparison using LC-IMS-MS and LC-HRMS. Xenobiotica 2019, 49, 945–952.
- Zuo, T.; Qian, Y.; Zhang, C.; Wei, Y.; Wang, X.; Wang, H.; Hu, Y.; Li, W.; Wu, X.; Yang, W. Data-dependent acquisition and database-driven efficient peak annotation for the comprehensive profiling and characterization of the multicomponents from compound xueshuantong capsule by UHPLC/IM-QTOF-MS. Molecules 2019, 24, 3431.
- Thomas, A.; Goergens, C.; Guddat, S.; Thieme, D.; Dellanna, F.; Schaenzer, W.; Thevis, M. Simplifying and expanding the screening for peptides <2 kDa by direct urine injection, liquid chromatography, and ion mobility mass spectrometry. J. Sep. Sci. 2016, 39, 333–341.
- Boschmans, J.; Lemiere, F.; Sobott, F. Analyzing complex mixtures of drug-like molecules: Ion mobility as an adjunct to existing liquid chromatography-(tandem) mass spectrometry methods. J. Chromatogr. A 2017, 1490, 80–88.
- Fresnais, M.; Muck, A.; Majewsky, M.; Statz, B.; Krausert, S.; Benzel, J.; Castel, D.; Le Dret, L.; Pfister, S.; Haefeli, W.E.; et al. Rapid and Sensitive Drug Quantification in Tissue Sections Using Matrix Assisted Laser Desorption Ionization-Ion Mobility-Mass Spectrometry Profiling. J. Am. Soc. Mass Spectrom. 2020, 31, 742–751.
- Chouinard, C.D.; Nagy, G.; Webb, I.K.; Garimella, S.V.B.B.; Baker, E.S.; Ibrahim, Y.M.; Smith, R.D. Rapid Ion Mobility Separations of Bile Acid Isomers Using Cyclodextrin Adducts and Structures for Lossless Ion Manipulations. Anal. Chem. 2018, 90, 11086–11091.
- Paglia, G.; Astarita, G. Traveling wave ion mobility mass spectrometry: Metabolomics applications. Methods Mol. Biol. 2019, 1978, 39–53.
- Poland, J.C.; Schrimpe-Rutledge, A.C.; Sherrod, S.D.; Flynn, C.R.; McLean, J.A. Utilizing Untargeted Ion Mobility-Mass Spectrometry To Profile Changes in the Gut Metabolome Following Biliary Diversion Surgery. Anal. Chem. 2019, 91, 14417–14423.
- Tebani, A.; Schmitz-Afonso, I.; Abily-Donval, L.; Heron, B.; Piraud, M.; Ausseil, J.J.; Brassier, A.; De Lonlay, P.; Zerimech, F.; Vaz, F.M.F.M.; et al. Urinary metabolic phenotyping of mucopolysaccharidosis type I combining untargeted and targeted strategies with data modeling. Clin. Chim. Acta 2017, 475, 7–14.
- Rainville, P.D.; Wilson, I.D.; Nicholson, J.K.; Issacs, G.; Mullin, L.; Langridge, J.I.; Plumb, R.S. Ion mobility spectrometry combined with ultra performance liquid chromatography/mass spectrometry for metabolic phenotyping of urine: Effects of column length, gradient duration and ion mobility spectrometry on metabolite detection. Anal. Chim. Acta 2017, 982, 1–8.
- Dodds, J.N.; Baker, E.S. Ion Mobility Spectrometry: Fundamental Concepts, Instrumentation, Applications, and the Road Ahead. J. Am. Soc. Mass Spectrom. 2019, 30, 2185–2195.
- Martinez-Lozano, P.; Rus, J. Separation of Isomers L-Alanine and Sarcosine in Urine by Electrospray Ionization and Tandem Differential Mobility Analysis-Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2010, 21, 1129–1132.
- Martinez-Lozano, P.; Criado, E.; Vidal, G.; Cristoni, S.; Franzoso, F.; Piatti, M.; Brambilla, P. Differential mobility analysis-mass spectrometry coupled to XCMS algorithm as a novel analytical platform for metabolic profiling. Metabolomics 2013, 9, 30–43.
- Purves, R.W. Enhancement of biological mass spectrometry by using separations based on changes in ion mobility (FAIMS and DMS). Anal. Bioanal. Chem. 2013, 405, 35–42.
- Fu, Y.; Xia, Y.-Q.; Flarakos, J.; Tse, F.L.S.; Miller, J.D.; Jones, E.B.; Li, W. Differential Mobility Spectrometry Coupled with Multiple Ion Monitoring in Regulated LC-MS/MS Bioanalysis of a Therapeutic Cyclic Peptide in Human Plasma. Anal. Chem. 2016, 88, 3655–3661.
- Arthur, K.L.; Turner, M.A.; Brailsford, A.D.; Kicman, A.T.; Cowan, D.A.; Reynolds, J.C.; Creaser, C.S. Rapid Analysis of Anabolic Steroid Metabolites in Urine by Combining Field Asymmetric Waveform Ion Mobility Spectrometry with Liquid Chromatography and Mass Spectrometry. Anal. Chem. 2017, 89, 7431–7437.
- Meng, X.; Xu, H.; Zhang, Z.; Fawcett, J.P.; Li, J.; Yang, Y.; Gu, J. Differential mobility spectrometry tandem mass spectrometry with multiple ion monitoring for the bioanalysis of liraglutide. Anal. Bioanal. Chem. 2017, 409, 4885–4891.
- Ren, T.; Li, R.; Meng, X.; Fawcett, J.P.; Sun, D.; Gu, J. Differential mobility spectrometry followed by tandem mass spectrometry with multiple ion monitoring for bioanalysis of eptifibatide in rat plasma. J. Pharm. Biomed. Anal. 2018, 151, 260–265.
- Bravo-Veyrat, S.; Hopfgartner, G. High-throughput liquid chromatography differential mobility spectrometry mass spectrometry for bioanalysis: Determination of reduced and oxidized form of glutathione in human blood. Anal. Bioanal. Chem. 2018, 410, 7153–7161.
- Dempsey, S.K.; Moeller, F.G.; Poklis, J.L. Rapid separation and quantitation of cocaine and its metabolites in human serum by differential mobility spectrometry-tandem mass spectrometry (DMS-MS-MS). J. Anal. Toxicol. 2018, 42, 518–524.
- Chen, P.-S.; Chen, S.-H.; Chen, J.-H.; Haung, W.-Y.; Liu, H.-T.; Kong, P.-H.; Yang, O.H.-Y. Modifier-assisted differential mobility-tandem mass spectrometry method for detection and quantification of amphetamine-type stimulants in urine. Anal. Chim. Acta 2016, 946, 1–8.
- Ruskic, D.; Hopfgartner, G. Modifier selectivity effect on differential ion mobility resolution of isomeric drugs and multidimensional liquid chromatography ion mobility Analysis. Anal. Chem. 2019, 91, 11670–11677.
- Zheng, X.; Cui, X.; Yu, H.; Jiang, J. Development of a quantitative method for four photocyanine isomers using differential ion mobility and tandem mass spectrometry and its application in a preliminary pharmacokinetics investigation. J. Chromatogr. A 2018, 1577, 109–119.
- Criado-Garcia, L.; Ruszkiewicz, D.M.; Eiceman, G.A.; Thomas, C.L.P. A rapid and non-invasive method to determine toxic levels of alcohols and γ-hydroxybutyric acid in saliva samples by gas chromatography-differential mobility spectrometry. J. Breath Res. 2016, 10, 017101.
- Berthias, F.; Wang, Y.; Alhajji, E.; Rieul, B.; Moussa, F.; Benoist, J.-F.; Maitre, P. Identification and quantification of amino acids and related compounds based on Differential Mobility Spectrometry. Analyst 2020, 145, 4889–4900.
- Szykula, K.M.; Meurs, J.; Turner, M.A.; Creaser, C.S.; Reynolds, J.C. Combined hydrophilic interaction liquid chromatography-scanning field asymmetric waveform ion mobility spectrometry-time-of-flight mass spectrometry for untargeted metabolomics. Anal. Bioanal. Chem. 2019, 411, 6309–6317.
- Wernisch, S.; Pennathur, S.; Pennathur, S. Application of differential mobility-mass spectrometry for untargeted human plasma metabolomic analysis. Anal. Bioanal. Chem. 2019, 411, 6297–6308.
- Ridgeway, M.E.; Lubeck, M.; Jordens, J.; Mann, M.; Park, M.A. Trapped ion mobility spectrometry: A short review. Int. J. Mass Spectrom. 2018, 425, 22–35.
- Adams, K.J.; Ramirez, C.E.; Smith, N.F.; Munoz-Munoz, A.C.; Andrade, L.; Fernandez-Lima, F. Analysis of isomeric opioids in urine using LC-TIMS-TOF MS. Talanta 2018, 183, 177–183.
- Adams, K.J.; Smith, N.F.; Ramirez, C.E.; Fernandez-Lima, F. Discovery and targeted monitoring of polychlorinated biphenyl metabolites in blood plasma using LC-TIMS-TOF MS. Int. J. Mass Spectrom. 2018, 427, 133–140.
- Neumann, E.K.; Migas, L.G.; Allen, J.L.; Caprioli, R.M.; Van de Plas, R.; Spraggins, J.M. Spatial Metabolomics of the Human Kidney using MALDI Trapped Ion Mobility Imaging Mass Spectrometry. Anal. Chem. 2020, 92, 13084–13091.
- Cornett, D.S.; Barsch, A.; Henkel, C.; Witt, M.; Szesny, M. Establishing a spatial metabolomics workflow that integrates MALDI imaging with new trapped ion mobility metabolomics for more comprehensive identification and validation. In Proceedings of the Abstracts of Papers, 258th ACS National Meeting & Exposition, San Diego, CA, USA, 25–29 August 2019; p. AGRO-0143.




