
Video Upload Options
Carbon nanotubes (CNTs) are seamless nanotubes made of single or multiple layers of graphene sheets rolled around a central axis with the advantages of being lightweight and having a perfect hexagonal connection structure.
1. Introduction
Chemical and biological sensors have attracted great attention recently, and have a wide range of applications in healthcare, environmental monitoring, food quality, and defense. These sensors can respond to specific chemical or biological compounds and convert this information into electrical signals. Many materials have been studied as the sensitive materials in the chemical/biological sensors, such as SnO2 [1][2], ZnO2 [3], Ag [4], and graphene [5]. Generally speaking, the ideal material in chemical and biological sensors should have a high chemical reactivity, a large surface to volume ratio or an easy fabrication at low cost.
Carbon nanotubes (CNTs) are seamless nanotubes made of single or multiple layers of graphene sheets rolled around a central axis with the advantages of being lightweight and having a perfect hexagonal connection structure. The unique electronic transport properties of CNTs make them potentially useful in nanodevices [6][7]. For example, CNTs are atomically thin in order to provide ideal electrostatic control over the channel, which is quite important when the device is scaled down. This unique atomically thin structure of CNTs also gives them many advantages when serving as the sensitive materials in sensors, and the electrical performance superiority of CNT-based field effect transistors (CNTFETs) has been extended in various chemical and biological sensors. Compared with other detecting technologies, CNTFET-based sensors have the advantages of high sensitivity, high selectivity, simple operation, low operating temperature, fast response speed, short recovery time, label-free detection, and good stability. Referring to the detection capability of various substances and the exceptional performance, CNTFETs are expected to play an increasing role in the field of sensing.
2. Surface Functionalization of CNTs for Sensing
2.1. Covaelent Modification
Covalent modification mainly involves chemical destruction of C-C bonds of the CNTs ports or sidewalls to generate more polar carboxyl groups or hydroxyl groups on the surfaces. Then, various functional groups can be introduced on the CNT surfaces to further attach the derivative reaction of the target product to the CNTs—for example, chemical groups, fluorescently labeled molecules, DNA, anticancer drugs, etc. The oxidants used for covalently modification include nitric acid, mixed acid (concentrated sulfuric acid/nitric acid), neutral hydrogen peroxide, and sodium hydroxide, as shown in Figure 1. The disadvantage of covalent modification is that it may destroy the integrity of CNTs, which will affect its mechanical and electrical properties to some extent.
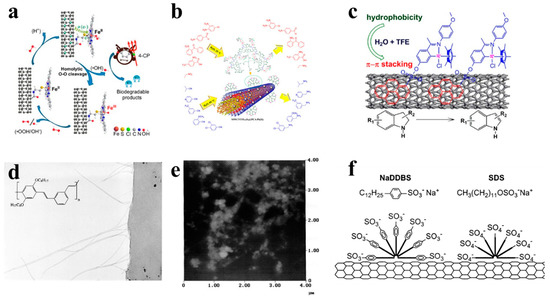
Figure 1. Surface functionalization of carbon nanotubes (CNTs). (a) The introduction of the -COOH group to the surface of the CNT [8]. (b) The introduction of-COOH on the surface of CNTs by the oxidation reaction [9]. (c) A metal iridium complex catalyst was coated on the surface of CNTs through non-covalent bond accumulation [10]. (d) TEM image which shows the nominal chemical structure of the polymer backbone [11]. (e) The atomic force microscopy image of the waxy corn amylopectin-single-walled CNT (SWCNT) film [12]. (f) Schematic representation of surfactants adsorb onto the CNT surfaces [13].
As shown in Figure 1a, Ni et al. [8] introduced the -COOH derivative group on the surface of the CNT by the oxidation reaction, and two different ligands are introduced on the CNT surface, including aminopyridine and aminoethyl mercaptan curing catalyst iron phthalocyanine (VE-H). As shown in Figure 1b, Rezaie et al. [9] also introduced -COOH to the surface of CNTs by oxidation reaction, then formed a dendrimer with poly(citric acid), and then attached a divalent platinum metal catalyst to the surface of CNTs. Finally, a magnetic catalyst was obtained. The catalyst can selectively reduce nitro and nitrile.
2.2. Non-Covalent Modification
Non-covalent modification means that covalent chemical bonds are not introduced for modification on the surface of CNTs, but are achieved through non-covalent bonding, including physical adsorption and surface coating. The non-covalent modification is mainly formed by the hybridization of carbon atoms sp2 in the graphene structure of the sidewall to form highly delocalized electrons and electrons of other compounds to generate non-covalent bonds. The non-covalent interactions include dispersion forces, hydrogen bonds, dipole-dipole forces, π-π stacking effects, and hydrophobic effects. Carbon atoms in CNTs are all SP2 hybrids, forming highly delocalized electrons, which can be modified with other π electron-rich compounds through π-π stacking. The non-covalently modified CNTs are structurally complete and can retain their original properties. The molecules for the non-covalent modification mainly include the surfactants, the molecules containing aromatic groups, and the polymers. For biological sensors, the non-covalent modification of the CNTs can not only improve their water solubility in biological systems, but also can avoid the non-specific adsorption of the biomolecules.
As shown in Figure 1c, Liu et al. [10] coated the metal iridium complex catalyst on the surface of CNTs through non-covalent bond accumulation, and the coating efficiency reached over 94%. The metal iridium complex catalyst is coated on CNT to make the catalytic dehydrogenation reaction of indole from organics such as methanol, ethanol, tetrahydrofuran, trifluoroethanol, etc. The transfer of solvents to water makes this organic reaction more environmentally friendly. The surfactant contains two parts, which are the lipophilic end and the hydrophilic end. When they are adsorbed on the surface by the CNTs, the charge repulsion disperses them. Under thermodynamics, water-soluble polymers such as sodium polystyrene sulfonate entangle CNTs, thereby exerting the role of surfactants and making them amphiphilic [14]. As shown in Figure 1d, Dalton et al. [11] used conjugated poly-phenylene vinylene to surface-coat the CNTs, and found that the CNTs were evenly dispersed in the polymer matrix. As shown in Figure 1e, Stobinski et al. [12] modified CNTs with sodium lauryl sulfate and dispersed them by ultrasound. It was found that increasing the ultrasonic time or decreasing the concentration of the suspension can obtain CNT solutions with better dispersion properties. As shown in Figure 1f, coating CNTs with sodium benzoate and sodium dodecyl benzenesulfonate can also increase their water solubility [13].
3. Configuration and Sensing Mechanism
3.1. General CNTFETs
CNTFETs have a variety of structures [15], but share similar characteristics: the conductive channel, source and drain electrodes, a gate electrode on the top or bottom of the channel, and a dielectric layer between the channel and the gate to separate the gate electrode from the CNTs. The operating principles of these CNTFETs are similar: the gate electrode uses a vertical electric field to control the amount of charge in the channel; the horizontal electric field between the source and drain electrodes provides driving force, and a current is made to flow from one electrode through the CNT to the other electrode [16].
Figure 2a shows a typical configuration of a CNTFET for sensing purpose. In general, the transport of carriers in a CNTFET can be attributed to four states, which are independent of the device structure [17]. The classification of these states depends on the comparison of the CNT length and the mean free path length of the CNT, and the type of contact between the CNT and the source and drain [18]. For example, an ohmic contact ballistic CNTFET means that carriers are injected into the CNTs from the source and the drain through an ohmic contact, and the carrier transport process in the CNT is not subjected to any scattering. In contrast, Schottky-type diffused CNTFETs mean that the carrier injection is affected by a Schottky barrier derived from the heterojunction of the electrode and the CNT, and the carriers are constantly scattered during transmission in the conductive channel. There are two kinds of carriers: holes and electrons. If the type of carriers is mainly electrons, then the FET is an n-type transistor; on the other hand, if the carriers are mainly holes, then the FET is a p-type transistor. In theory, the type of metal-CNT contact depends on the difference in work function between the metal electrode and the CNTs. However, due to the physical and chemical properties of the electrodes in contact with CNTs [19], p-type CNTFETs are more common.
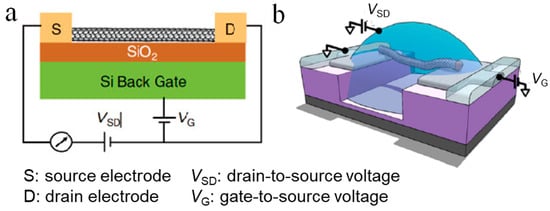
Figure 2. Illustration of CNT-based field-effect transistors (FETs) for sensing. (a) Schematic representation of a general CNTFET [17]. (b) Schematic diagram of an electrolyte-gated CNTFET [20].
CNTs have the characteristics of nanometer size, huge specific surface area, and surface effects. When a specific molecule is adsorbed on the surface of a CNTs, it causes the energy band of the CNTs to bend and affect its electronic structure, which would further cause the change of transport characteristics of CNTs. The change provides the possibility of CNTs to work as sensitive materials.
The FET-based chemical sensor and biosensor use the basic characteristics of the transistor to convert difficult-to-detect high-resistance changes into easily-detectable changes in current. The sensitivity of the sensor can be adjusted by appropriately selecting the gate operating voltage of the device. The single-walled CNTs (SWCNTs) can be divided into metal type and semiconductor type, and the FET-based chemical sensor and biosensor are prepared by using the resistance response characteristic of semiconductor SWCNTs to the adsorbed chemical.
When the chemical molecules or biological material are adsorbed on the surface of semiconductor-type CNTs, electron transfer occurs, which changes their electrical conductivity, which provides a theoretical basis for CNTs as good sensor materials. The multi-walled CNTs (MWCNTs) have a multi-layered tubular structure, so the MWCNTs have a more complex chemical adsorption mechanism than the SWCNTs. In addition, the MWCNTs lack carbon band gaps or have narrow band gaps; the tube is mainly metallic so that the adsorption of the chemical molecules has little effect on the electrical conductivity. Therefore, the conductivity of the MWCNTs is not as sensitive as that of SWCNTs, but it has been shown that the MWCNTs still have excellent sensing characteristics to substances such as water vapor [21], NH3 [22][23], NO2 [18], and O2 [24][25].
The SWCNTs have shown great advantages in FET-based nanosensors. Paolo et al. [26] reported the application of CNTFETs in gas sensors. Whether for a single CNT FET or a CNT-thin film FET, the main sensing mechanism is derived from gas adsorption on the carbon tubes/metals—the modulation of the Schottky barrier at the electrodes.
3.2. Electrolyte-Gated CNTFETs
The biosensors based on electrolyte-gated FETs, also known as liquid gate FETs, have attracted increasing attention due to their advantages of easy processing [27], low cost [28], good flexibility [29], good biocompatibility, and low operating voltage. Figure 2b shows a typical configuration of a electrolyte-gated CNTFET [20], in which the electrolyte is used instead of the dielectric layer material to directly contact the gate electrode and the channel. The biggest difference between the working principle of the electrolyte-gated FET and the conventional FET is that the gate electrode regulates the channel current through the electrolyte solution. The biggest advantage of electrolyte-gated FETs is the huge two-electron layer effect of the electrolyte [30][31], which enables the sensor to obtain the same current with a smaller gate voltage and usually can work at a rather low voltage (1V). This can avoid undesired electrochemical reactions, such as decomposition of water, damage to biological activity, etc.; thus, it can be used to detect important biological samples in a solution environment [32][33].
According to whether the ions in the electrolyte can penetrate the semiconductor channel layer, the electrolyte-gated FET can be divided into an electrostatically coupled FET and an electrochemically doped FET. Taken the p-type electrolyte-gated FET as an example, we explain these two different working mechanisms as follows. (1) Electrostatically coupled FETs. Under negative gate bias, cations in the electrolyte migrate to the gate/electrolyte interface, while the anions move to the electrolyte/channel interface, and an electric double layer is formed at the respective interface. The capacitance of the entire sensor can be equivalent to two electric double-layer capacitors connected in series. Usually, the capacitance at the electrolyte/channel interface is small, so this capacitance determines the total capacitance. Under negative bias, anions accumulated at the electrolyte/channel interface in the electrolyte induce holes of equal charge amount in the p-type channel. Under the action of the source-drain voltage, holes move in the channel to form a source-drain current. In this process, the anions in the electrolyte are always at the electrolyte/channel interface and do not penetrate the semiconductor channel layer. The holes in the channel are completely generated by the electrostatic coupling of the electric double-layer capacitance. (2) Electrochemically doped FETs. Under negative bias, cations in the electrolyte migrate to the gate/electrolyte interface, while anions migrate to the electrolyte/channel and penetrate the interface into the semiconductor channel layer. The entering anions would cancel or compensate some of the holes. This process is called electrochemical doping and occurs mostly at the electrolyte–polymer semiconductor interface. Both these two methods are often used in CNTFETs.
According to different analytes, the application of electrolyte-gated CNTFETs in chemical and biological sensors mainly includes ion sensors, small molecule sensors, protein sensors, DNA sensors, bacterial sensors, cell sensors, etc. Because the electrolyte-gated FET sensor works in a solution environment, it is particularly important to investigate the interaction between ions and CNTs. According to the interaction between ions and CNTs, ion redox, chloride ion detection, nucleic acid aptamers, and ion-selective membranes can be used for electrolyte-gated CNTFET for ion detection. For example, electrolyte-gated FET with single-walled CNTs could be used to detect redox ions [34]. It was found that ions with redox ability affect the conductivity of CNT channels mainly by adjusting the electrochemical potential of the solution. Boussaad et al. [35] prepared electrolyte-gated CNTFET sensors and studied their response to K3Fe(CN)6/K4Fe(CN)6, K2IrCl6/K3IrCl6. It was found that redox ions can not only regulate the electrochemical potential of the solution, but also directly conduct a redox reaction with the CNT, thereby regulating the conductivity of the CNT channel.
References
- Khamfoo, K.; Inyawilert, K.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Formaldehyde sensor based on FSP-made AgOx-doped SnO2 nanoparticulate sensing films. Sensor. Actuat. B Chem. 2020, 309, 127705.
- Nam, B.; Ko, T.-K.; Hyun, S.-K.; Lee, C. CO Sensing Properties of Chemiresistive In2O3/SnO2 Composite Nanoparticle Sensors. J. Nanosci. Nanotechno. 2020, 20, 4344–4348.
- Sebok, D.; Dekany, I. ZnO2 nanohybrid thin film sensor for the detection of ethanol vapour at room temperature using reflectometric interference spectroscopy. Sensor. Actuat. B Chem. 2015, 206, 435–442.
- Baccarin, M.; Ciciliati, M.A.; Oliveira, O.N., Jr.; Cavalheiro, E.T.; Raymundo-Pereira, P.A. Pen sensor made with silver nanoparticles decorating graphite-polyurethane electrodes to detect bisphenol-A in tap and river water samples. Mater. Sci. Eng. C 2020, 114, 110989.
- Raymundo-Pereira, P.A.; Baccarin, M.; Oliveira, O.N., Jr.; Janegitz, B.C. Thin films and composites based on graphene for electrochemical detection of biologically-relevant molecules. Electroanalysis 2018, 30, 1888–1896.
- Cui, J.; Zhang, J.; He, X.; Mei, X.; Wang, W.; Yang, X.; Xie, H.; Yang, L.; Wang, Y. Investigating interfacial contact configuration and behavior of single-walled carbon nanotube-based nanodevice with atomistic simulations. J. Nanopart. Res. 2017, 19, 110.
- Yang, W.D.; Li, Y.D.; Wang, X. Scale-dependent dynamic-pull-in of functionally graded carbon nanotubes reinforced nanodevice with piezoelectric layer. J. Aerospace Eng. 2017, 30, 04016096.
- Ni, D.; Zhang, J.; Wang, X.; Qin, D.; Li, N.; Lu, W.; Chen, W. Hydroxyl radical-dominated catalytic oxidation in neutral condition by axially coordinated iron phthalocyanine on mercapto-functionalized carbon nanotubes. Indus. Eng. Chem. R. 2017, 56, 2899–2907.
- Rezaei, S.J.T.; Khorramabadi, H.; Hesami, A.; Ramazani, A.; Amani, R.; Ahmadi, R. Chemoselective reduction of nitro and nitrile compounds with magnetic carbon nanotubes-supported Pt(II) catalyst under mild conditions. Ind. Eng. Chem. Res. 2017, 56, 12256–12266.
- Liu, H.; Chen, J.-G.; Wang, C.; Liu, Z.-T.; Li, Y.; Liu, Z.-W.; Xiao, J.; Lu, J. Immobilization of cyclometalated iridium complex onto multiwalled carbon nanotubes for dehydrogenation of indolines in aqueous solution. Ind. Eng. Chem. Res. 2017, 56, 11413–11421.
- Dalton, A.B.; Stephan, C.; Coleman, J.N.; McCarthy, B.; Ajayan, P.M.; Lefrant, S.; Bernier, P.; Blau, W.J.; Byrne, H.J. Selective interaction of a semiconjugated organic polymer with single-wall nanotubes. J. Phys. Chem. B 2000, 104, 10012–10016.
- Stobinski, L.; Tomasik, P.; Lii, C.Y.; Chan, H.H.; Lin, H.M.; Liu, H.L.; Kao, C.T.; Lu, K.S. Single-walled carbon nanotube—Amylopectin complexes. Carbohyd. Polym. 2003, 51, 311–316.
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett. 2003, 3, 269–273.
- Cui, D.; Ozkan, C.S.; Ravindran, S.; Kong, Y.; Gao, H. Encapsulation of pt-labelled DNA molecules inside carbon nanotubes. Mech. Chem. Biosyst. MCB 2004, 1, 113–121.
- Kim, H.; Seo, J.; Seong, N.; Lee, S.; Lee, S.; Kim, T.; Hong, Y. Multidipping technique for fabrication time reduction and performance improvement of solution-processed single-walled carbon nanotube thin-film transistors. Adv. Eng. Mater. 2020, 22, 1901413.
- Chen, J.; Zhang, B.; Dang, X.; Zheng, D.; Ai, Y.; Chen, H. A nanocomposite consisting of etched multiwalled carbon nanotubes, amino-modified metal-organic framework UiO-66 and polyaniline for preconcentration of polycyclic aromatic hydrocarbons prior to their determination by HPLC. Mikrochim. Acta 2020, 187, 78.
- Moghaddam, S.; Ghoreishi, S.S.; Yousefi, R.; Aderang, H. Quantum simulation of a junctionless carbon nanotube field-effect transistor under torsional strain. Superlattice. Microst. 2020, 138, 106239.
- Sacco, L.; Forel, S.; Florea, I.; Cojocaru, C.-S. Ultra-sensitive NO2 gas sensors based on single-wall carbon nanotube field effect transistors: Monitoring from ppm to ppb level. Carbon 2020, 157, 631–639.
- Choi, Y.; Kim, J.-H.; Qian, C.; Kang, J.; Hersam, M.C.; Park, J.-H.; Cho, J.H. Gate-tunable synaptic dynamics of ferroelectric-coupled carbon-nanotube transistors. ACS Appl. Mater. Inter. 2020, 12, 4707–4714.
- Sharf, T.; Wang, N.-P.; Kevek, J.W.; Brown, M.A.; Wilson, H.; Heinze, S.; Minot, E.D. Single electron charge sensitivity of liquid-gated carbon nanotube transistors. Nano Lett. 2014, 14, 4925–4930.
- Lapointe, F.; Ding, J.; Lefebvre, J. Carbon Nanotube Transistors as Gas Sensors: Response differentiation using polymer gate dielectrics. ACS Appl. Poly. Mater. 2019, 1, 3269–3278.
- Eberle, S.; Roman, C.; Hierold, C. Effect of varying gate distance on the threshold voltage shift in carbon nanotube field effect transistor gas sensors. Microelectron. Eng. 2018, 193, 86–90.
- Jeon, M.; Choi, B.; Yoon, J.; Kim, D.M.; Kim, D.H.; Park, I.; Choi, S.-J. Enhanced sensing of gas molecules by a 99.9% semiconducting carbon nanotube-based field-effect transistor sensor. Appl. Phys. Lett. 2017, 111, 022102.
- Sivasathya, S.; Thiruvadigal, D.J. Ab Initio study of carbon nanotube transistor-based gas sensor for NO2 detection. Asian J. Chem. 2013, 25, S411–S413.
- Wei, L.; Chen, H.; Wang, J.; Yuang, W.; Zhao, J.; Xu, D.; Zhang, Y. Gas Sensors based on single-walled carbon nanotube field-effect transistor. Sensor. Mater. 2014, 26, 9–17.
- Bondavalli, P.; Legagneux, P.; Pribat, D. Carbon nanotubes based transistors as gas sensors: State of the art and critical review. Sensor. Actuat. B Chem. 2009, 140, 304–318.
- Delgado, K.P.; Raymundo-Pereira, P.A.; Campos, A.M.; Oloveira, O.N., Jr.; Janegitz, R.C. Ultralow cost electrochemical sensor made of potatoStarch and carbon black nanoballs to detect tetracyclinein waters and milk. Electroanalysis 2018, 30, 2153–2159.
- Raymundo-Pereira, P.A.; Shimizu, F.M.; Coelho, D.; Piazzeta, M.H.; Gobbi, A.L.; Machado, S.A.; Oliveira, O.N., Jr. A nanostructured bifunctional platform for sensing of glucose biomarker in artificial saliva: Synergy in hybrid Pt/Au surfaces. Biosens. Bioelectr. 2016, 86, 369–376.
- Scuratti, F.; Bonacchini, G.E.; Bossio, C.; Salazar-Rios, J.M.; Talsma, W.; Loi, M.A.; Antognazza, M.R.; Caironi, M. Real-time monitoring of cellular cultures with electrolyte-gated carbon nanotube transistors. ACS Appl. Mater. Interfaces 2019, 11, 37966–37972.
- Dorfman, K.D.; Adrahtas, D.Z.; Thomas, M.S.; Frisbie, C.D. Microfluidic opportunities in printed electrolyte-gated transistor biosensors. Biomicrofluidics 2020, 14, 011301.
- Tin Phan, N.; Hayakawa, R.; Kilinc, V.; Petit, M.; Yemineni, S.L.V.N.; Higuchi, M.; Raimundo, J.M.; Charrier, A.M.; Wakayama, Y. Electrolyte-gated-organic field effect transistors functionalized by lipid monolayers with tunable pH sensitivity for sensor applications. Appl. Phys. Expre. 2020, 13, 011005.
- Jo, I.Y.; Park, J.-G.; Moon, J.-H.; Jung, J.Y.; Kim, D.E.; Baeg, K.-J. Low-voltage-operating complementary-like circuits using ambipolar organic-inorganic hybrid thin-film transistors with solid-state-electrolyte gate insulator. Org. Electron. 2019, 75, 105358.
- Neuper, F.; Chandresh, A.; Singaraju, S.A.; Aghassi-Hagmann, J.; Hahn, H.; Breitung, B. Tailoring threshold voltages of printed electrolyte-gated field-effect transistors by chromium doping of indium oxide channels. ACS Omega 2019, 4, 20579–20585.
- Larrimore, L.; Nad, S.; Zhou, X.; Abruna, H.; McEuen, P.L. Probing electrostatic potentials in solution with carbon nanotube transistors. Nano Lett. 2006, 6, 1329–1333.
- Boussaad, S.; Diner, B.A.; Fan, J. Influence of redox molecules on the electronic conductance of single-walled carbon nanotube field-effect transistors: Application to chemical and biological sensing. J. Am. Chem. Soc. 2008, 130, 3780–3787.




