
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sara Spelta | + 1671 word(s) | 1671 | 2021-03-16 10:55:51 | | | |
| 2 | Dean Liu | -11 word(s) | 1660 | 2021-03-29 05:21:57 | | |
Video Upload Options
AK is relatively rare among corneal infections, with an estimated prevalence of 1–9 cases per 100,000. However, in Western countries, the incidence of AK has been steadily rising in direct correlation with contact lens wearing, which is the predominant risk factor.
1. Introduction
In humans, Acanthamoeba spp. are responsible for a painful and sight-threatening disease that has a significant negative impact on patient quality of life. The diagnosis of Acanthamoeba keratitis (AK) is often delayed and patients frequently experience protracted treatment courses that can require surgical intervention in an attempt to restore corneal integrity and health [1][2][3][4].
AK is relatively rare among corneal infections, with an estimated prevalence of 1–9 cases per 100,000. However, in Western countries, the incidence of AK has been steadily rising in direct correlation with contact lens wearing, which is the predominant risk factor [3][5][6][7][8]. Approximately 93% of all cases of AK are reported to occur in contact lens wearers [9][10][11][12]. Poor contact lens hygiene, such as overnight wear or wearing lenses during swimming and showering, is a known risk factor for AK [4][6][12][13][14]; there is also an increased risk amongst monthly disposable contact lens wearers [6]. In addition, orthokeratology is considered a major risk factor for AK, with an annual incidence of 7.7 cases per 10,000 [8].
Acanthamoeba spp. is a free-living, ubiquitous protozoan that is commonly found in freshwater and soil [7][12][15]. It exists in two forms: motile, which replicate trophozoites, and dormant cysts, which have minimal metabolic activity and are much more resistant to adverse conditions, such as extremes in temperature, dryness, and pH, as well as antiamoebic drugs [7][16][17][18].
2. Treatment of AK
The treatment course for AK is often long and challenging, and while the trophozoite form of Acanthamoeba spp. is susceptible to multiple therapies, the cystic form is highly drug resistant and may persist for months [8]. The principal initial treatment is the administration of a topical biguanide, such as polyhexamethylene biguanide (PHMB) 0.02–0.08% [19] or chlorhexidine 0.02–0.06% [20], along with or without a topical diamidine, such as propamidine isethionate 0.1% [2][3][4][6][7][8][11][12][21][22][23]. Initially, these medications should be administered every hour around the clock for the first 48–72 h and then tapered gradually. The maintenance therapy of PHMB and propamidine, each 3–4 times per day, is then continued for 4–6 weeks. Both biguanides and diamidines can be toxic to the cornea, often causing corneal epitheliopathy. In cases of toxicity, a decrease in the dosage or allowing for a medication holiday may be required.
The fact that Acanthamoeba spp. has two forms, namely, trophozoites and cysts, has implications for the management strategy in AK. An overarching theme to AK management is that the initial empiric treatment must be aggressive, as trophozoites and immature cysts are significantly more responsive to treatment than mature cysts [11][23]. Topical PHMB and chlorhexidine are effective medications against the cyst form of Acanthamoeba spp. [3][11], while propamidine has cystostatic but not cystocidal activity, and thus cannot be used as monotherapy [11][24]. Chlorhexidine 0.02% used as both monotherapy and combination therapy also demonstrated therapeutic efficacy against Acanthamoeba spp. without adverse effects [20]. Moreover, low concentrations of benzalkonium chloride (BAK) and povidone iodine seem to exhibit significant antiacanthamoebal activity in vitro [25][26]. In some studies, monotherapy with 0.02% PHMB for the initial AK treatment has been shown to be as effective as combination therapies, including a biguanide plus a diamidine. This therapeutic approach has shown promising cure rates and is an attractive option, as the use of a single medication can improve patient compliance and lower costs as compared to combined therapy [27].
Several other classes of medications have shown promise in the treatment of AK. Systemic antifungal drugs, such as voriconazole and posaconazole, may be useful against the cyst form of Acanthamoeba spp., as they inhibit the synthesis of ergosterol, one component of the Acanthamoeba spp. cell membrane, making it a potential cystostatic treatment option [18][23]. Miltefosine is an oral medication that is used to treat leishmaniasis and amoebas and has been used in the management of AK. Although at this time it can be expensive and/or difficult to obtain, it has demonstrated efficacy in the treatment of AK [3][12][28]. Other medications, such as topical tea tree oil [1] and neomycin, may also hold promise as accompanying therapies, although prolonged treatment with the latter is not recommended because of its adverse effects on the corneal epithelium, including toxicity and hypersensitivity reactions [2][21]. One important adjunct to topical medications is epithelial debridement, which in addition to providing a tissue sample for culture, can also physically remove trophozoites and cysts limited to the corneal epithelium and enhance topical drug penetration. In cases of intraepithelial infection, epithelium debridement combined with three to four months of antiamoebic treatment may be enough for the successful resolution of disease [2][11][12].
The use of steroids in the treatment of AK remains controversial and should, in general, be used with extreme caution. Steroids can increase the total length of treatment and are known to increase Acanthamoeba spp. pathogenicity by promoting the transition of cysts to trophozoites and by increasing trophozoite proliferation [7][8][23]. Nonetheless, steroids are sometimes used in cases of AK when there are intense inflammation and pain out of proportion to what is found in the exam, such as when there is concomitant scleritis and in the presence of deep corneal neovascularization [2][3][4][6][7][8][11][12][21][22][23]. In cases of limbitis and scleritis, steroids have been used to reduce persistent inflammation of the anterior segment and sclera, along with systemic non-steroidal anti-inflammatory drugs (NSAIDs), in order to transition to more long-term immunomodulatory therapy [2][8][11][29]. Early in the course of AK, there is generally no role for steroids, but in cases of prolonged treatment courses with severe corneal inflammation and an inadequate response to topical antiamoebic therapy, topical corticosteroids have been reported to help with the resolution of the disease [3][7][11][23]. AK infection is considered to be eradicated when there is a demonstration of clinical stability after a 2-week suspension of antiamoebic therapy (free period, Figure 3) [11]. Interestingly, anecdotal reports have described the use of topical low-frequency steroids following the suspension of antiamoebic therapy to manage corneal sequelae and also unmask residual amoebic cysts. When used, steroids should always be used with simultaneous antiamoebic coverage.
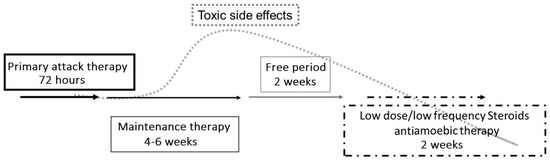
Figure 3. Proposed therapeutic strategy for Acanthamoeba keratitis. An initial aggressive approach to treatment involves hourly topical eye drops (polyhexamethylene biguanide (PHMB) 0.02% and propamidine isethionate 0.1%), followed by tapering to maintenance therapy using PHMB and propamidine 3–4 times per day for 6 weeks. A stable clinical exam after a 2-week antiamoebic free period reduces the risk of medication toxicity and can also unmask the continued presence of trophozoites or cysts. If the infection is still present, the treatment protocol must be repeated. Topical low-dose and low-frequency steroid eye drops (such as loteprednol etabonate and fluorometholone acetate) have been suggested in cases of severe ocular pain, limbitis, or scleritis, and must be used with extreme caution. Topical steroids should only be used with concomitant antiamoebic therapy.
In cases of AK that are poorly responsive to medical treatment, surgical interventions, including deep anterior lamellar keratoplasty (DALK) or penetrating keratoplasty (PK), may be required. DALK performed within 30–60 days of the onset of symptoms [30] has been shown to be beneficial in eradicating infection in conjunction with antiamoebic treatment before, during, and after surgery, and can yield a statistically significant improvement in final postoperative best-corrected visual acuity (BCVA) compared to preoperative BCVA (average postoperative Snellen visual acuity of approximately 20/25) [31]. While DALK presents less risk of rejection and graft failure when compared with PK, it is relatively technically more difficult and it can be less effective than PK in eradicating the infection, particularly when performed in inflamed eyes or late in the disease course [31].
Full-thickness PK is used to prevent scleral extension and is the most useful and definitive surgical treatment in cases of severe and progressive AK that are unresponsive to medical therapy [15][31]. PK is also indicated in cases of corneal perforation and fulminant corneal abscesses [3][11][23]. However, PK must be performed judiciously as transplants in eyes with severe AK tend to have a poorer prognosis [11]. Grafts must be large enough to remove all affected tissue and minimize the chances of recurrence, but because larger grafts have a higher risk of rejection and failure, surgeons must balance these two factors to appropriately size the corneal transplant. Recurrence occurs most commonly in the first two weeks after surgery, but late recurrences, taking place several months after surgery, also occur [11]. In order to minimize disease recurrence, a good goal to aim for is a 1 mm margin of healthy tissue [24]. In addition, it is recommended to continue antiamoebic treatment for 2–4 weeks following surgery [3][11]. Of note, optical keratoplasties (PK or DALK) that are performed for corneal scarring and irregular astigmatism have better outcomes than therapeutic keratoplasties [27]; therefore, a goal of AK management, whenever possible, should be to utilize medical therapies and delay surgical interventions until Acanthamoeba spp. eradication can be achieved. Then, following the resolution of inflammation, perform an optical DALK or PK.
In addition to DALK and PK, amniotic membrane transplantation is an additional procedure that can be used to facilitate complete corneal recovery. In cases of progressive stromal lesions and persistent epithelial defects, amniotic membrane transplantation may be effective in controlling inflammation and promoting stromal and epithelial healing. However, complete stromal and epithelial recovery may require multiple amniotic membrane transplantations and a PK may still be needed [3][4][8][15].
A recent meta-analysis studied the role of photoactivated chromophores for keratitis-corneal cross-linking (PACK-CXL), in addition to standard antimicrobial treatment (SAT), as a therapy for infectious keratitis versus SAT alone [32]. The results showed that in bacterial or fungal keratitis, PACK-CXL may be a useful adjunct therapy for reducing the time to complete corneal healing, but PACK-CXL was not useful compared to SAT alone in reducing the infiltrate size, improving visual acuity, or reducing the risk of adverse effects, such as the worsening of infectious keratitis, corneal melt, or perforations [32]. The microbicidal effect of PACK-CXL likely arises from ultraviolet A (UVA) induced DNA damage and reactive oxygen species release [15][33][34], while pain reduction may be secondary to the suppression of inflammation and nociception by subepithelial nerves [33]. PACK-CXL has been used along with standard antimicrobial therapy to treat persistent cases of Acanthamoeba spp. infection [33]; however, there is currently insufficient evidence to support its use in the setting of AK [32][35][36].
References
- Hadaś, E.; Derda, M.; Cholewiński, M. Evaluation of the effectiveness of tea tree oil in treatment of Acanthamoeba infection. Parasitol. Res. 2017, 116, 997–1001.
- Lindquist, T.D. Treatment of Acanthamoeba keratitis. Cornea 1998, 17, 11–16.
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10.
- Maycock, N.J.R.; Jayaswal, R. Update on Acanthamoeba Keratitis. Cornea 2016, 35, 713–720.
- Ross, J.; Roy, S.L.; Mathers, W.D.; Ritterband, D.C.; Yoder, J.S.; Ayers, T.; Shah, R.D.; Samper, M.E.; Shih, C.Y.; Schmitz, A.; et al. Clinical Characteristics of Acanthamoeba Keratitis Infections in 28 States, 2008 to 2011. Cornea 2014, 33, 161–168.
- McKelvie, J.; Alshiakhi, M.; Ziaei, M.; Patel, D.V.; McGhee, C.N. The rising tide of Acanthamoeba keratitis in Auckland, New Zealand: A 7-year review of presentation, diagnosis and outcomes (2009–2016). Clin. Exp. Ophthalmol. 2018, 46, 600–607.
- Alkharashi, M.; Lindsley, K.; Law, H.A.; Sikder, S. Medical interventions for acanthamoeba keratitis. Cochrane Database Syst. Rev. 2015, 2, CD010792.
- Hammersmith, K.M. Diagnosis and management of Acanthamoeba keratitis. Curr. Opin. Ophthalmol. 2006, 17, 327–331.
- Radford, C.F.; Lehmann, O.J.; Dart, J.K.G. Acanthamoeba keratitis: Multicentre survey in England 1992-6. Br. J. Ophthalmol. 1998, 82, 1387–1392.
- Carnt, N.; Robaei, D.; Minassian, D.C.; Dart, J.K.G. Acanthamoeba keratitis in 194 patients: Risk factors for bad outcomes and severe inflammatory complications. Br. J. Ophthalmol. 2018, 102, 1431–1435.
- Dart, J.K.; Saw, V.P.; Kilvington, S. Acanthamoeba Keratitis: Diagnosis and Treatment Update 2009. Am. J. Ophthalmol. 2009, 148, 487–499.
- Padhi, T.R.; Das, S.; Sharma, S.; Rath, S.; Rath, S.; Tripathy, D.; Panda, K.G.; Basu, S.; Besirli, C.G. Ocular parasitoses: A comprehensive review. Surv. Ophthalmol. 2017, 62, 161–189.
- Shimmura-Tomita, M.; Takano, H.; Kinoshita, N.; Toyoda, F.; Tanaka, Y.; Takagi, R.; Kobayashi, M.; Kakehashi, A. Risk factors and clinical signs of severe Acanthamoeba keratitis. Clin. Ophthalmol. 2018, 12, 2567–2573.
- Scruggs, B.A.; Quist, T.S.; Salinas, J.L.; Greiner, M.A. Notes from the Field: Acanthamoeba Keratitis Cases—Iowa, 2002–2017. Morb. Mortal. Wkly. Rep. 2019, 68, 448–449.
- Szentmáry, N.; Daas, L.; Shi, L.; Laurik, K.L.; Lepper, S.; Milioti, G.; Seitz, B. Acanthamoeba keratitis—Clinical signs, differential diagnosis and treatment. J. Curr. Ophthalmol. 2019, 31, 16–23.
- Clarke, D.W.; Niederkorn, J.Y. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol. 2006, 22, 175–180.
- Füst, Á.; Tóth, J.; Simon, G.; Imre, L.; Nagy, Z.Z. Specificity of in vivo Confocal Cornea Microscopy in Acanthamoeba Keratitis. Eur. J. Ophthalmol. 2017, 27, 10–15.
- Iovieno, A.; Miller, D.; Ledee, D.R.; Alfonso, E.C. Cysticidal Activity of Antifungals against Different Genotypes of Acanthamoeba. Antimicrob. Agents Chemother. 2014, 58, 5626–5628.
- Papa, V.; Van Der Meulen, I.; Rottey, S.; Sallet, G.; Overweel, J.; Asero, N.; Minassian, D.C.; Dart, J.K.G. Safety and tolerability of topical polyhexamethylene biguanide: A randomised clinical trial in healthy adult volunteers. Br. J. Ophthalmol. 2020.
- Lim, N.; Goh, D.; Bunce, C.; Xing, W.; Fraenkel, G.; Poole, T.R.; Ficker, L. Comparison of Polyhexamethylene Biguanide and Chlorhexidine as Monotherapy Agents in the Treatment of Acanthamoeba Keratitis. Am. J. Ophthalmol. 2008, 145, 130–135.
- Hargrave, S.L.; McCulley, J.P.; Husseini, Z. Results of a trial of combined propamidine isethionate and neomycin therapy for acanthamoeba keratitis. Ophthalmology 1999, 106, 952–957.
- Illingworth, C.D.; Cook, S.D. Acanthamoeba Keratitis. Surv. Ophthalmol. 1998, 42, 493–508.
- Garg, P.; Kalra, P.; Joseph, J. Non-contact lens related Acanthamoeba keratitis. Indian J. Ophthalmol. 2017, 65, 1079–1086.
- Roozbahani, M.; Hammersmith, K.M.; Rapuano, C.J.; Nagra, P.K.; Zhang, Q. Therapeutic penetrating keratoplasty for acanthamoeba keratitis: A review of cases, complications and predictive factors. Int. Ophthalmol. 2019, 39, 2889–2896.
- Tu, E.Y.; Shoff, M.E.; Gao, W.; Joslin, C.E. Effect of Low Concentrations of Benzalkonium Chloride on Acanthamoebal Survival and Its Potential Impact on Empirical Therapy of Infectious Keratitis. JAMA Ophthalmol. 2013, 131, 595–600.
- Padzik, M.; Baltaza, W.; Conn, D.B.; Szaflik, J.P.; Chomicz, L. Effect of povidone iodine, chlorhexidine digluconate and toyocamycin on amphizoic amoebic strains, infectious agents of Acanthamoeba keratitis—A growing threat to human health worldwide. Ann. Agric. Environ. Med. 2018, 25, 725–731.
- Di Zazzo, A.; Kheirkhah, A.; Abud, T.B.; Goyal, S.; Dana, R. Management of high-risk corneal transplantation. Surv. Ophthalmol. 2017, 62, 816–827.
- Dewan, N.; Ming, W.; Holland, S.P.; Yeung, S.N.; Iovieno, A. Oral Miltefosine as Adjunctive Treatment for Recalcitrant Acanthamoeba Keratitis. Cornea 2019, 38, 914–917.
- Iovieno, A.; Gore, D.M.; Carnt, N.; Dart, J.K. Acanthamoeba Sclerokeratitis. Ophthalmology 2014, 121, 2340–2347.
- Bonini, S.; Di Zazzo, A.; Varacalli, G.; Coassin, M. Acanthamoeba Keratitis: Perspectives for Patients. Curr. Eye Res. 2020, 1–6.
- Sarnicola, E.; Sarnicola, C.; Sabatino, F.; Tosi, G.M.; Perri, P.; Sarnicola, V. Early Deep Anterior Lamellar Keratoplasty (DALK) for Acanthamoeba Keratitis Poorly Responsive to Medical Treatment. Cornea 2016, 35, 1–5.
- Ting, D.S.J.; Henein, C.; Said, D.G.; Dua, H.S. Photoactivated chromophore for infectious keratitis—Corneal cross-linking (PACK-CXL): A systematic review and meta-analysis. Ocul. Surf. 2019, 17, 624–634.
- Pettersson, M.N.; Lagali, N.; Mortensen, J.; Jofré, V.; Fagerholm, P. High fluence PACK-CXL as adjuvant treatment for advanced Acanthamoeba keratitis. Am. J. Ophthalmol. Case Rep. 2019, 15, 100499.
- Bonzano, C.; Di Zazzo, A.; Barabino, S.; Coco, G.; Traverso, C.E. Collagen Cross-Linking in the Management of Microbial Keratitis. Ocul. Immunol. Inflamm. 2018, 27, 507–512.
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the Management of Infectious Keratitis. Ophthalmology 2017, 124, 1678–1689.
- Prajna, N.V.; Radhakrishnan, N.; Lalitha, P.; Austin, A.; Ray, K.J.; Keenan, J.D.; Porco, T.C.; Lietman, T.M.; Rose-Nussbaumer, J. Cross-Linking–Assisted Infection Reduction. Ophthalmology 2020, 127, 159–166.




