
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio De La Vieja | + 4294 word(s) | 4294 | 2021-03-19 12:42:47 | | | |
| 2 | Peter Tang | Meta information modification | 4294 | 2021-03-27 15:36:26 | | |
Video Upload Options
Thyroid radioiodide or radioiodine therapy (RAI) is one of the oldest known and used targeted therapies. In thyroid cancer, it has been used for more than eight decades and is still being used to improve thyroid tumor treatment to eliminate remnants after thyroid surgery, and tumor metastases. Knowledge at the molecular level of the genes/proteins involved in the process has led to improvements in therapy, both from the point of view of when, how much, and how to use the therapy according to tumor type. The effectiveness of this therapy has spread into other types of targeted therapies, and this has made sodium/iodide symporter (NIS) one of the favorite theragnostic tools.
1. Radioactive Iodide (RAI) Therapy
Radioactive iodide (RAI) therapy has been a treatment option for patients with benign and malignant thyroid disease since the 1940s [1][2]. This post-surgical treatment after thyroidectomy is the oldest targeted therapy and contributes significantly to differentiated thyroid cancer (DTC) patients’ life expectancy. The potential secondary effects such as damage to salivary glands or tear ducts, or soreness and swelling in glands, are generally temporary and do not outweigh the benefits for thyroid malignancies. RAI is also used in benign thyroid diseases such as hyperthyroidism [3].
RAI is based on radiation capacity. In the case of 131I, high energy nuclear electron emissions are used to destroy target cells (Table 1) (Figure 1). Ionizing radiation leads to DNA damage, which is primarily caused by both the direct and indirect effects of radiation. This leads to molecular damage such as single-strand breaks, double-strand breaks, base damage, and DNA–protein cross links [4]. In the direct effects, 131I significantly inhibits cell proliferation, enhances cell apoptosis by downregulating the effector of cell cycle checkpoint Bcl2 gene, and promotes cell cycle arrest by upregulating the B-cell translocation gene 2-mediated activation of JNK/NF-κB pathways [5]. The sodium/iodide symporter (NIS) is also susceptible to DNA damage involving ataxia telangiectasia mutated kinase (ATM)-mediated mechanisms [6]. Indirect effects of radiation occur in the surrounding cells through non-targeted (bystander and abscopal) effects [7].
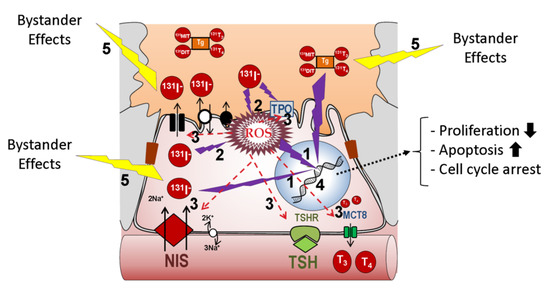
Figure 1. Putative effects of radio-iodide in the cell. Two types of effects can be experienced: direct and indirect. Four main types of damage can occur as a result of direct effects: (1) direct DNA damage such as single-strand and double-strand breaks, (2) increased ROS, (3) inactivation of DNA repair proteins to compensate for elevated ROS, (4) elevated ROS-mediated protein inactivation of proteins directly implicated in iodide transport, such as NIS, or in general thyroid differentiation (TPO, Tg, Duox2, TSH-R, Pendrin, etc.). Indirect non-targeted effects of radiation (5) occur in the surrounding cells as a result of bystander and abscopal effects. In any case, damage intensity would depend on radioiodine concentration and subcellular localization. Abbreviations: NIS: sodium/iodide symporter; TPO: thyroid peroxidase; Tg: thyroglobulin; TSH-R: Thyroid Stimulating Hormone receptor; ROS: reactive oxygen species; Duox2: Dual oxidase 2; MCT8: Monocarboxylate transporter 8.
Table 1. Radioactive isotopes used in clinical medicine for thyroid cancer. 18F-FDG: 2-deoxy-2-[18F]-Fluor-D-glucose; 18F-BF4-: [18F]-tetrafluoroborate; EC: electronic capture; γ: gamma radiation (high energy photons); β+: positrons from the nucleus; β-: electrons from the nucleus; IT: isometric transition; PET: positron emission tomography; SPECT: single-photon emission computed tomography; TG: thyroglobulin; GLUT: Glucose Transporter.
|
Isotope |
Detection Technique |
Transporter |
TG Organification? |
Energy Emitted Type |
Energy Emitted (KeV) |
Half Life |
Tissue Penetration/Spatial Resolution |
|---|---|---|---|---|---|---|---|
|
123I |
SPECT |
NIS |
Yes |
EC/γ |
159 |
13 h |
|
|
124I |
PET |
NIS |
Yes |
β+ |
510 |
4.17 d |
5.0 mm |
|
125I |
SPECT |
NIS |
Yes |
EC/γ |
27 |
59.4 d |
17 µm |
|
131I |
SPECT |
NIS |
Yes |
β-/γ |
364 |
8.02 d |
0.44–2.4 mm |
|
211At |
PET |
NIS, others |
No |
EC/α |
27 y 6900 (α) |
7.2 h |
65 µm |
|
99mTcO4− |
SPECT |
NIS |
No |
IT/γ |
140 |
6.03 h |
|
|
188ReO4− |
SPECT |
NIS |
No |
β-/γ |
155 |
17 h |
10.8 mm |
|
18F-BF4− |
PET |
NIS |
No |
β+ |
511 |
110 min |
|
|
18F-FDG |
PET |
GLUT |
No |
β+ |
511 |
110 min |
4.2 mm |
2. Iodide Accumulation in Normal, Tumor, and Metastatic Thyroid Cells
RAI therapy is a broad term that encompasses three different treatments associated with the administered activity of 131I [8]. The first, remnant ablation, is where 131I is given to destroy normal residual functioning thyroid tissue after surgery. This increases the sensitivity of detection of putative locoregional and/or metastatic disease on whole-body scans, maximizes therapeutic effects, and identifies additional sites of tumor cells. The second, adjuvant treatment, is where 131I is used to destroy unknown microscopic thyroid tumor cells and potentially decrease the chance of recurrence and patient mortality. Finally, RAI treatment of known disease is where 131I is given to destroy locoregional and distant metastasis to cure patients more efficiently, to reduce recurrence and mortality, and in cases of palliative care. RAI therapy is usually recommended for patients who have DTC. There are still issues to resolve regarding the therapeutic use of 131I for DTC once implementing RAI in clinical practice is considered [9]. Among others, these include the best method of preparation (thyroid hormone (TH) deprivation vs. recombinant human Thyroid Stimulating Hormone (rhTSH)), the amount of 131I used (low vs. high doses) depending on tumor risk stratification, assessment of post-operative disease status, precise definition of successful therapy, the radioisotopes chosen in diagnosis to avoid stunning, the use of personalized dosimetry, the management of refractory RAI cases, and the evaluation of putative side effects in the risk–benefit ratio of RAI to optimize the decision-making process. However, RAI therapy has provided undoubted benefits for DTC patients, and its success in their treatment has turned RAI into a potential therapeutic tool for other extra-thyroid tumors that express NIS [10][11][12][13]. Today, RAI through NIS is one of the favored theragnostic tools in gene therapies [13][14][15][16][17]. Nevertheless, RAI therapy success essentially depends on the capacity of the cell to accumulate radioactive iodide.
One of the main questions when approaching treatment is how radioiodine is incorporated into the tumor cell. To understand this process, it is necessary to describe the incorporation of iodine by NIS into a thyroid cell in normal physiology. The main role of iodine in metabolism is the synthesis of TH, which occurs in the thyroid gland [18][19]. Therefore, it is essential to know the molecular mechanisms involved in order to understand the incorporation of stable iodine (127I−), its radioactive isotopes (123I−, 124I−, 125I−, 131I−), and other thyroid-accumulated radioisotopes such as 99mTcO4−, 188ReO4−, 18F-FDG, and 18F-TFB which are currently used in clinical medicine (Table 1) [20].
3. Thyroid Hormone Deprivation vs. Recombinant Human TSH: Pros and Cons
3.1. Which TSH Stimulation Treatment Obtains Higher Radioiodide Accumulation and Organification in Tumor Cells?
RAI therapy strategies in DTC are summarized in Figure 2. Given the high capacity of NIS to accumulate iodine against its concentration gradient [21], a very high expression of NIS is not required, but it must be located in the plasma membrane. This could explain, at least in part, why recent studies show that the use of only 30 mCi of 131I can have as effective an outcome on remnant ablation as the most common clinically used dose of 100 mCi [22][23]. To achieve maximum iodine organification in TG, and also to keep follicular structures, the presence of the oxidative enzymes TPO and Duox2 is important. Also, in case of TH deprivation, a low iodine diet is very important before radioiodide treatment so that the accumulated/stored TG-I is as low as possible before the therapeutic dose of radioiodide is administered [24].
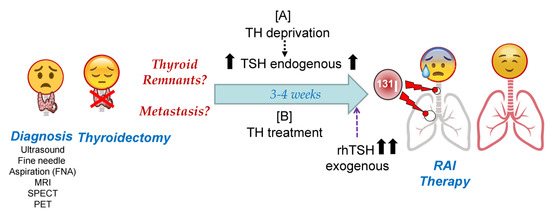
Figure 2. Schematic representation of Radioactive Iodide (RAI) therapy in patients with differentiated thyroid cancer (DTC). After tumor diagnosis, the final goal of both pre-RAI treatments is to achieve maximum radioiodine accumulation in tumor cells, with maximum residence time during 131I therapy. Molecularly speaking, this translates to the maximum accumulation of radioiodine through NIS and the maximum iodine organification in TG or other molecules that are able to oxidize iodide. Abbreviations: TH: Thyroid Hormones; TG: thyroglobulin; TSH: Thyroid Stimulating Hormone; rhTSH: recombinant human TSH; MRI: Magnetic resonance imaging; SPECT: Single-photon emission computed tomography; PET: Positron emission tomography.
Both hormonal deprivation and rhTSH can be extrapolated, in part, to in vitro cultures of thyroid cells. In the case of hypothyroidism induced by hormonal deprivation, TSH levels remain chronically high. In in vitro cultures, this chronic TSH stimulation has been shown to result in a continuous synthesis of NIS, TPO, TG, and Duox2, in addition to faster cell growth. However, the chronic induction of these genes results in relatively low expression levels [25]. Additionally, patients need to maintain a significantly low iodine intake because if they did not, part of the expressed TG would be iodized, and subsequent radioiodine organization would be much less effective during therapy. On the other hand, TH deprivation induces high concentrations of TG in colloids and/or in the blood. High TG concentrations result in lower NIS levels [26], and this could affect the efficiency of the organization process during therapy (Table 2). This situation occurs during TH deprivation. Furthermore, the TG in the colloid undergoes different oligomerization processes to allow better storage [27], which can later partially prevent the incorporation of radioiodine into TG, or at least reduce the efficiency of the process.
To prepare for rhTSH treatment, patients are treated with TH to keep endogenous TSH levels very low, and TSH-R protein at low or insignificant levels (Figure 2). Then, there is a high peak of serum TSH with rhTSH treatment. However, the time before the 131I treatment (in which TSH levels are high in the patient’s body) is short. In cell culture, the lack of TSH in the medium usually results in the decrease, or even absence, of NIS, both in the plasma membrane and in the cytoplasm [25], and also a considerable reduction in TG expression. Subsequently, the treatment with high TSH produces de novo synthesis of NIS, TG, and TPO, with maximum expression levels after 24–72 h [21]. These expression levels are higher than those obtained with chronic TSH treatment, at least in the case of NIS [25]. Additionally, because the temporal rhTSH stimulus is short, cell growth rate is lower compared to chronic treatment. This could be beneficial for the patient when it comes to tumor cells.
TH treatment inhibits TSH synthesis, and consequently, NIS is not synthesized in the thyroid follicle cell. Therefore, TG will not be iodized before radioiodine treatment and, after TSH stimulation, the efficiency of the organification of radioiodine in TG synthesized de novo will be higher. The duration of the stimulation of rhTSH is shorter than chronic TSH, and therefore a reduction in cell proliferation rate (and, as a consequence, the overall amount of TG protein synthesized), will be lower both in the thyroid cells and in the serum, but proportionally less iodinated.
The amount of radioiodine typically used for 131I therapy, regardless of the type of TSH pretreatment, is very high, and only part of it will be transported, accumulated, and organified in normal or tumor thyroid cells. This implies that with large doses of radiation, the relative observed differences in the incorporation and organization in both TSH treatments are not very relevant in terms of the final ablation response. However, there is controversy in the literature regarding dose effects. Different studies compared 30 vs. 100 mCi doses of RAI for post-thyroidectomy low-risk DTC patients, showing similar results in ablation independently of the TSH pre-therapy treatment used [22][23][28][29]. More recent studies did not show similar results and determine that the ablation rate was better with a higher dose of RAI [30]. In intermediate-risk or high-risk DTC patients, low doses appeared inadequate for achieving successful ablation [31]. However, the follow-up period for all these studies was too short (less than one year) to determine whether long-term disease-free survival (DFS) or overall survival (OS) was equivalent.
With respect to differences between TSH stimulation treatments pre-RAI, these tend to be minimal. Clinical data showed that there is a higher, but not significant, initial accumulation of iodine with TH deprivation. On the other hand, some studies showed that rhTSH treatment obtains a significantly greater retention of radioiodine during 131I therapy [32][33], probably as a consequence of the greater efficiency of organification, and of a lesser discharge of TG into the bloodstream. This could be observed as a greater amount of radioiodine in whole-body scan images. These differences could become significantly more relevant if the amount of therapeutic radioiodine used is lowered (Table 2).
Table 2. Main benefits and disadvantages in the use of TH deprivation vs. stimulation with recombinant human TSH before RAI therapy.
|
|
|
Benefit |
Disadvantages | |
|---|---|---|---|---|
|
|
TH deprivation for 2–4 weeks |
|
|
|
|
|
Recombinant human TSH |
|
|
|
In summary, TH deprivation keeps serum TSH chronically elevated (Figure 2), leading to the constant expression of NIS and TG in addition to high tumor cell proliferation. Globally, TG expression levels are high, although a significant proportion will be discharged into the bloodstream and part of the TG in the colloid may be organified with cold iodine. This translates into relatively inefficient radioiodine organization during 131I treatment.
In recombinant human TSH treatment, endogenous TSH levels are very low or not present, as are NIS and TG expression levels. After treatment with rhTSH, high de novo synthesis of NIS and TG is achieved. This allows for very high NIS radio-iodide uptake and a greater efficiency of radioiodine TG organization in the colloid, increasing radioiodine residence time in thyroid remnants. This approach can compensate for the lower overall expression of TG and prevent the constant proliferation of tumor cells as compared to TH deprivation.
3.2. Which TSH Stimulation Treatment Obtains Longer Radioiodine Residence Time in Tumor Cells?
As previously mentioned, in the case of TH deprivation, TG levels are higher in addition to increased proliferation and growth (Table 2). Therefore, the global TG amount will be greater than in thyroid remnants treated with recombinant human TSH. However, part of this TG may be iodized and/or in tertiary structures stored in the colloid, where the incorporation of radioiodine will be less efficient than in the case of de novo synthetized TG, as occurs in the case of stimulation with rhTSH. This would explain why there is a greater amount of organized radioiodine in TH deprivation, and yet the period of time that this radioiodine stays in the remaining thyroid tissue is low [32][33]. Furthermore, chronic TSH will stimulate the proliferation and growth of tumor cells [34]. In the case of TH deprivation, it has been observed that a large part of TG is discharged into the blood before treatment, again decreasing the effectiveness of radioiodine incorporation, and thus allowing a greater exposure of TG to the immune system, which may increase the probability that new anti-TG antibodies are generated. In any case, clinical data indicate that there are no significant differences regarding ablation results obtained in both situations [22][23][32]. This could be explained because the amount of radio-iodide typically used is very high. No significant differences were observed with respect to the recurrence risk in patients treated with either treatment [35][36].
Another important aspect may be kidney function. In TH deprivation, and therefore chronic elevated TSH, kidney function is affected. Since NIS is also expressed in the kidney, normal metabolism iodide flow can be altered [13]. This is important and explains, at least in part, why in TH deprivation the levels of radioiodine in blood, and in general in whole-body scans, are higher than in euthyroid patients treated with rhTSH. However, because kidney function is altered, blood radioiodine levels could increase the risk of side effects in other organs (stomach, ovary, salivary glands, etc.) where NIS is also expressed [13][37]. This would decrease potential radioiodine accumulation in thyroid remnants and/or metastases. In this sense, recent studies demonstrate that DTC patients treated with 131I after TSH stimulation have lower abdominal (liver, stomach, ascending colon, transverse colon, descending colon, rectum, and small intestine) radioiodine activity, both in terms of activity ratio and absorbed dose ratios, than patients with TH withdrawal [38]. These results could be relevant to prevent possible gastrointestinal side effects after therapy and also in post-therapy patient management (i.e., length of hospital stay, health cost, and quality of life) [38].
In summary, kinetic data from patients indicate that there are differences in the amount of radioiodine that can accumulate and/or be organified depending on whether the treatment prior to radioiodine is via TH deprivation or by exogenous stimulation with TSH. There is a greater quantity of TG and therefore radioiodine organification in a situation of TH deprivation, but a large part of that TG-I is discharged into the bloodstream, becoming a relatively inefficient process. TH treatment and recombinant human TSH stimulation achieve lower total organification but allow a more efficient accumulation in thyroid remnants. The opposite occurs with radio-iodide residence in blood and other tissues, minimizing possible side effects in other organs (Table 2).
3.3. Do Different TSH Stimulation Treatments Affect Negative Scans in Diagnosis?
The management of DTC patients with rising TG levels and negative radioiodine whole body scans is still controversial [39]. A radioiodine negative scan can be mainly due to two factors: (i) NIS expression is very low or null, and (ii) even if there is expression, even over-expression of NIS, the protein is delocalized and does not reach the plasma membrane. Both cases can occur regardless of the type of previous TSH treatment and will depend more on the degree of dedifferentiation of tumor cells and the expression of TSH-R. Iodide negative scans will be more frequent in more de-differentiated cases within the umbrella of those considered DTC. The new classification of DTC from panels of mutated and/or altered genes after sequencing of many patients [40] may help to correlate tumor subtypes more clearly and predict the response to radioiodine treatment. Alternative radioelements, i.e., those not transported by NIS, have been proposed to scan thyroid remnants and/or metastases [41][42][43]. The treatment of those cases may also differ from RAI and strategies used in PDTC and ATC, previously mentioned, could be more promising [44].
3.4. What TSH Stimulation Treatment Could Be Better for DTC Metastases?
As in non-metastatic tumor thyroid tissue, the efficacy of radioiodine therapy will depend mainly on the presence of NIS to accumulate iodine and on the TPO/Duox2 system to oxidize radio-iodide into TG radioiodine. However, the organification of iodine can be carried out by other oxidative protein systems such as Lactoperoxidase (LPO)/NOX, and protein targets could be different than TG. This last aspect becomes more important in the treatment of metastasis since higher concentrations of radioiodine are used.
There is still debate regarding the efficacy of the treatment of DTC thyroid metastases when comparing TH deprivation vs. rhTSH [45]. In metastatic patients where radioiodine therapy was used compassionately, both pre-therapy methods were found to be equally effective in dosimetry measurements of patients [45]. Nevertheless, the studies are mostly retrospective, and groups of patients were heterogeneous. In addition, some studies did not find significant differences in the efficacy of eliminating locoregional and pulmonary metastases [46][47], or bone metastases [46][48]. Better programmed epidemiological studies that use a higher number of patients are needed in order to obtain adequate scientific evidence in this regard. One of the most recent studies [49] indicates that radio-iodide retention rate and effective half-time in metastatic lymph nodes were significantly lower than in thyroid remnants of primary tumors. This is concordant to lower levels of expression or delocalization of NIS in metastatic cells (Table 3). Additionally, this study indicates that the retention rate and the effective half-time of thyroid remnants and in metastatic lymph nodes in the rhTSH pre-RAI treatment group were higher than those in the TH deprivation group, although not statistically significant for metastatic cases [49]. Taking into account the clinical response after RAI, overall patient survival after 5.5 years was similar when the pre-RAI treatment was either TH deprivation or rhTSH [48].
In summary, although there are no available large comparative epidemiological studies of RAI treatment of metastases after TH deprivation or rhTSH, most studies show similar effectiveness.
4. Stunning Phenomenon in RAI Therapy
Thyroid stunning was first reported in 1951 [50]. This phenomenon is characterized by less 131I accumulation at therapy than was predicted from the radioiodine accumulation measurement during dose planning at diagnosis. There is quite a bit of controversy regarding the existence of such an effect, and the possible underlying mechanisms in the stunning process. A wide variety of results both in vitro [51][52][53] and in vivo [54][55][56] have been published, sometimes contradictory; at other times, procedures used differ and results are difficult to compare [57].
Two different effects would explain a large part of the observed results. (1) If radioiodine, either 131I, 123I, or 124I, is used in the initial screening, it will end up oxidized, mostly in TG. This organified radioiodine will be able, depending on quantity and radiation energy levels, to affect the cell where it has been incorporated and even destroy it. Surrounding cells could also be affected through non-targeted reactions (bystander and abscopal effects) [7] as occurs in typical 131I therapeutic doses. Therefore, these cells would no longer be able to accumulate or incorporate radioiodine during treatment. Consequently, these cells will have been successfully treated, even with apparently low radioiodine doses, and thyroid stunning would be an artifact due to an early therapeutic effect of ablative 131I, and not a clinical problem. In this sense, using 131I during diagnosis would be more effective than 123I, since 131I has higher levels of radiation emission (Table 1). (2) If radioiodine does not destroy the cell, it would be stored as TG-131I/123I, and this would diminish the future capacity of that TG to organize radioiodine during treatment. In this sense, both radioisotopes would interfere similarly. In these two effects, there would be an additional indirect effect produced by the increase of ROS [58], which would affect the cell depending on accumulated levels. Again, 131I would be more effective than 123I here. Given that tumor cells already have high levels of ROS, the increased ROS could additionally contribute to the elimination of the cell regardless of radiation. This would be equal in diagnosis and treatment. Using 123I and 124I may have some disadvantages, such as lower sensitivity, higher costs, shorter half-life, and availability problems [59]. 124I has an additional disadvantage because it is a positron emitter which can cause methodological problems.
Other radioisotopes used in diagnosis [51][60] that can be transported by NIS, such as 99mTcO4, 211At, and 18F-BF4−, will not be organified in TG (Table 1). Therefore, the first effect would not occur, and the other two effects would be greatly diminished. As there is no organification in TG and they have short half-lives (Table 1), diagnostic images should be recorded shortly after radioisotope administration. ROS production using theses isotopes, except 211At, will be lower than using 131I, 123I, or 124I. Therefore, the isotopes that could cause a minor stunning effect would be those that are not organified by TG, such as 99mTcO4− and 18F-BF4. This was demonstrated for 99mTcO4−, where the stunning effect in mice could only be observed at extremely high thyroid absorbed dose thresholds (above 20 Gy), a level unlikely to be found in clinical practice [61]. However, 99mTcO4− has the drawback of low sensitivity, especially for metastatic images, so a negative diagnosis cannot be absolutely guaranteed [62]. Perhaps the most promising is 18F-BF4−, which provides more sensitive images using positron emission tomography (PET), although more studies are needed to confirm this.
Concerning the first effect, in addition to cellular damage, ROS could generate an effect similar to the Wolff–Chaikoff effect during diagnosis or treatment [63][64][65]. This would cause not only an oxidation of iodide (both TG and lipid oxidation), but also a very rapid inhibition of NIS present at the plasma membrane, even in times as short as 1 h, and could last up to 48–72 h [63][65]. In this case it would be necessary to wait for the escape of the Wolff–Chaikoff effect so that the cells are able to accumulate 131I again [63]. Given that ROS are high in tumor cells before diagnostic 131I is applied, this phenomenon could be possible even if relatively low doses of 131I are used [66].
Clinical results show that using 131I at concentrations between 1–3 mCi for diagnosis [67] and applying the therapeutic dose within 24–48 h prevents the stunning effect [57]. However, this effect is observed when using 2–5 mCi of 123I for diagnosis. Recent studies have demonstrated the stunning effect in benign thyroid diseases [54], and that it is dependent on pre-therapeutic radiation doses. A correction factor for the therapeutic dose, to avoid the stunning effect, has been proposed [54][68].
One of the controversies in RAI is the most appropriate time-window to administer 131I. It has been shown in clinical studies that the greatest accumulation of radioiodine occurs 24–48 h after the injection of rhTSH [69]; therefore, this would be the most appropriate window to use radioiodine for diagnosis and/or treatment. According to this, the closer the doses are given, the better the therapeutic outcome [69].
In summary, based on current data, the best way to avoid stunning would be to use radioisotopes that are not organified in the TG and that are also transported by NIS, such as 18F-BF4−, in diagnostic tests. In clinical studies, it has been shown that an initial dose of 1–3 mCi of 131I or 2–5 mCi 123I does not generate, or minimally generates, the stunning effect. It is most appropriate to give radioiodine doses prior to 72 h post-injection of recombinant human TSH.
References
- Hertz, S.; Roberts, A. Radioactive Iodine as an Indicator in Thyroid Physiology. V. The Use of Radioactive Iodine in the Differential Diagnosis of Two Types of Graves’ Disease. J. Clin. Investig. 1942, 21, 31–32.
- Seidlin, S.M.; Marinelli, L.D.; Oshry, E. Radioactive Iodine Therapy; Effect on Functioning Metastases of Adenocarcinoma of the Thyroid. J. Am. Med. Assoc. 1946, 132, 838–847.
- Gronich, N.; Lavi, I.; Rennert, G.; Saliba, W. Cancer Risk after Radioactive Iodine Treatment for Hyperthyroidism: A Cohort Study. Thyroid 2020, 30, 243–250.
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. 2013, 25, 578–585.
- Zhao, L.M.; Pang, A.X. Iodine-131 treatment of thyroid cancer cells leads to suppression of cell proliferation followed by induction of cell apoptosis and cell cycle arrest by regulation of B-cell translocation gene 2-mediated JNK/NF-kappaB pathways. Braz. J. Med Biol. Res. Rev. Bras. Pesqui. Med. Biol. 2017, 50, e5933.
- Lyckesvard, M.N.; Kapoor, N.; Ingeson-Carlsson, C.; Carlsson, T.; Karlsson, J.O.; Postgard, P.; Himmelman, J.; Forssell-Aronsson, E.; Hammarsten, O.; Nilsson, M. Linking loss of sodium-iodide symporter expression to DNA damage. Exp. Cell Res. 2016, 344, 120–131.
- Pouget, J.P.; Georgakilas, A.G.; Ravanat, J.L. Targeted and Off-Target (Bystander and Abscopal) Effects of Radiation Therapy: Redox Mechanisms and Risk/Benefit Analysis. Antioxid. Redox. Signal. 2018, 29, 1447–1487.
- Ylli, D.; Van Nostrand, D.; Wartofsky, L. Conventional Radioiodine Therapy for Differentiated Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019, 48, 181–197.
- Tuttle, R.M.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Daniels, G.H.; Dillehay, G.; Draganescu, C.; Flux, G.; Fuhrer, D.; et al. Controversies, Consensus, and Collaboration in the Use of 131I Therapy in Differentiated Thyroid Cancer: A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid 2019, 29, 461–470.
- Micali, S.; Maggisano, V.; Cesinaro, A.; Celano, M.; Territo, A.; Reggiani Bonetti, L.; Sponziello, M.; Migaldi, M.; Navarra, M.; Bianchi, G.; et al. Sodium/iodide symporter is expressed in the majority of seminomas and embryonal testicular carcinomas. J. Endocrinol. 2013, 216, 125–133.
- Riesco-Eizaguirre, G.; Leoni, S.G.; Mendiola, M.; Estevez-Cebrero, M.A.; Gallego, M.I.; Redondo, A.; Hardisson, D.; Santisteban, P.; De la Vieja, A. NIS mediates iodide uptake in the female reproductive tract and is a poor prognostic factor in ovarian cancer. J. Clin. Endocrinol. Metab. 2014, 99, E1199-1208.
- Tazebay, U.H.; Wapnir, I.L.; Levy, O.; Dohan, O.; Zuckier, L.S.; Zhao, Q.H.; Deng, H.F.; Amenta, P.S.; Fineberg, S.; Pestell, R.G.; et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat. Med. 2000, 6, 871–878.
- De la Vieja, A.; Santisteban, P. Role of iodide metabolism in physiology and cancer. Endocr. Relat. Cancer 2018, 25, R225–R245.
- Ravera, S.; Reyna-Neyra, A.; Ferrandino, G.; Amzel, L.M.; Carrasco, N. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu. Rev. Physiol. 2017, 79, 261–289.
- Spitzweg, C.; Morris, J.C. Gene therapy for thyroid cancer: Current status and future prospects. Thyroid 2004, 14, 424–434.
- Chung, J.K.; Cheon, G.J. Radioiodine therapy in differentiated thyroid cancer: The first targeted therapy in oncology. Endocrinol. Metab. 2014, 29, 233–239.
- Ahn, B.C. Sodium iodide symporter for nuclear molecular imaging and gene therapy: From bedside to bench and back. Theranostics 2012, 2, 392–402.
- De La Vieja, A.; Dohan, O.; Levy, O.; Carrasco, N. Molecular analysis of the sodium/iodide symporter: Impact on thyroid and extrathyroid pathophysiology. Physiol. Rev. 2000, 80, 1083–1105.
- Rousset, B.; Dupuy, C.; Miot, F.; Dumont, J. Chapter 2 Thyroid Hormone Synthesis And Secretion. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText. com, Inc.: South Dartmouth, MA, USA, 2000.
- Jiang, H.; DeGrado, T.R. [18F]Tetrafluoroborate ([18F]TFB) and its analogs for PET imaging of the sodium/iodide symporter. Theranostics 2018, 8, 3918–3931.
- Dohan, O.; De la Vieja, A.; Paroder, V.; Riedel, C.; Artani, M.; Reed, M.; Ginter, C.S.; Carrasco, N. The sodium/iodide Symporter (NIS): Characterization, regulation, and medical significance. Endocr. Rev. 2003, 24, 48–77.
- Mallick, U.; Harmer, C.; Yap, B.; Wadsley, J.; Clarke, S.; Moss, L.; Nicol, A.; Clark, P.M.; Farnell, K.; McCready, R.; et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N. Engl. J. Med. 2012, 366, 1674–1685.
- Schlumberger, M.; Catargi, B.; Borget, I.; Deandreis, D.; Zerdoud, S.; Bridji, B.; Bardet, S.; Leenhardt, L.; Bastie, D.; Schvartz, C.; et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N. Engl. J. Med. 2012, 366, 1663–1673.
- Zwarthoed, C.; Chatti, K.; Guglielmi, J.; Hichri, M.; Compin, C.; Darcourt, J.; Vassaux, G.; Benisvy, D.; Pourcher, T.; Cambien, B. Single-Photon Emission Computed Tomography for Preclinical Assessment of Thyroid Radioiodide Uptake Following Various Combinations of Preparative Measures. Thyroid 2016, 26, 1614–1622.
- Riedel, C.; Levy, O.; Carrasco, N. Post-transcriptional regulation of the sodium/iodide symporter by thyrotropin. J. Biol. Chem. 2001, 276, 21458–21463.
- Sellitti, D.F.; Suzuki, K. Intrinsic regulation of thyroid function by thyroglobulin. Thyroid 2014, 24, 625–638.
- Di Jeso, B.; Arvan, P. Thyroglobulin From Molecular and Cellular Biology to Clinical Endocrinology. Endocr. Rev. 2016, 37, 2–36.
- Bal, C.; Chandra, P.; Kumar, A.; Dwivedi, S. A randomized equivalence trial to determine the optimum dose of iodine-131 for remnant ablation in differentiated thyroid cancer. Nucl. Med. Commun. 2012, 33, 1039–1047.
- Prior-Sanchez, I.; Muñoz-Jimenez, C.; Moreno-Moreno, P.; Rebollo-Roman, A.; Barrera-Martín, A.; Moreno-Ortega, E.; Vallejo-Casas, J.A.; Galvez-Moreno, M.A. Our experience with low doses of radioactive iodine (30 mCi) in patients with differentiated thyroid cancer. In Proceedings of the 18th European Congress of Endocrinology, Munich, Germany, 28–31 May 2016; p. EP1139.
- Albano, D.; Bonacina, M.; Durmo, R.; Bertagna, F.; Giubbini, R. Efficacy of low radioiodine activity versus intermediate-high activity in the ablation of low-risk differentiated thyroid cancer. Endocrine 2020, 68, 124–131.
- Abe, K.; Ishizaki, U.; Ono, T.; Horiuchi, K.; Kanaya, K.; Sakai, S.; Okamoto, T. Low-dose radioiodine therapy for patients with intermediate- to high-risk differentiated thyroid cancer. Ann. Nucl. Med. 2020, 34, 144–151.
- Hanscheid, H.; Lassmann, M.; Luster, M.; Thomas, S.R.; Pacini, F.; Ceccarelli, C.; Ladenson, P.W.; Wahl, R.L.; Schlumberger, M.; Ricard, M.; et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: Procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J. Nucl. Med. 2006, 47, 648–654.
- Taieb, D.; Sebag, F.; Farman-Ara, B.; Portal, T.; Baumstarck-Barrau, K.; Fortanier, C.; Bourrelly, M.; Mancini, J.; De Micco, C.; Auquier, P.; et al. Iodine biokinetics and radioiodine exposure after recombinant human thyrotropin-assisted remnant ablation in comparison with thyroid hormone withdrawal. J. Clin. Endocrinol. Metab. 2010, 95, 3283–3290.
- Garcia-Jimenez, C.; Santisteban, P. TSH signalling and cancer. Arq. Bras. Endocrinol. Metabol. 2007, 51, 654–671.
- Elisei, R.; Schlumberger, M.; Driedger, A.; Reiners, C.; Kloos, R.T.; Sherman, S.I.; Haugen, B.; Corone, C.; Molinaro, E.; Grasso, L.; et al. Follow-up of low-risk differentiated thyroid cancer patients who underwent radioiodine ablation of postsurgical thyroid remnants after either recombinant human thyrotropin or thyroid hormone withdrawal. J. Clin. Endocrinol. Metab. 2009, 94, 4171–4179.
- Tuttle, R.M.; Brokhin, M.; Omry, G.; Martorella, A.J.; Larson, S.M.; Grewal, R.K.; Fleisher, M.; Robbins, R.J. Recombinant human TSH-assisted radioactive iodine remnant ablation achieves short-term clinical recurrence rates similar to those of traditional thyroid hormone withdrawal. J. Nucl. Med. 2008, 49, 764–770.
- Portulano, C.; Paroder-Belenitsky, M.; Carrasco, N. The Na+/I− symporter (NIS): Mechanism and medical impact. Endocr. Rev. 2014, 35, 106–149.
- Campenni, A.; Amato, E.; Laudicella, R.; Alibrandi, A.; Cardile, D.; Pignata, S.A.; Trimarchi, F.; Ruggeri, R.M.; Auditore, L.; Baldari, S. Recombinant human thyrotropin (rhTSH) versus Levo-thyroxine withdrawal in radioiodine therapy of differentiated thyroid cancer patients: Differences in abdominal absorbed dose. Endocrine 2019, 65, 132–137.
- Chao, M. Management of differentiated thyroid cancer with rising thyroglobulin and negative diagnostic radioiodine whole body scan. Clin. Oncol. 2010, 22, 438–447.
- The Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690.
- Elboga, U.; Karaoglan, H.; Sahin, E.; Kalender, E.; Demir, H.D.; Basibuyuk, M.; Zeki Celen, Y.; Yilmaz, M.; Ozkaya, M. F-18 FDG PET/CT imaging in the diagnostic work-up of thyroid cancer patients with high serum thyroglobulin, negative I-131 whole body scan and suppressed thyrotropin: 8-year experience. Eur. Rev. Med Pharmacol. Sci. 2015, 19, 396–401.
- Riesco-Eizaguirre, G.; Galofre, J.C.; Grande, E.; Zafon Llopis, C.; Ramon y Cajal Asensio, T.; Navarro Gonzalez, E.; Jimenez-Fonseca, P.; Santamaria Sandi, J.; Gomez Saez, J.M.; Capdevila, J. Spanish consensus for the management of patients with advanced radioactive iodine refractory differentiated thyroid cancer. Endocrinol. Nutr. 2016, 63, e17-24.
- Zakani, A.; Saghari, M.; Eftekhari, M.; Fard-Esfahani, A.; Fallahi, B.; Esmaili, J.; Assadi, M. Evaluation of radioiodine therapy in differentiated thyroid cancer subjects with elevated serum thyroglobulin and negative whole body scan using 131I with emphasize on the thallium scintigraphy in these subgroups. Eur. Rev. Med Pharmacol. Sci. 2011, 15, 1215–1221.
- Buffet, C.; Wassermann, J.; Hecht, F.; Leenhardt, L.; Dupuy, C.; Groussin, L.; Lussey-Lepoutre, C. Redifferentiation of radioiodine-refractory thyroid cancers. Endocr. Relat. Cancer 2020.
- Klubo-Gwiezdzinska, J.; Burman, K.D.; Van Nostrand, D.; Mete, M.; Jonklaas, J.; Wartofsky, L. Potential use of recombinant human thyrotropin in the treatment of distant metastases in patients with differentiated thyroid cancer. Endocr. Pract. 2013, 19, 139–148.
- Liepe, K. Sensitivity of preparation with rhTSH or thyroid hormone withdrawal using 131I-whole body scans to identify metastases of differentiated thyroid cancer. Int. J. Surg. 2015, 16, 107–112.
- Tuttle, R.M.; Lopez, N.; Leboeuf, R.; Minkowitz, S.M.; Grewal, R.; Brokhin, M.; Omry, G.; Larson, S. Radioactive iodine administered for thyroid remnant ablation following recombinant human thyroid stimulating hormone preparation also has an important adjuvant therapy function. Thyroid 2010, 20, 257–263.
- Tala, H.; Robbins, R.; Fagin, J.A.; Larson, S.M.; Tuttle, R.M. Five-year survival is similar in thyroid cancer patients with distant metastases prepared for radioactive iodine therapy with either thyroid hormone withdrawal or recombinant human TSH. J. Clin. Endocrinol. Metab. 2011, 96, 2105–2111.
- Hong, C.M.; Kim, C.Y.; Son, S.H.; Jung, J.H.; Lee, C.H.; Jeong, J.H.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. I-131 biokinetics of remnant normal thyroid tissue and residual thyroid cancer in patients with differentiated thyroid cancer: Comparison between recombinant human TSH administration and thyroid hormone withdrawal. Ann. Nucl. Med. 2017, 31, 582–589.
- Rawson, R.W.; Rall, J.E.; Peacock, W. Limitations in the treatment of cancer of the thyroid with radioactive iodine. Trans. Assoc. Am. Physicians 1951, 64, 179–198.
- Lundh, C.; Lindencrona, U.; Postgard, P.; Carlsson, T.; Nilsson, M.; Forssell-Aronsson, E. Radiation-induced thyroid stunning: Differential effects of 123I, 131I, 99mTc, and 211At on iodide transport and NIS mRNA expression in cultured thyroid cells. J. Nucl. Med. 2009, 50, 1161–1167.
- Meller, B.; Gaspar, E.; Deisting, W.; Czarnocka, B.; Baehre, M.; Wenzel, B.E. Decreased radioiodine uptake of FRTL-5 cells after 131I incubation in vitro: Molecular biological investigations indicate a cell cycle-dependent pathway. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1204–1212.
- Postgard, P.; Himmelman, J.; Lindencrona, U.; Bhogal, N.; Wiberg, D.; Berg, G.; Jansson, S.; Nystrom, E.; Forssell-Aronsson, E.; Nilsson, M. Stunning of iodide transport by 131I irradiation in cultured thyroid epithelial cells. J. Nucl. Med. 2002, 43, 828–834.
- Happel, C.; Kranert, W.T.; Ackermann, H.; Binse, I.; Bockisch, B.; Groner, D.; Herrmann, K.; Grunwald, F. Thyroid stunning in radioiodine-131 therapy of benign thyroid diseases. Endocrine 2019, 63, 537–544.
- Lassmann, M.; Luster, M.; Hanscheid, H.; Reiners, C. Impact of 131I diagnostic activities on the biokinetics of thyroid remnants. J. Nucl. Med. 2004, 45, 619–625.
- Morris, L.F.; Waxman, A.D.; Braunstein, G.D. Thyroid stunning. Thyroid 2003, 13, 333–340.
- McDougall, I.R.; Iagaru, A. Thyroid stunning: Fact or fiction? Semin. Nucl. Med. 2011, 41, 105–112.
- Vrndic, O.B.; Radivojevic, S.D.; Jovanovic, M.D.; Djukic, S.M.; Teodorovic, L.C.; Simonovic, S.T. Oxidative stress in patients with differentiated thyroid cancer: Early effects of radioiodine therapy. Indian J. Biochem. Biophys. 2014, 51, 223–229.
- Ruhlmann, M.; Sonnenschein, W.; Nagarajah, J.; Binse, I.; Herrmann, K.; Jentzen, W. Pretherapeutic 124I dosimetry reliably predicts intratherapeutic blood kinetics of 131I in patients with differentiated thyroid carcinoma receiving high therapeutic activities. Nucl. Med. Commun. 2018, 39, 457–464.
- Watanabe, K.; Igarashi, T.; Ashida, H.; Ogiwara, S.; Ohta, T.; Uchiyama, M.; Ojiri, H. Diagnostic value of ultrasonography and TI-201/Tc-99m dual scintigraphy in differentiating between benign and malignant thyroid nodules. Endocrine 2019, 63, 301–309.
- Cambien, B.; Franken, P.R.; Lamit, A.; Mauxion, T.; Richard-Fiardo, P.; Guglielmi, J.; Crescence, L.; Mari, B.; Pourcher, T.; Darcourt, J.; et al. 99mTcO4−-, auger-mediated thyroid stunning: Dosimetric requirements and associated molecular events. PLoS ONE 2014, 9, e92729.
- Kueh, S.S.; Roach, P.J.; Schembri, G.P. Role of Tc-99m pertechnetate for remnant scintigraphy post-thyroidectomy. Clin. Nucl. Med. 2010, 35, 671–674.
- Leoni, S.G.; Kimura, E.T.; Santisteban, P.; De la Vieja, A. Regulation of thyroid oxidative state by thioredoxin reductase has a crucial role in thyroid responses to iodide excess. Mol. Endocrinol. 2011, 25, 1924–1935.
- Leoni, S.G.; Sastre-Perona, A.; De la Vieja, A.; Santisteban, P. Selenium Increases Thyroid-Stimulating Hormone-Induced Sodium/Iodide Symporter Expression Through Thioredoxin/Apurinic/Apyrimidinic Endonuclease 1-Dependent Regulation of Paired Box 8 Binding Activity. Antioxid. Redox. Signal. 2016, 24, 855–866.
- Arriagada, A.A.; Albornoz, E.; Opazo, M.C.; Becerra, A.; Vidal, G.; Fardella, C.; Michea, L.; Carrasco, N.; Simon, F.; Elorza, A.A.; et al. Excess iodide induces an acute inhibition of the sodium/iodide symporter in thyroid male rat cells by increasing reactive oxygen species. Endocrinology 2015, 156, 1540–1551.
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496.
- Yap, B.K.; Murby, B. No adverse affect in clinical outcome using low preablation diagnostic 131I activity in differentiated thyroid cancer: Refuting thyroid-stunning effect. J. Clin. Endocrinol. Metab. 2014, 99, 2433–2440.
- Happel, C.; Kranert, W.T.; Groner, D.; Bockisch, B.; Sabet, A.; Vardarli, I.; Gorges, R.; Herrmann, K.; Grunwald, F. Correction for hyperfunctioning radiation-induced stunning (CHRIS) in benign thyroid diseases. Endocrine 2020.
- Fast, S.; Nielsen, V.E.; Grupe, P.; Bonnema, S.J.; Hegedus, L. Optimizing 131I uptake after rhTSH stimulation in patients with nontoxic multinodular goiter: Evidence from a prospective, randomized, double-blind study. J. Nucl. Med. 2009, 50, 732–737.





