| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yanhua Wang | + 1334 word(s) | 1334 | 2020-05-11 08:48:04 | | | |
| 2 | Camila Xu | -1 word(s) | 1333 | 2020-05-29 04:19:19 | | | | |
| 3 | Camila Xu | -58 word(s) | 1275 | 2020-10-30 06:54:47 | | | | |
| 4 | Camila Xu | -58 word(s) | 1275 | 2020-10-30 06:55:15 | | |
Video Upload Options
Aldosterone is a steroid hormone that is produced in the adrenal cortex. Its major renal effect is to regulate electrolyte and water homeostasis in the distal tubule, thus maintaining blood pressure and extracellular fluid homeostasis through the activation of mineralocorticoid receptors (MR) in epithelial cells [2]. Aldosterone enters an epithelial cell and binds to the MR. The complex of aldosterone and MR translocates into the nucleus and regulates gene transcription of, among others, the epithelial sodium channel (ENaC) and the signaling proteins and kinases that impact channel and transporter activity, such as serum/glucocorticoid kinases (SGKs).
1. Introduction
Aldosterone is a steroid hormone that is produced in the adrenal cortex. Its major renal effect is to regulate electrolyte and water homeostasis in the distal tubule, thus maintaining blood pressure and extracellular fluid homeostasis through the activation of mineralocorticoid receptors (MR) in epithelial cells [2]. Aldosterone enters an epithelial cell and binds to the MR. The complex of aldosterone and MR translocates into the nucleus and regulates gene transcription of, among others, the epithelial sodium channel (ENaC) and the signaling proteins and kinases that impact channel and transporter activity, such as serum/glucocorticoid kinases (SGKs). This results in increased apical membrane accumulation and activity of ENaC, thus increasing sodium reabsorption and subsequent water reabsorption (reviewed in [3][4]).
In addition to the genomic effects, aldosterone has rapid actions that are independent of transcription and translation. Aldosterone can have rapid effects on sodium chloride cotransport (NCC) in distal convoluted tubules [5], bicarbonate reabsorption in proximal tubules [6], natriuresis [7], and several signaling processes in inner medullary collecting ducts (IMCDs) and other nephron segments [8][9][10][11]. Aldosterone increases calcineurin activity in rat cortical collecting ducts through an MR‐dependent but transcription‐independent mechanism [12]. Our published data show that inhibiting calcineurin alters the phosphorylation and the activity of the urea transporter UT‐A1 in the IMCD [13][14].
Aldosterone has a complex interaction with vasopressin (AVP). Vasopressin is the major hormone regulating water permeability in the kidney [15]. Vasopressin binds to V2 receptors on the IMCD basolateral plasma membrane, resulting in stimulation of adenylyl cyclase and cyclic AMP (cAMP) generation. Stimulation of the cAMP dependent protein kinase A (PKA) promotes phosphorylation of key transporters and channels, resulting in an increase in water permeability[16]. Vasopressin stimulation of water permeability is due, in large part, to stimulation of the aquaporin 2 (AQP2) water channel [17][18]. AQP2, located at the apical plasma membrane and the subapical vesicles in collecting duct principal cells, is regulated by vasopressin‐stimulated increases in both its phosphorylation and its apical plasma membrane accumulation [19]. Through the cAMP‐PKA pathway, vasopressin also increases urea transport by stimulating UT‐A1 [15][20][21].
Although aldosterone indirectly regulates water reabsorption in distal tubules, its effect on vasopressin‐regulated water and urea permeability in rat IMCDs has not been tested, nor has whether there is a sex difference been examined. Our published data show that aldosterone decreases UT‐A1 protein in the inner medullary tip [22]. Nielsen et al. reported that aldosterone increases urine production and decreases apical membrane AQP2 expression in rats with diabetes insipidus [23]. These findings suggest that aldosterone may decrease vasopressin‐stimulated osmotic water permeability.
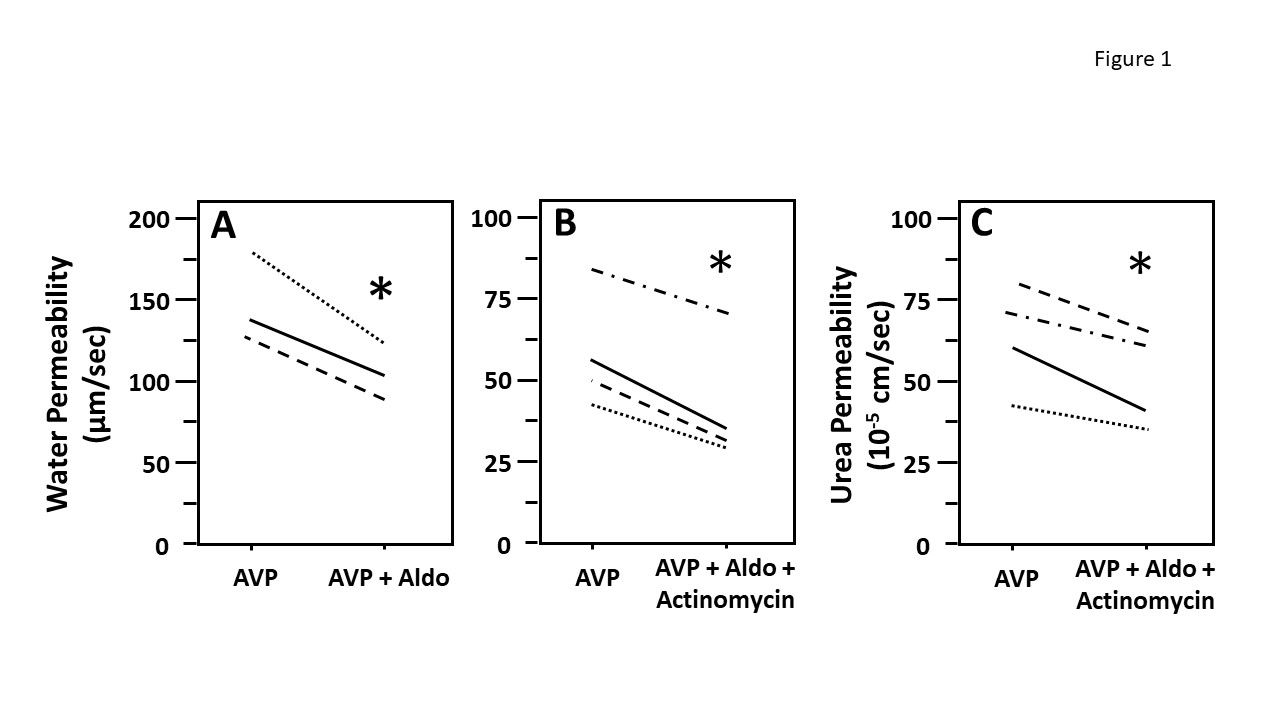
Figure 1: Aldosterone decreased vasopressin-stimulated osmotic water and urea permeability in terminal inner medullary collecting ducts (IMCDs) in the presence of actinomycin D. Terminal IMCDs were perfused with 50 pM vasopressin (AVP) for 20 min, samples were taken for analysis, then 500 nM aldosterone (Aldo) was added to the bath for 30 min, after which samples were taken for analysis (A). In a separate experiment, terminal IMCDs were perfused with 50 pM vasopressin (AVP), then treated with 1 µM actinomycin for 30 min before adding 500 nM aldosterone (Aldo) for 30 min. Samples were analyzed for either raffinose (water permeability marker, panel B) or urea content (panel C). Each line represents a separate IMCD from a different rat. * P < 0.05 AVP control vs. AVP with Aldo plus actinomycin, N = 4 rats/condition.
2. Discussion
Our research group expanded the knowledge to this field of research by investigating the effect of aldosterone on water and urea transport in isolated perfused rat IMCDs. We also tested the corresponding changes in the levels of phosphorylated AQP2. Our results showed that aldosterone inhibits vasopressin-stimulated water and urea reabsorption.
To determine whether aldosterone decreases osmotic water permeability we first perfused with vasopressin and then perfused with aldosterone in the presence of vasopressin. Aldosterone decreased vasopressin‐stimulated osmotic water permeability. We compared the water permeability in both male and female rat IMCDs. It appears that there may be a greater difference in aldosterone inhibition in female rats versus the male rats. However, further experiments need to be performed to examine whether the female rat is more susceptible to the inhibition of osmotic water permeability by aldosterone.
To test the role of aldosterone in the phosphorylation of AQP2, IM tissues were incubated with and without aldosterone. Tissue lysates were analyzed by western blot, probing for total AQP2, pSer256 AQP2, and pSer261 AQP2. Aldosterone decreased AQP2 phosphorylation at serine 256 in the presence of vasopressin, which may decrease AQP2 accumulation in the apical membrane. There was no observed change in the phosphorylation of serine 261 AQP2. Thus, the decrease in vasopressin‐stimulated water permeability by aldosterone may be related to the decrease in AQP2 trafficking to the plasma membrane rather than an increase in AQP2 endocytosis.
To test whether aldosterone attenuated osmotic water permeability through a genomic mechanism we compared the effect of aldosterone on water permeability in the absence and presence of actinomycin D (an inhibitor of gene transcription) (Figure 1). IMCDs were first perfused with vasopressin, then perfused with aldosterone in the absence (A) or presence (B) of actinomycin D. The decrease of vasopressin‐stimulated osmotic water permeability in response to aldosterone was not changed by the presence of actinomycin suggesting that aldosterone was working through a non‐genomic pathway. To test whether aldosterone decreases urea permeability through a genomic action, IMCDs were first perfused with vasopressin, then perfused with aldosterone in the presence of vasopressin and actinomycin D. Aldosterone significantly decreases vasopressin‐stimulated urea permeability in the presence of actinomycin D, suggesting that the aldosterone‐attenuated urea permeability is a non‐genomic action (Figure 1, panel C).
In addition, our data show that aldosterone inhibited water and urea reabsorption in 30 min. Since activation of genomic mechanisms is highly unlikely to happen in such a short period, we surmise that aldosterone rapidly inhibits water and urea absorption initially by a non‐genomic pathway. However, we cannot exclude the possibility that effects are attributed to aldosterone and not to actinomycin D based on the current data. The consistency of the results for aldosterone‐regulated water and urea permeability suggest that aldosterone can decrease urine concentrating ability in a rapid manner that does not involve genomic changes.
Our observations suggest that aldosterone is an independent regulator of water permeability in both male and female rats. The fact that aldosterone also reduces urea permeability in a rapid fashion suggests that the immediate regulation of urine concentrating ability involves the non-genomic action of aldosterone since water and urea transport processes are interdependent in the kidney inner medulla. The decrease in water permeability appears to be a result of a decrease in AQP2 phosphorylation at serine 256, which may decrease AQP2 accumulation at the plasma membrane. It is unclear whether the decrease in the urea transporter is due to a change in its phosphorylation state, but it seems likely based on the responses of the water channel. Aldosterone‐attenuated osmotic water and urea permeability are non‐genomic processes. The figure in this entry conveys data directly excised from our recent paper (1) that is the first report of aldosterone directly affecting vasopressin‐stimulated water and urea transport in the IMCD.
References
- Wang Y, Ma F, Rodriguez EL, Klein JD, Sands JM. Aldosterone Decreases vasopressin-Stimulated Water Reabsorption in Rat Inner Medullary Collecting Ducts. Cells. 2020 Apr 14;9(4). pii: E967
- Terker, A.S.; Ellison, D.H. Renal mineralocorticoid receptor and electrolyte homeostasis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309. R1068–R1070.
- Fuller, P.J.; Young, M.J. Mechanisms of mineralocorticoid action. Hypertension 2005, 46, 1227–1235.
- Scott, J.H.; Dunn, R.J. Physiology, Aldosterone. In StatPearls. Treasure Island, FL, , 2019.
- Cheng, L.; Poulsen, S.B.; Wu, Q.; Esteva-Font, C.; Olesen, E.T.B.; Peng, L.; Olde, B.; Leeb-Lundberg, L.M.F.; Pisitkun, T.; Rieg, T.; Dimke, H.; Fenton, R.A. Rapid Aldosterone-Mediated Signaling in the DCT Increases Activity of the Thiazide-Sensitive NaCl Cotransporter. J. Am. Soc. Nephrol. 2019, 30, 1454–1470.
- Pergher, P.S.; Leite-Dellova, D.; de Mello-Aires, M. Direct action of aldosterone on bicarbonate reabsorption in in vivo cortical proximal tubule. Am. J. Physiol. Renal Physiol. 2009, 296, F1185–F1193.
- Aboudehen, K.; Noureddine, L.; Cobo-Stark, P.; Avdulov, S.; Farahani, S.; Gearhart, M.D.; Bichet, D.G.; Pontoglio, M.; Patel, V.; Igarashi, P. Hepatocyte Nuclear Factor-1beta Regulates Urinary Concentration and Response to Hypertonicity. J. Am. Soc. Nephrol. 2017, 28, 2887–2900.
- Boldyreff, B.; Wehling, M. Non-genomic actions of aldosterone: Mechanisms and consequences in kidney cells. Nephrol. Dial. Transplant. 2003, 18, 1693–1695.
- Boldyreff, B.; Wehling, M. Rapid aldosterone actions: From the membrane to signaling cascades to gene transcription and physiological effects. J. Steroid Biochem. Mol. Biol. 2003, 85, 375–381.
- Good, D.W. Nongenomic actions of aldosterone on the renal tubule. Hypertension 2007, 49, 728–739.
- Sheader, E.A.; Wargent, E.T.; Ashton, N.; Balment, R.J. Rapid stimulation of cyclic AMP production by aldosterone in rat inner medullary collecting ducts. J. Endocrinol. 2002, 175, 343–347.
- Tumlin, J.A.; Lea, J.P.; Swanson, C.E.; Smith, C.L.; Edge, S.S.; Someren, J.S. Aldosterone and dexamethasone stimulate calcineurin activity through a transcription-independent mechanism involving steroid receptor-associated heat shock proteins. J. Clin. Invest. 1997, 99, 1217–1223.
- Ilori, T.O.; Wang, Y.; Blount, M.A.; Martin, C.F.; Sands, J.M.; Klein, J.D. Acute calcineurin inhibition with tacrolimus increases phosphorylated UT-A1. Am. J. Physiol. Renal Physiol. 2012, 302, F998–F1004.
- Ren, H.; Yang, B.; Ruiz, J.A.; Efe, O.; Ilori, T.O.; Sands, J.M.; Klein, J.D. Phosphatase inhibition increases AQP2 accumulation in the rat IMCD apical plasma membrane. Am. J. Physiol. Renal Physiol. 2016, 311, F1189–F1197.
- Sands, J.M.; Nonoguchi, H.; Knepper, M.A. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am. J. Physiol. 1987, 253, F823–F832.
- Brown, D.; Fenton, R.A. The cell biology of vasopressin action. In: Brenner and Rector’s The Kidney, Taal, M.W., Chertow, G.M., Marsden, P.A., Skorecki, K., Yu, A.S.L., Brenner, B.M., Eds; Elsevier: Philadelphia, PA, USA, 2011; pp. 353–383.
- Chou, C.L.; Yip, K.P.; Michea, L.; Kador, K.; Ferraris, J.D.; Wade, J.B.; Knepper, M.A. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J. Biol. Chem. 2000, 275, 36839–36846.
- Knepper, M.A.; Inoue, T. Regulation of aquaporin-2 water channel trafficking by vasopressin. Curr. Opin. Cell Biol. 1997, 9, 560–564.
- Hoffert, J.D.; Pisitkun, T.; Wang, G.; Shen, R.F.; Knepper, M.A. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: Regulation of aquaporin-2 phosphorylation at two sites. Proc. Natl. Acad. Sci. USA 2006, 103, 7159–7164.
- Wall, S.M.; Suk Han, J.; Chou, C.-L.; Knepper, M.A. Kinetics of urea and water permeability activation by vasopressin in rat terminal IMCD. Am. J. Physiol. 1992, 262, F989–F998.
- Zhang, C.; Sands, J.M.; Klein, J.D. Vasopressin rapidly increases phosphorylation of UT-A1 urea transporter in rat IMCDs through PKA. Am. J. Physiol. Renal Physiol. 2002, 282, F85–F90.
- Gertner, R.A.; Klein, J.D.; Bailey, J.L.; Kim, D.-U.; Luo, X.H.; Bagnasco, S.M.; Sands, J.M. Aldosterone decreases UT-A1 urea transporter expression via the mineralocorticoid receptor. J. Am. Soc. Nephrol. 2004, 15, 558–565.
- Nielsen, J.; Kwon, T.H.; Praetorius, J.; Frokiaer, J.; Knepper, M.A.; Nielsen, S. Aldosterone increases urine production and decreases apical AQP2 expression in rats with diabetes insipidus. Am. J. Physiol. Renal Physiol. 2006, 290, F438–F449.