
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gizem Gulfidan | + 1262 word(s) | 1262 | 2021-03-23 06:46:40 | | | |
| 2 | Catherine Yang | Meta information modification | 1262 | 2021-03-26 02:53:31 | | |
Video Upload Options
Pancreatic cancer is one of the most fatal malignancies and the seventh leading cause of cancer-related deaths related to late diagnosis, poor survival rates, and high incidence of metastasis.
1. Introduction
Pancreatic cancer is one of the most fatal malignancies and the seventh leading cause of cancer-related deaths considering both sexes worldwide according to the latest global cancer statistics reported in 2018 [1]. Pancreatic cancer has a difficult diagnosis at an early stage and a 5 year survival rate of 10% at the time of diagnosis in the United States, where the poor survival rates have hardly changed for almost 40 years since most patients reporting to the hospital have either unresectable or metastatic disease. Only 10.8% of these patients are at a locally advanced stage at the time of diagnosis [2][3]. Unfortunately, pancreatic cancer is projected to become the third leading cause of cancer deaths in the future [1].
Pancreatic cancer can be divided into two large groups; (a) endocrine pancreatic tumors, including gastrinoma, glucagonoma, and insulinoma, and (b) exocrine (non-endocrine) pancreatic tumors, including adenoma, ductal adenocarcinoma, acinar cell carcinoma, cystadenocarcinoma, adenosquamous carcinoma, signet ring cell carcinoma, hepatoid carcinoma, colloid carcinoma, undifferentiated carcinoma, pancreatoblastoma, and pancreatic mucinous cystic neoplasm [4][5]. Most of the pancreatic cancers are exocrine types—namely, ductal adenocarcinoma, which comprises 80–90% of all pancreatic cancers; whereas endocrine (neuroendocrine) pancreatic tumors are rare with 1–2% of all pancreatic cancers [6].
Like most cancer types, pancreatic cancer has also several known risk factors, such as cigarette smoking, diabetes, obesity, lack of physical activity, and chronic pancreatitis [7][8]. Currently, computed tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasound (EUS), positron emission tomography (PET), and other imaging methods are used in the diagnosis and prognosis of pancreatic cancer [7][8][9].
Unsurprisingly, early detection of PDAC by effective screening approaches is crucial to improve a better prognosis of the disease. The absence of clinical symptoms in the early stage of pancreatic cancer could lead to a delay in confirmed diagnosis even though tumor biomarkers and imaging techniques are being developed. Therefore, using circulating biomarkers for primary screening and its combination with imaging and histopathologic results might be the future strategy for diagnosing PDAC. Candidate circulating biomarkers in PDAC are not limited to circulating tumor cells (CTC) but also consist of metabolites, cell-free DNA and non-coding RNA, exosomes, autoantibodies, and inflammatory or growth factors, which are recently summarized [10]. The presence of CTCs in the blood usually correlates with the systemic spread of the tumor, and the characteristics of these CTCs could be used as potential biomarkers. Moreover, the challenging tasks of CTC isolation and detection are being overcome [11][12], and the emerging area of profiling CTCs has been recognized in prognosis of pancreatic cancer [13].
The most common way to get pancreatic tumor samples is by fine-needle aspiration (FNA). However, a core needle biopsy using a larger needle than an FNA can provide a larger sample, often useful for molecular profiling. These biopsies can be taken with an EUS. Other biopsy types, like brush biopsy or forceps biopsy, can be done during an endoscopic cholangiopancreatography (ERCP). However, body fluids such as blood, cyst fluid, pancreatic juice, bile, as well as urine are characteristically enriched with biomarkers that can be a potential source of diagnostic, predictive, and/or prognostic biomarkers in PDAC. As a source of pancreatic cancer biomarker, saliva has also been used. In omics biomarker studies, blood is a frequently preferred sample source due to its easy accessibility, noninvasiveness, and cost-effectiveness [14]. As an alternative rich source for the discovery of biomarkers, pancreatic juice has recently been identified. Pancreatic juice contains pancreatic cancer-specific markers such as DNA, RNA, proteins, and cancer cells, but the collection procedure for this sample source is invasive [15]. Although urine contains limited protein, DNA, and RNA, it can be considered as an ideal source sample for proteomic and genomic biomarkers [16]. Furthermore, accurate staging is very important for providing appropriate treatment. The majority of the time, surgical excision is used for treatment, and traditional chemoradiotherapy has very restricted effectiveness, despite the development of novel therapy options [6]. A systems-level outlook of PDAC biomarkers from different “omics” levels (Figure 1) as well as a comprehensive overview of methodology and sampling used in biomarker studies for PDAC.
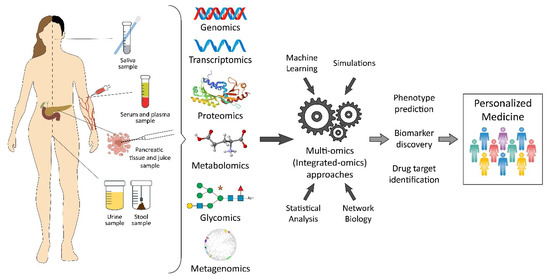
Figure 1. Pancreatic cancer biomarkers from a variety of “omics” levels.
2. Recent Insights from Different Omics Levels
Despite the substantial advancement in pancreatic cancer research, there has not been any remarkable reduction in the mortality-to-incidence ratio. This is mainly a result of the limited early diagnostic characteristic symptoms and reliable biomarkers, besides the unresponsiveness to the treatments due to the tumor heterogeneity, plasticity, and the aggressive metastasis that presents in more than 50% of the diagnosed patients [17].
Systems biology studies of pancreatic cancer rely on the integration of omics data from different biological levels. With the frequently arising challenges regarding cancer diagnosis and treatment—mainly due to its complex pathogenic landscape and cellular heterogeneity—the holistic view provided by the systems biology approach allowed for having a global understanding of the mechanisms of the disease and gaining more insight toward diagnostic or prognostic biomarkers and drug target discovery [18][19].
Likewise, systems biology also augments current diagnosis and therapy options. Aggressiveness and chemoresistance of PDAC are caused by the desmoplastic reactions induced by immune cells, stromal cells, neural cells, and the extracellular matrix surrounding and forming the bulk of the tumor mass. Therefore, single-cell sequencing may shed a better insight into cellular differences. Moreover, altered metabolism is caused by limited delivery of the needed oxygen and nutrients in such a hypoxic and acidic microenvironment; a direct impact on the drug delivery mechanisms is common [20][21].
3. Genomic Signatures
Next-generation sequencing (NGS) provides support for the early diagnosis and screening of PDAC as well as many other diseases. Genomics techniques may assist in the early diagnosis of pancreatic cancer in patients with specific alleles that predispose them to cancer development. Different potential biomarkers discovered by genomics methods can be categorized as chromosomal aberrations, driver changes, single nucleotide polymorphisms (SNPs), or copy-number alterations.
Previous studies pointed out the most prominent genetic features of PDAC, such as oncogenic activation of K-RAS, which is a standard feature in more than 90% of the patients, and with the early onset mutation of that gene, it is considered a critical driver of PDAC initiation and progression [22]. Along with the oncogenic activation, inactivating mutations of the tumor suppressor gene CDKN2A/2B are also observed in more than 80% of the early-stage lesions, while later stages of PDAC exhibit inactivating mutations and deletions of tumor suppressor genes most prominently including TP53 and SMAD4 [23].
Metabolic reprogramming is considered a prominent hallmark of PDAC. Therefore, tackling this aggressive cancer might be possible through establishing a clear understanding regarding its metabolism in addition to genomics [24]. Recent studies have shown the crucial role of both glucose and glutamine metabolism in the progression of PDAC tumors that are regulated by the K-RAS oncogene to maintain tumor growth [25][26][27]. Inducible oncogenic K-RAS mouse model of PDAC showed—in addition to being a key driver of PDAC initiation—that it plays a central role in rewiring the tumor glucose metabolism by stimulating the glucose uptake and driving glycolysis intermediates toward nonoxidative pentose phosphate pathways [26]. It was also reported that the PDAC cells maintain the tumor growth by relying on the distinct pathway of glutamine metabolism and that this reprogramming is mediated by K-RAS [25].
Therefore, not only genomics biomarkers but also network reconstructions [28], including different omics levels, become an essential tool for exploring the disease under the systems biology perspective. Network models and computational platforms for integrating and analyzing these data, as well as investigating more thoroughly into these networks by simulations, are prominent efforts.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30.
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060.
- Wolfgang, C.L.; Herman, J.M.; Laheru, D.A.; Al, E. Recent progress in pancreatic cancer. CA Cancer J. Clin. 2013, 63, 318–348.
- Goral, V. Pancreatic cancer: Pathogenesis and diagnosis. Asian Pac. J. Cancer Prev. 2015, 16, 5619–5624.
- Zhang, Q.; Zeng, L.; Chen, Y.; Lian, G.; Qian, C.; Chen, S.; Li, J.; Huang, K. Pancreatic Cancer Epidemiology, Detection, and Management. Gastroenterol. Res. Pract. 2016, 2016.
- Decker, G.A.; Batheja, M.J.; Collins, J.M.; Silva, A.C.; Mekeel, K.L.; Moss, A.A.; Nguyen, C.C.; Lake, D.F.; Miller, L.J. Risk factors for pancreatic adenocarcinoma and prospects for screening. Gastroenterol. Hepatol. 2010, 6, 246–254.
- Becker, A.E.; Hernandez, Y.G.; Frucht, H.; Lucas, A.L. Pancreatic ductal adenocarcinoma: Risk factors, screening, and early detection. World J. Gastroenterol. 2014.
- Costache, M.I.; Costache, C.A.; Dumitrescu, C.I.; Tica, A.A.; Popescu, M.; Baluta, E.A.; Anghel, A.C.; Saftoiu, A.; Dumitrescu, D. Which is the Best Imaging Method in Pancreatic Adenocarcinoma Diagnosis and Staging—CT, MRI or EUS? Curr. Health Sci. J. 2017, 43, 132–136.
- Zhang, X.; Shi, S.; Zhang, B.; Ni, Q.; Yu, X.; Xu, J. Circulating biomarkers for early diagnosis of pancreatic cancer: Facts and hopes. Am. J. Cancer Res. 2018, 8, 332–353.
- Tjensvoll, K.; Nordgård, O.; Smaaland, R. Circulating tumor cells in pancreatic cancer patients: Methods of detection and clinical implications. Int. J. Cancer 2014, 134, 1–8.
- Martini, V.; Timme-Bronsert, S.; Fichtner-Feigl, S.; Hoeppner, J.; Kulemann, B. Circulating tumor cells in pancreatic cancer: Current perspectives. Cancers 2019, 11, 1659.
- Amantini, C.; Morelli, M.B.; Nabissi, M.; Piva, F.; Marinelli, O.; Maggi, F.; Bianchi, F.; Bittoni, A.; Berardi, R.; Giampieri, R.; et al. Expression Profiling of Circulating Tumor Cells in Pancreatic Ductal Adenocarcinoma Patients: Biomarkers Predicting Overall Survival. Front. Oncol. 2019, 9, 1–13.
- Zhang, W.H.; Wang, W.Q.; Han, X.; Gao, H.L.; Li, T.J.; Xu, S.S.; Li, S.; Xu, H.X.; Li, H.; Ye, L.Y.; et al. Advances on diagnostic biomarkers of pancreatic ductal adenocarcinoma: A systems biology perspective. Comput. Struct. Biotechnol. J. 2020, 18, 3606–3614.
- Bhat, K.; Wang, F.; Ma, Q.; Li, Q.; Mallik, S.; Hsieh, T.; Wu, E. Advances in Biomarker Research for Pancreatic Cancer. Curr. Pharm. Des. 2012, 18, 2439–2451.
- Young, M.R.; Wagner, P.D.; Grosh, S.; Al, E. Validation of Biomarkers for Early Detection of Pancreatic Cancer: Summary of the Alliance of Pancreatic Cancer Consortia for Biomarkers for Early Detection Workshop. Pancreas 2019, 47, 135–141.
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic ductal adenocarcinoma: Current and evolving therapies. Int. J. Mol. Sci. 2017, 18, 1338.
- Tian, Q.; Price, N.D.; Hood, L. Systems Cancer Medicine: Towards Realization of Predictive, Preventive, Personalized, and Participatory (P4) Medicine. J. Intern. Med. 2012, 271, 111–121.
- Westerhoff, H.V.; Palsson, B.O. The evolution of molecular biology into systems biology. Nat. Biotechnol. 2004, 22, 1249–1252.
- Sousa, C.M.; Kimmelman, A.C. The complex landscape of pancreatic cancer metabolism. Carcinogenesis 2014, 35, 1441–1450.
- Liang, C.; Qin, Y.; Zhang, B.; Ji, S.; Shi, S.; Xu, W.; Liu, J.; Xiang, J.; Liang, D.; Hu, Q.; et al. Metabolic plasticity in heterogeneous pancreatic ductal adenocarcinoma. Bioch. Biophys. Acta Rev. Cancer 2016, 1866, 177–188.
- Löhr, M.; Klöppel, G.; Maisonneuve, P.; Lowenfels, A.B.; Lüttges, J. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: A meta-analysis. Neoplasia 2005, 7, 17–23.
- Bardeesy, N.; DePinho, R.A. Pancreatic cancer biology and genetics. Nat. Rev. Cancer 2002, 2, 897–909.
- Perera, R.M.; Bardeesy, N. Pancreatic Cancer Metabolism—Breaking it down to build it back up. Cancer Discov. 2015, 5, 1247–1261.
- Son, J.; Lyssiotis, C.A.; Ying, H.; Wang, X.; Hua, S.; Ligorio, M.; Perera, R.M.; Ferrone, C.R.; Mullarky, E.; Shyh-, N.; et al. Glutamine supports pancreatic cancer growth through a Kras- regulated metabolic pathway. Nature 2013, 496, 101–105.
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell 2012, 149, 656–670.
- Le, A.; Cooper, C.R.; Gouw, A.M.; Dinavahi, R.; Maitra, A.; Deck, L.M.; Royer, R.E.; Vander Jagt, D.L.; Semenza, G.L.; Dang, C.V. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA 2010, 107, 2037–2042.
- Alian, O.; Philip, P.; Sarkar, F.; Azmi, A. Systems Biology Approaches to Pancreatic Cancer Detection, Prevention and Treatment. Curr. Pharm. Des. 2014.





