
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | DAVID NALIN | + 2391 word(s) | 2391 | 2021-03-17 04:50:18 | | | |
| 2 | Bruce Ren | -148 word(s) | 2243 | 2021-03-25 02:44:37 | | |
Video Upload Options
The original studies demonstrating the efficacy of oral glucose-electrolytes solutions in reducing or eliminating the need for intravenous therapy to correct dehydration caused by acute watery diarrheas (AWD) were focused chiefly on cholera patients. Later research adapted the oral therapy (ORT) methodology for treatment of non-cholera AWDs including for pediatric patients. These adaptations included the 2:1 regimen using 2 parts of the original WHO oral rehydration solution (ORS) formulation followed by 1 part additional plain water, and a “low sodium” packet formulation with similar average electrolyte and glucose concentrations when dissolved in the recom-mended volume of water.
1. Introduction
Since 1990, controversies in the field of oral rehydration therapy (ORT) have arisen concerning efforts to preserve a single formulation for cholera and non-cholera acute watery diarrheas (AWD) in patients of all ages, while modifying the original WHO (Oralyte) oral rehydration solution (ORS) formulation containing 90 mEq/L Na+, to address three goals: (1) to safely provide effective rehydration and maintenance therapy for both cholera and non-cholera AWDs; (2) to reduce duration and volume of diarrhea and (3) to reduce the need for restarting intravenous fluids after completing rehydration, during the oral maintenance period [1][2]. The treatment of dysentery and inflammatory diarrheal diseases in which dehydration is not the main focus of therapy is beyond the scope of this report.
2. Basic Principles of Oral Therapy Methodology
Oral rehydration and maintenance therapy for significantly dehydrating AWD is not a magic bullet which works simply by giving patients ORS to drink; achieving optimal results requires administering the oral solutions according to the following well established ORT methodologic principles. Effective and safe ORT rests on the basic principle of AWD treatment: timely replacement of the water and electrolyte losses of AWD with matching volumes of an absorbable ORS with electrolyte content sufficient to replace the electrolyte losses of AWD. Deviation from this principle has resulted either in higher ORT failure rates with reversion to I.V. therapy and/or electrolyte abnormalities with potentially serious and avoidable sequelae of severe hyponatrema or hypernatremia.
Gross diarrhea rate (GDR), or the volume of diarrheal stool passed over a given unit of time, is far less important for successful ORT and optimal success rates than net gut balance, or the difference between diarrheal volume and volume of ORS intake in a given observation period. An excess of ORS intake volume over diarrheal volume during a given observation period, usually 4 or 6 h, is called positive net gut balance (PNGB), and early achievement of PNGB is key to successful ORT [3]. Moderate increases in GDR are of negligible consequence if exceeded by ORS intake.
Initial diarrhea rates after hospital admission for AWD determine subsequent total stool volume [4]. Two methods of prestratification of patients entered in research studies ensure validity of comparison groups: entry only of patients in shock due to dehydration, or, more elegantly, allocating patients after confirming comparable GDR rates during the several hours required for initial intravenous rehydration. Allocation based on any other criteria often leads to an imbalance in disease severity between comparison groups which can bias the outcomes and accounts for a significant amount of variability seen between different results from the different centers conducting these studies.
When severely dehydrated patients arrive at treatment centers for rehydration, they are generally at or past their peak diarrhea rate. At this point vomiting, sometimes massive at disease onset (e.g., cholera [5]), is waning, and may wane more rapidly with correction of acidosis using ORS or, if patients are in shock due to dehydration, using I.V. fluids containing bicarbonate or a base precursor [6]. Vomiting quickly subsides soon after initiating treatment of most patients arriving at treatment centers, and measured vomitus volumes are generally small in relation to diarrhea volumes in most patients. Failure to correct acidosis in severely dehydrated patients leads to increased risk of pulmonary edema during I.V. rehydration [7].
Maintaining PNGB using proper ORT methodology will allow sufficient net fluid absorption to replace insensible losses and, in addition, promote sufficient net absorption to permit use of ORS with sodium content modestly lower than that of diarrhea fluid (e.g., 120 mEq/L. in cholera patients), while maintaining positive electrolyte balance [8].
If inadequate oral intake results in delayed or failed achievement of PNGB, additional I.V. fluid will be needed. This can arise rarely from excess vomiting or more commonly from inadequate monitoring or supervision, leading to failure of pediatric patients to be given or to drink sufficient ORS. Also, pediatric diarrhea patients in endemic areas not uncommonly have malabsorption of glucose and dietary sugars [9][10] and will be at higher risk of ORT failure and hypernatremia due to excess water loss in diarrheal stools. Caregivers should be alert to such patients, who may require I.V. therapy, though they can respond to ORS formulations with glycine replacing glucose. Researchers must be careful to avoid disproportionately allocating such patients among comparison groups, to avoid confounding interpretation of outcome results.
Claims have been made, without presentation of objective quantitative evidence, that mothers are aware of gross stool volume. In the writer’s opinion, based on extensive experience, mothers are aware of time since onset and of subsequent duration of diarrhea, and of course would prefer to see it stop if asked; but this is different from perception of the small difference in stool volume measured in most studies of modified ORS formulations [11].
Even trained clinicians are unable to accurately guess diarrheal volumes without stool volume measurements, as shown when they were asked to guess the volume of synthetic diarrhea fluid tossed onto a bedsheet. Estimates were wildly inaccurate (Dr. Norbert Hirschhorn, personal communication). Mothers (and clinicians) would doubtless like to have available a drug which could stop the diarrhea in minutes, but no currently available ORS formulation does that.
Experience from many studies have confirmed that the taste and appearance of plain ORS do not influence ORS acceptability among moderately and severely dehydrated patients, consistent with a recent report regarding milder cases [12]. Sweetening the ORS has had a negative effect [13]. The enormous benefits of ORS result from preventing and quickly correcting signs of dehydration, which parents fully appreciate.
3. Home Therapy with the WHO 90 ORS Using the 2:1 Regimen
When no I.V. is available, patients need the early positive balance and superior retention this regimen affords, to replace pretreatment deficits and maintain positive water and electrolyte balance (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7).
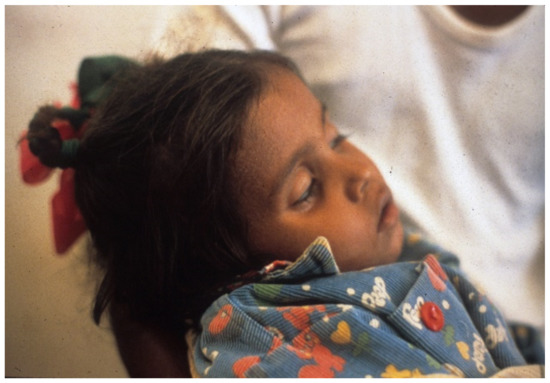
Figure 1. Severely dehydrated child in Greentown, Lahore, Pakistan. Note deeply sunken eyes and obtunded appearance. Etiology unknown.
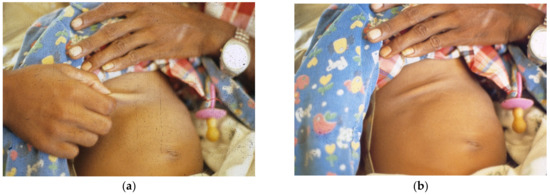
Figure 2. (a) Father pinches abdominal skin as instructed, (b) showing tenting indicating decreased elasticity after withdrawing hand.
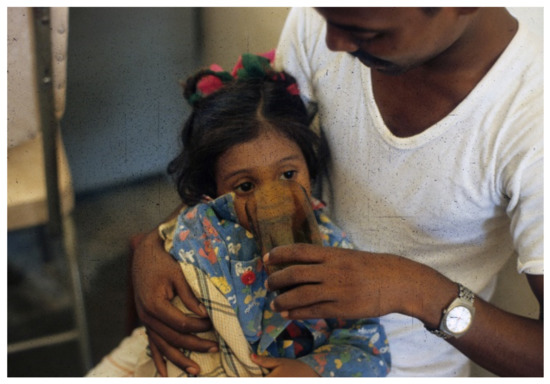
Figure 3. Father begins to offer patient oral rehydration solution (ORS) (WHO 90 formulation) to drink.
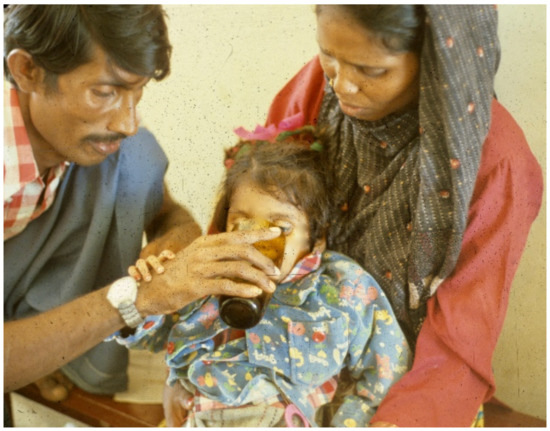
Figure 4. Patient continues to drink, using hand to keep ORS coming.
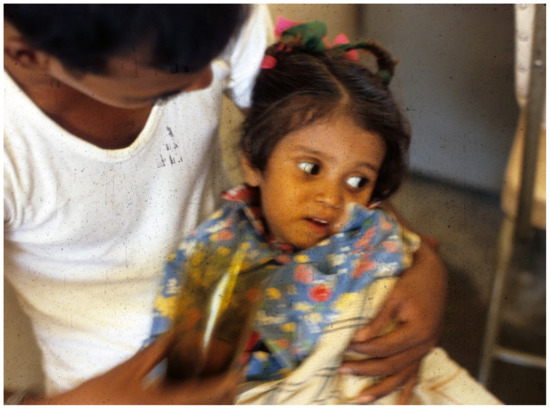
Figure 5. Patient now more alert, eyes less sunken at 1 h after starting ORS.
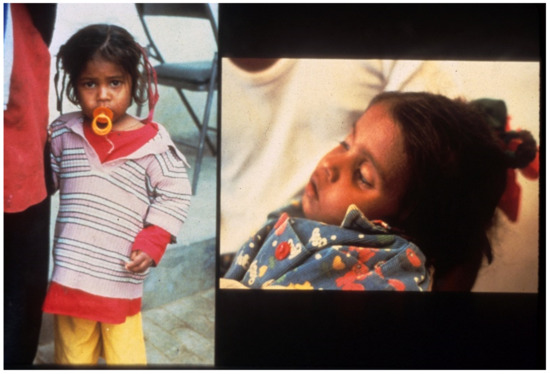
Figure 6. Patient after recovery, with pretreatment appearance on right.

Figure 7. The ultimate goal: another child with acute watery diarrheas (AWD) starting ORS to prevent becoming dehydrated.
4. The Evolution of ORT and ORS
The first successful clinical trial of ORT [8] was successful based on the combination of an appropriate ORS and, importantly, an effective method of administering the solution. The ORS formulation used had an electrolyte content approximating that of cholera diarrheal fluid losses, with sodium halfway between that of pediatric and adult cholera diarrhea fluid, and 110 mMol/L. glucose, without which the patients could not absorb the sodium, chloride and water essential to effective therapy.
ORS formulations using a range of sodium levels, chiefly 90 mEq/L, with glucose as the substrate and additional water intake (or breast milk feedings) permitted, have proven effective in treating dehydration in patients with cholera, nonvibrio cholera (caused by enterotoxigenic Escherichia coli and other organisms producing cholera toxin analogs) and non-cholera AWD including rotavirus diarrhea [14] and have decimated global under-five AWD mortality [15][16]. However, ORS formulations with Na+ content significantly below patients’ stool Na+ losses lead to negative sodium balance, Na+ depletion with hyponatremia and heightened risk of hyponatremic complications, whether combined with glucose, rice or other substrates [17][18].
The therapeutic method which proved essential [3][8] to overcoming the failures of earlier ORT trials [19] consisted of rapid initial correction of shock when present on admission, using I.V. rehydration. Oral therapy began as soon as shock was corrected, generally after administering I.V. fluid equivalent to 10% of admission body weight (in populations with BMIs (body mass index) below Western levels.) Initially, oral therapy was administered at a rate of 0.5 to 0.75 L/h in adults, based on body weight [8]. If the GNB (gut net balance: see PNGB above) monitored during the first 4 h indicated a fluid requirement greater than that estimated on admission, oral therapy was increased to match the volume of losses. Hydration status was monitored by checking plasma sp. gr. during the transition to oral maintenance. A rise over 1.030 was an indication for additional I.V. fluids to avoid progression to severe dehydration, estimation of hydration status based on clinical signs alone being less sensitive and more dependent on variable subjective criteria.
The ORS formulation used contained 120 mEq/L of Na+ (ORS 120), suitable for treating cholera and nonvibrio cholera patients. Using this method, total I.V. fluid requirements of cholera patients admitted in shock averaged 80% less than in controls and plasma Na+ remained normal. Patients not in severe shock were rehydrated and maintained in water and electrolyte balance using ORT alone without I.V. fluids [20][21]. Since cholera patients given effective adjunct antibiotic therapy [22], and most hospitalized non-cholera AWD patients, have steadily declining stool volume after treatment begins [4], closely matching ORS volume and composition imbibed in each sequential 4 or 6 h period to volume and composition of losses in the prior period by using fluids of appropriate composition ensures maintenance of PNGB of both water and electrolytes [3]. In the first large-scale field trial using this method [23], total I.V. requirements averaged 3.0 L in cholera patients arriving in shock whose average admission weight was 40 kg. Nonvibrio cholera patients not in shock on admission were successfully treated using glucose or glucose+glycine ORT alone with no I.V. fluids. Plasma sodium remained normal [21].
The ORS 90 formulation with 90 mEq/L of sodium (abbreviated “WHO 90” here because the Oralyte name has been copied by other ORS brands) was devised as a “compromise” between a formulation approximating the mean composition of cholera diarrhea fluid and that of noncholera pediatric AWDs. Since diarrheal stool content of sodium (directly) and potassium (inversely) correlate closely with diarrhea rate [24] (Figure 8) and cholera diarrhea rates are greatly in excess of average non-cholera AWD rates, the “compromise” provided excess sodium and insufficient potassium for pediatric non-cholera AWD patients and insufficient sodium for pediatric and adult cholera patients, if the method of matching fluid losses with equal volumes of ORS was used. After the initial clinical trial of the WHO ORS 90 formulation [25], it was noted [26] that, to maintain positive sodium balance using this formulation in cholera patients, the patients would have to drink an amount of the ORS equivalent to one and one-half times their diarrhea volume of the previous intake and output period, rather than simply matching that volume, in order to avoid negative Na+ balance and hyponatremia. Some patients would be unable to imbibe such large volumes over 24–32 h, leading to increased failure rates. However, the 1.5 X losses requirement was not promoted for general use, though it obviated the need for a separate ORS formulation for cholera. In research studies, however, it has been often matched or exceeded, somewhat confounding the conclusions regarding formulation impact per se [27][28][18][29].

Figure 8. Relationship of diarrheal sodium and potassium losses (mEq/L) to stooling rate in 37 cholera patients during a period of maximum diarrhea 12–24 h after admission. At all ages, stool sodium tends to rise and potassium to fall at higher diarrhea rates. The numbers of patients were: 12 (0–4 yrs old), 10 (5–9 yrs), 6 (10–14 yrs), 2 (15–19 yrs), 7 (20 yrs and over). From Lancet, 30 October 1976, p. 957.
The WHO 90 formulation was nonetheless highly successful in reducing global diarrheal mortality, since it was within the range of effective formulations suitable for most mild and moderate AWDs, in which ORS intake is limited by low diarrhea volume and short duration; any “extra” sodium in that formulation is needed to replace pretreatment losses when ORS 90 is used for both rehydration and maintenance without I.V. rehydration. In addition, allowance of extra free water (or breast milk [30]) given to pediatric AWD patients, either permissively (“ad libitum”) or in the fixed 2:1 ratio [31] prevented hypernatremia, the 2:1 method having the added safety factor of offering protection against instances of wrongly mixed hyperconcentrated ORS preparations. Also, the 90 mEq/L ORS proved safe and effective for treatment of noncholera AWDs in neonates and of children with hypo- or hypernatremia on admission when the 2:1 regimen was used [32]. The 2:1 regimen permitted use of the WHO 90 ORS packet in noncholera pediatric diarrhea patients and had the advantage of promoting early PNGB while avoiding transient hypernatremia [33].
In cholera patients, the inevitable high incidence of negative sodium balance, hyponatremia and, in some patients, seizures and other complications of hyponatremia, was overlooked until 2006 (10) due to lack of sodium balance studies plus inadequate safety surveillance for several decades in those areas where cholera was highly prevalent. The fundamental differences between cholera (including nonvibrio cholera) and other AWDs in magnitude of losses and pathophysiology underline the inferior efficacy for maintenance of Na+ balance and for avoidance of hyponatremia when ORS formulations with glucose or rice with ORS 90 or less are used to treat cholera patients.
References
- WHO. Reduced Osmolarity Oral Rehydration Salts (ORS) Formulation; Department of Child and Adolescent Health and Development. Available online: (accessed on 20 October 2020).
- Musekiwa, A.; Volmink, J.; Cochrane Infectious Diseases Group. Oral Rehydration Salt Solution for Treating Cholera: ≤270 mOs/L solutions vs. ≤310 mOsm/L Solutions. Cochrane Database Syst. Rev. 2011, 2011, CD003754.
- Nalin, D.R. Oral therapy for diarrheal diseases. J. Diarrheal Dis. Res. 1987, 5, 283–292.
- Hirschhorn, N.; Kinzie, J.I.; Sachar, D.B.; Northrup, R.S.; Taylor, J.O.; Ahmad, S.Z.; Philllips, R.A. Decrease in net stool output in cholera during intestinal perfusion with glucose-containing solutions. NEJM 1968, 279, 176–181.
- Nalin, D.R.; Levine, M.M.; Hornick, R.; Bergquist, E.; Hoover, D.; Holley, H.P.; Waterman, D.; Van Blerk, J.; Matheny, S.; Sotman, S.; et al. The problem of emesis during oral glucose-electrolytes therapy given from the onset of severe cholera. Trans. Roy. Soc. Trop. Med. Hyg. 1979, 73, 10–14.
- Cash, R.A.; Nalin, D.R.; Toaha, K.M.M.; Huq, Z. Acetate in the correction of acidosis secondary to diarrhea. Lancet 1969, 294, 302–303.
- Harvey, R.M.; Enson, Y.; Lewis, M.L.; Greenough, W.B.; Ally, K.; Panno, R.A. Hemodynamic studies on cholera, Effects of hypovolemia and acidosis. Circulation 1968, 37, 709–728.
- Nalin, D.R.; Cash, R.A.; Islam, R.; Molla, M.; Phillips, R.A. Oral maintenance therapy for cholera in adults. Lancet 1968, 292, 370–373.
- El-Mougi, M.; Hendaw, A.; Koura, H.; Hegazi, E.; Fontain, O.; Pierce, N.F. Efficacy of standard glucose-based and reduced-osmolarity maltodextrin-based oral rehyration solutions: Effect of sugar malabsorption. Bull. WHO 1996, 74, 471–477.
- Lifschitz, F.; Coello-Ramirez, P.; Gutierrez, L.L.C. Monosaccharide intolerance and hypoglycemia in infants with diarrh74, 471-477.ea: Metabolic studies in 23 infants. J. Peds 1970, 77, 604–612.
- Hirschhorn, N.; Nalin, D.R.; Cash, R.A. CHOICE Study Group Trial. Pediatrics 2002, 109, 713–715.
- Saniel, M.C.; Zimicki, S.; Carlos, C.C.; Maria, A.C.S.; Balis, A.C.; Malacad, C. J. Diarrhoeal Dis. Res. 1997, 15, 47–52.
- Isolauri, E. Evaluation of an oral rehydration solution with Na+ 60 mmol/L. in infants hospitalized for acute diarrhea or treated as outpatients. Acta Paediatr. Scand 1985, 74, 643–649.
- Nalin, D.R.; Levine, M.M.; Mata, L.; de Cespedes, C.; Vargas, W.; Lizano, C.; Loria, A.R.; Simhon, A.; Mohs, E. Oral rehydration and maintenance of children with rotavirus and bacterial diarrheas. Bull. WHO 1979, 57, 453–459.
- Snyder, J.D.; Merson, M.H. The magnitude of the problem of acute diarrheal disease: A review of active surveillance data. Bull. WHO 1982, 60, 506–513.
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional and national morbidity, mortality and aetiologies of diarrhea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 18, 1211–1228.
- Alam, N.H.; Majumder, R.N.; Fuchs, G.J.; The CHOICE study group. Efficacy and safety of oral rehydration solution with reduced osmolarity in adults with cholera: A randomized double-blind clinical trial. Lancet 1999, 354, 296–299.
- Dutta, M.K.; Bhattacharya, M.K.; Deb, A.K.; Sarkar, A.; Chatterjee, A.; Biswas, A.B.; Chatterjee, K.; Nair, G.B.; Bhattacharya, S.K. Evaluation of oral hypo-osmolar glucose-based and rice-based oral rehydration solutions in the treatment of cholera in children. Acta Paediatr. 2000, 89, 787–790.
- Ruxin, J. Magic Bullet: The history of oral rehydration therapy. Med. Hist. 1994, 38, 363–397.
- Cash, R.A.; Nalin, D.R.; Forrest, J.; Abrutyn, E. Rapid correction of the acidosis and dehydration of cholera with an oral solution. Lancet 1970, 296, 549–550.
- Nalin, D.R.; Cash, R.A. Oral or nasogastric maintenance therapy for diarrheas of unknown etiology resembling cholera. Trans. Roy. Soc. Trop. Med. Hyg. 1970, 64, 769–771.
- Greenough, W.B.; Gordon, R.S., Jr.; Rosenberg, I.S.; David, B.I.; Benenson, A.H. Tetracycline in the treatment of cholera. Lancet 1964, 1, 335–337.
- Cash, R.A.; Nalin, D.R.; Rochat, R.; Reller, B.; Haque, E.; Rahman, M. A clinical trial of oral therapy in a rural cholera treatment center. Am. J. Trop. Med. Hyg. 1970, 19, 653–656.
- Nalin, D.R.; Cash, R.A. Sodium content in oral therapy for diarrhea. Lancet 1976, 2, 957.
- Pierce, N.F.; Banwell, J.G.; Mitra, R.C.; Caranasos, G.J.; Keimowitz, R.I.; Mondal, A.; Manji, P.M. Effect of intragastric glucose-electrolyte infusion upon water and electrolyte balance in Asiatic cholera. Gastroenterology 1968, 55, 333–344.
- Nalin, D.R. Oral Cholera Therapy. Ann. Intern. Med. 1970, 72, 288–289.
- Faruque, A.S.G.; Mahalanabis, D.; Hamadani, J.D.; Zetterstrom, R. Reduced osmolarity oral rehydration salt in cholera. Scan. J. Infect Dis. 1996, 28, 87–90.
- Bhattacharya, M.K.; Bhattacharya, S.K.; Dutta, D.; Deb, A.K.; Deb, M.; Dutta, A.; Saha, C.A.; Nair, G.B.; Mahalanabis, D. Efficacy of oral hyposmolar glucose-based and rice-based oral rehydration salt solutions in the treatment of cholera in adults. Scan. J. Gastroent. 1998, 33, 159–163.
- Molla, A.M.; Ahmed, S.M.; Greenough, W.B., III. Rice-based oral rehydration solution decreases the stool volume in acute diarrhea. Bull. WHO 1985, 63, 751–756.
- Roy, S.K.; Rabbani, G.H.; Black, R.E. Oral rehydration solution safely used in breast-fed children without additional water. J. Trop. Med. Hyg. 1984, 87, 11–13.
- Nalin, D.R.; Levine, M.M.; Mata, L.; de Cespedes, C.; Vargas, W.; Lizano, C.; Loria, A.R.; Simhon, A.; Mohs, E. Comparison of sucrose with glucose in oral therapy of infant diarrheas. Lancet 1978, 312, 277–279.
- Pizarro, D.; Possada, G.; Villavicencio, N.; Mohs, E.; Levine, M.M. Hypernatremic and hyponatremic diarrheal dehydration. Treatment with oral glucose-electrolyte solution. Am. J. Dis. Child. 1983, 137, 730–734.
- Nalin, D.R.; Harland, E.; Ramlal, A.; Swaby, D.; McDonald, J.; Gangarosa, R.; Levine, M.; Akierman, A.; Antonine, M.; Mackenzie, K.; et al. Comparison of low and high sodium and potassium content in oral rehydration solutions. J. Pediatr. 1980, 97, 848–853.




