
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dong-Soon Im | + 3780 word(s) | 3780 | 2021-02-10 05:07:28 | | | |
| 2 | Camila Xu | Meta information modification | 3780 | 2021-03-25 07:04:04 | | |
Video Upload Options
Oleoylethanolamide and palmitoylethanolamide are members of the fatty acid ethanolamide family, also known as acylethanolamides. Their physiological effects, including glucose homeostasis, anti-inflammation, anti-anaphylactic, analgesia, and hypophagia, have been reported. They have affinity for different receptor proteins, including nuclear receptors such as PPARα, channels such as TRPV1, and membrane receptors such as GPR119 and GPR55. In the present review, the pathophysiological functions of fatty acid ethanolamides have been discussed from the perspective of receptor pharmacology and drug discovery.
1. Introduction
Arachidonylethanolamide (AEA, also known as anandamide), oleoylethanolamide (OEA), and palmitoylethanolamide (PEA) are members of the acylethanolamide or fatty acid ethanolamide family, and are each composed of a fatty acid and an ethanolamine linked together by an amide bond [1]. These acylethanolamides are present in both animals and plants [2][3]. In particular, the biosynthesis, degradation, and pharmacological actions of acylethanolamides have been studied [4].
Although all acylethanolamides have a common ethanolamide structure, they differ with respect to the attached fatty acid. Therefore, specific receptor proteins recognize them by their differences in the chain length and degree of unsaturation of the fatty acids, which act as agonists/antagonists or activators/blockers. AEA has been extensively studied, and has been identified as an endogenous ligand of cannabinoid CB1 and CB2 receptors, along with another ligand, 2-arachidonyl glycerol [5]. Two G protein-coupled receptors, GPR119 and GPR55, are implicated as membrane surface receptors for OEA and PEA, respectively [6][7].
2. OEA and GPR119
2.1. Physiological Actions of OEA
2.1.1. Anorectic Action of OEA
OEA was initially described as a lipid that induces anorexia and body weight loss via altering peripheral signals in rodent models [8][9][10]. Currently, OEA is known to be generated from hydrolysis of dietary lipids in the intestinal lumen [8][9][10]. OEA suppresses appetite via various mechanisms, and thus, reduces food intake. Indeed, several targets, such as transient receptor potential vanilloid 1 (TRPV1), peroxisome proliferator-activated receptor-α (PPARα), and GPR119, have been investigated for the anorectic action of OEA.
Further, OEA delays meal initiation and causes reduction in meal size and meal frequency [11]. However, it did not influence food intake when injected into cerebral ventricles; its anorexic action was inhibited by blockage of peripheral sensory fibers upon treatment with capsaicin, implying peripheral regulation on feeding [8]. Food intake stimulates mucosal cells in the duodenum and jejunum to generate OEA. Localized OEA production through food intake in the small intestine of rodents is sufficient to induce a satiety state similar to that induced by systemic OEA treatment [12][13], suggesting that OEA serves as a local messenger for satiety that excites peripheral vagal sensory nerves.
OEA also enhances the magnitude of currents evoked by TRPV1 [14][15]. OEA was reported to induce increase in Ca2+ concentrations in capsaicin-sensitive sensory neurons, which were inhibited by capsazepine, a TRPV1 blocker [14][15]. Therefore, TRPV1 was proposed as a potential target of OEA and might be responsible for an excitatory action of OEA on sensory nerves, resulting in satiety response [14][15].
OEA also showed high affinity to PPARα, a nuclear receptor that regulates lipid metabolism. Its administration also induced satiety and body weight loss in wild-type mice but not in PPARα knockout mice, suggesting its role in PPARα activation [16][17].
Weight loss has also been observed after in vivo administration of several GPR119 agonists, such as AR231453, PSN632408, and YH18421 [18][19][20][21]. However, the anorectic action of OEA seems not to be mediated through GPR119 because intraperitoneal injection of OEA suppressed food intake in both wild-type and GPR119 knockout mice; moreover, off-target effects were proposed for AR231453 and PSN632408 [21][22][23]. Recently, OEA was also reported to suppress food intake via activating central oxytocin transmission [24].
Therefore, endogenous formation of OEA and other acylethanolamines in the small intestine may play an important role in food intake regulation through PPARα signaling and vagus nerve stimulation to the appetite center in the brain (Figure 1). A chronic high-fat diet causes a decrease in the endogenous levels of anorectic OEA in the intestine and increase in food intake or a hyperphagic effect [13][25].
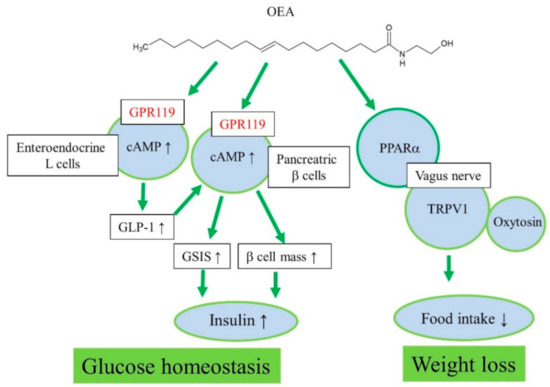
Figure 1. Proposed action mechanisms of OEA for regulation of glucose homeostasis and weight loss.
2.1.2. Analgesic and Other Actions of OEA
OEA showed analgesic action in an experimental animal model; it reduced nociceptive responses that were generated by administration of acetic acid and formalin [26]. It has also been reported to suppress inflammatory and visceral responses through a PPARα-activation independent mechanism [26]. Further, administration of sub-analgesic doses of MK-801 (N-methyl-D-aspartate receptor antagonist) and OEA together induced an analgesic effect, implying the contribution of glutamatergic transmission in the antinociceptive action of OEA [26].
Luminal or blood-borne GPR119 agonism by OEA and another agonist, 2-oleoyl-glycerol, can stimulate peptide tyrosine-tyrosine (PYY) release from enteroendocrine L-cells; subsequent paracrine PYY signaling through the Y1 receptor retards colonic motility [27]. The modulation of colonic motility by GPR119 activation was not observed in GPR119 knockout tissue [27]. Plasma levels of OEA and AEA were increased in patients with Crohn’s disease and ulcerative colitis, and levels of lysophosphatidylinositols 18:0 and 20:4 were higher in patients with inflammatory bowel disease than in control subjects. In addition, levels of GPR119 and CB1 transcripts were decreased in patients with Crohn’s disease [28], implying the significance of OEA and GPR119 in inflammatory bowel diseases.
OEA treatment has also been shown to increase the abundance of Akkermansia muciniphila, a mucin-degrading bacterium in the human intestine having an anti-inflammatory effect, suggesting its use in the treatment of obesity [29]. OEA inhibits osteoclast resorptive function by disrupting cytoskeletal organization, not related to RANKL-induced osteoclast differentiation [30]. It also enhances the differentiation of SZ95 human sebocytes, resulting in increased lipogenesis, promotion of granulated tissue formation, and induction of early apoptotic events; furthermore, OEA shifted the cells to a pro-inflammatory phenotype, resulting in increased production of pro-inflammatory cytokines by these cells [31]. GPR119 expression was downregulated in the sebaceous glands of patients with acne, implying OEA/GPR119 signaling as a positive regulator of sebocyte differentiation [31]. Therefore, OEA and GPR119 have been implicated in analgesia, colon motility and colitis, regulation of microbiota, and bone and skin biology.
2.2. Pharmacology of GPR119
2.2.1. GPR119 Expression
Initially, GPR119 was cloned as an orphan GPCR [32] and its expression was found to be predominantly high in insulin-secreting β-cell and GLP-1-producing L-cells, present in the pancreas and the gastrointestinal tract, respectively [21][33][34][35][36]. Additionally, GPR119 mRNA was highly expressed in GIP-producing K-cells and CCK-producing I-cells derived from murine small intestinal cells [37][38][39]; its expression was also reported in rodent brain and human fatal liver [40]. In addition, pancreatic polypeptide (PP)-producing cells and glucagon-producing islet α-cells were suggested to express GPR119 [36][41][42].
2.2.2. GPR119 Ligands
In 2002, Bonini et al. observed constitutive activation of Gαs proteins in GPR119-expressing cells [40], which was consistently observed in later studies as well [21]. In 2005, Soga et al. first reported oleoyl-lysophosphatidylcholine as a ligand for GPR119, along with other lysolipids [33]. In 2006, Overton et al. reported OEA as a ligand for GPR119, along with other acylethanolamides [18]. Later, N-oleoyldopamine and 5-hydroxy-eicosapentaenoic acid were also considered as endogenous GPR119 agonists [43][44]. The most potent natural ligands reported were OEA and N-oleoyldopamine [18][43]. Therefore, the derivatives of oleic acid showed similar low micromolar potency and full efficacy at GPR119 receptors [18][43]. 2-Oleoyl-glycerol has also been proposed as an endogenous GPR119 ligand, formed during digestion of fats, which activates L-cells to produce GLP-1 in humans [45][46].
2.2.3. GPR119 Functions
GPR119 expression in pancreatic β-cells and intestinal L-cells makes it as an attractive therapeutic target in type 2 diabetes; stimulation of glucose-dependent insulin secretion from β-cell by increased plasma GLP-1 released by L-cells provides new avenues for diabetes treatment using GLP-1 analogs and dipeptidyl peptidase 4 inhibitors.
Pancreas
Initially, oleoyl-lysophosphatidylcholine was found to induce insulin secretion from a mouse pancreatic β-cell line, NIT-1, in a GPR119-dependent manner [33]. Oleoyl-lysophosphatidylcholine and OEA increased cAMP and insulin secretion in MIN6c4 and RINm5f insulinoma cells (Figure 1) [23]. Chu et al. reported that activation of GPR119 by AR231453, a specific GPR119 agonist, improved glucose homeostasis via cAMP-mediated enhancement of glucose-dependent insulin release in both rodent islets and the hamster β-cell line, HIT-T15 cells [21]. Further, AR231453 promoted glucose-dependent insulin release in vivo and improved oral glucose tolerance in wild-type mice, but not in GPR119 knockout mice [21]. N-Oleoyldopamine induced cAMP accumulation in GPR119-transfected cells as effectively as OEA [43]. Oral administration of N-oleoyldopamine to C57bl/6 mice induced significant improvement in glucose tolerance, whereas GPR119 knockout mice were not responsive [43].
Further, GPR119 mRNA levels were elevated in islets of obese hyperglycemic db/db mice compared with those in control islets, implying a possible involvement of GPR119 in the development of diabetes and obesity [41]. In addition, maintaining β-cell mass is very important for glucose homeostasis in diabetic patients. OEA and PSN632408 could stimulate the replication of β-cells in vitro and in vivo, improving islet graft function. GPR119 is a novel therapeutic target for increasing β-cells mass and improving islet graft function via enhancing β-cell replication [20]. However, the cytoprotective actions of OEA in BRIN-BD11 or INS-1E cells do not require GPR119 activation; rather, internalized and hydrolyzed free oleate mediate the cytoprotection [47].
GI Tract
GPR119 mRNA was detected in all L-cell models, including murine GLUTag, human NCI-H716, and primary fetal rat intestinal L-cells [35]. OEA increased GLP-1 secretion from intestinal L-cells through GPR119 activation in vitro and in vivo (Figure 1) [35]. In GLUTag-Fla cells, AR231453 induced cAMP accumulation and GLP-1 release [34]. Moreover, AR231453 increased active GLP-1 levels within 2 min after oral glucose delivery in mice, and substantially enhanced the levels of glucose-dependent insulinotropic peptide, but no such induction was observed in GPR119 knockout mice [34]. Nutrient-stimulated GLP-1 release was attenuated in GPR119 knockout mice [22]. Thus, GPR119 regulates glucose tolerance by acting on both intestinal L-cells and pancreatic β-cells [34]. 2-Oleoyl-glycerol activated GPR119 and caused an increase in GLP-1 in the human intestine [45]. Similar results have been observed with other GPR119 agonists in vitro and in vivo [18][48][49][50][51]. AR231453 also induced an increase in GPR119-activation dependent plasma GIP levels [34]. In various animal models of obesity and type 2 diabetes, orally available, potent, selective, synthetic GPR119 agonists lowered blood glucose without hypoglycemia, slowed diabetes progression, and reduced food intake and body weight [46][52][53][54][55][56][57].
2.3. Drug Development
Restricted expression and distribution of GPR119 in pancreatic β-cell and intestinal L-cells have drawn great attention in the pharmaceutical industry owing to its potential role in glucose homeostasis. Many drug candidates have been developed based on the dual mechanism of action of GPR119—direct insulin secretion from β-cells and indirect secretion through GLP-1 activation, respectively. In addition, GPR119 agonists with unique scaffolds and signaling properties have been developed [18][48][49][50][51][53][54][55][56][57]. As mentioned above, GPR119 is coupled to Gαs proteins in the gut and pancreas and activated to induce release of GLP-1 and GIP from L-cells and K-cells and of insulin from β-cells. However, basal constitutive activity of GPR119 has been observed, and OEA has demonstrated differential modulation of GPR119-mediated signaling; the potency profiles of OEA with regard to Gαq, Gαi, β-arrestin recruitment, and downstream cAMP response element and serum response element are variable [58]. Many GPR119 agonists, such as GSK1292263, APD668, APD5997, MBX-2982, BMS-903452, LEZ763, ZYG-19, and PSN821, have been developed for type 2 diabetes and obesity [53][54][55][56][57]. However, several clinical trials have failed because repeated administration did not reduce the mean glucose levels in these trials [56][58][59][60].
Recently, continuing efforts on new GPR119 agonist development has resulted in novel structures with better potency and efficacy [19][48][61][62][63]. In particular, DS-8500a has shown positive clinical outcomes for 12 weeks in Japanese patients with type 2 diabetes; the outcomes included HbA1c lowering, glucose lowering, and lipid profile improvement [63]. The sustained efficacy of DS-8500a on glucose control for 12 weeks has revealed an opportunity to overshadow previously failed clinical trials of JNJ-38431055 and GSK1292263 [56][59]. DS-8500a administration also showed improvement in lipid profile, which has been supported by previous preclinical and clinical studies of GSK1292263, PSN821, and JTP-109192 [50][64][65]. These results were also consistent with the results of OEA treatment significantly decreasing plasma triglyceride levels [66].
In addition to the hypophagic effect of OEA, AR231453 induced a reduction in feeding behavior, but only at higher doses than those for glucose homeostasis [21]; also, the hypophagic effects did not disappear in GPR119 knockout mice [34]. In rat models, administration of PSN632408, a synthetic GPR119 agonist, suppressed food intake and reduced body weight gain and white adipose tissue deposition [18], but through GPR119-independent pathways [23]. These results exclude the role of GPR119 as a therapeutic target for the hypophagic action of OEA. However, plasma levels of PYY, an anorexigenic peptide, were increased significantly in patients treated with GSK1292263 and DS-8500a [56][63]. Furthermore, GPR119 expression in PP-cells was reported. GPR119 agonists might have stimulated PP secretion and induced satiety in patients with type 2 diabetes and obesity [41]. Recently, GPR119 activation in pancreatic α-cells was shown to increase glucagon secretion during insulin-induced hypoglycemia [36][42]. In addition, application of GPR119 agonists in non-alcoholic steatohepatitis has been suggested [67]. Further research is needed to confirm the role of GPR119 activation in PYY increase, glucagon secretion, and non-alcoholic fatty liver.
3. PEA and GPR55
3.1. Pharmacological Actions of PEA
Pharmacological actions of PEA have been investigated in three categories of analgesia, anti-anaphylaxis, and anti-inflammation. The first anti-inflammatory activity of PEA was described by Ganley and Robinson in 1958 [68] and the authors also reported anti-anaphylactic and anti-serotonin activities of PEA obtained from egg yolk, peanut oil, and soybean lecithin [69]. Later, exogenous PEA was reported to exert anti-inflammatory action in a chronic inflammatory disease, Freund’s adjuvant-induced arthritis, in 1971 [70].
3.1.1. Implications of PEA in Anti-Anaphylactic Activity
Aloe et al. reported the pharmacological effects of PEA on mast cells in 1993 [71]. In the study, PEA, when systemically administrated, reduced mast cell degranulation induced by local injection of substance P in the ear pinna of rats [71]. Subsequently, PEA was reported to suppress IgE-triggered activation of mast cells, which was suggested to be mediated by agonism on peripheral CB2 receptors in the mast cells. This has been evidenced by suppression of binding of [3H]WIN 55212-2b, the full agonist of CB1/CB2 receptors, by PEA in mast cells expressing CB2 only [72][73]. PEA agonist activity on CB2 receptor was also observed in cerebellar granule cells in glutamate-induced neurotoxicity [74]. In addition, PEA was found to accumulate in inflamed tissues after injury [75]. Later, the in vivo anti-anaphylactic action of PEA was shown to be mediated by downregulation of serotonin release from basophils and mast cells [76]. In addition, PEA reduced plasma extravasation induced by passive cutaneous anaphylaxis reaction in a dose-dependent manner [76].
Therefore, the acronym ALIA (autacoid local inflammation antagonism) was proposed for the mechanism of action of PEA in the early 1990s [71]. The activation of mast cells led to the production of endogenous PEA, which in turn acted as a local autocrine or paracrine regulator for the negative feedback mechanism [71]. PEA supplementation prevented ovalbumin-induced bronchial hyperreactivity, but it did not affect plasma IgE level [77]. In addition, PEA could act as an entourage compound for endocannabinoids by inhibiting the inactivation of endogenous cannabinoids, thereby increasing their levels [78]. PEA counteracted substance P-stimulated degranulation and histamine release in RBL-2H3 cells, in a manner antagonized by AM630, a CB2 antagonist, and by OMDM188, a diacylglycerol lipase enzyme inhibitor (DAGL) [79]. PEA significantly stimulated DAGL-α and -β activity and, consequently, increased biosynthesis of 2-arachidonyl glycerol, which might have contributed to the PEA action [79]. Additionally, PEA levels were significantly reduced in the bronchi of ovalbumin-treated animals, and upregulation of CB2 and GPR55 receptors was observed [77].
Therefore, PEA may negatively regulate anaphylactic responses, such as mast cell degranulation, and its mechanism of action might be related to the endocannabinoid-like system, including CB2, GPR55, and 2-arachidonyl glycerol (Figure 2).
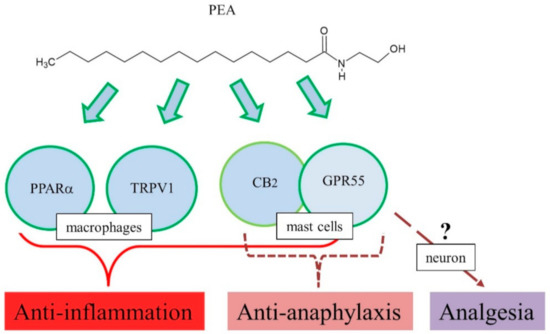
Figure 2. Proposed action mechanisms of PEA for anti-inflammation, anti-anaphylaxis, and analgesia.
3.1.2. Implications of PEA in Anti-Inflammatory Effects
Oral administration of PEA reduced carrageenan-induced hind paw edema in a time- and dose-dependent manner, and significantly decreased carrageenan-induced mechanical hyperalgesia [76]. The anti-inflammatory properties of PEA were shared by the endogenous CB2 receptor ligands [78][80]. However, CB2 involvement could not be observed in the later study [80].
The anti-inflammatory action of PEA in a rat model of carrageenan-induced acute hind paw inflammation was compared with that of the non-steroidal anti-inflammatory drug, indomethacin [81]. PEA and indomethacin reduced the inflammatory parameters in a time-dependent manner, and the selective CB2 antagonist (SR 144528) prevented the anti-edema and anti-hyperalgesic effects of PEA, suggesting its interaction with a yet uncharacterized CB2-like cannabinoid receptor [81].
Consistently, the above-mentioned results with CB2 agonists and antagonists suggest involvement of CB2 receptor in pharmacological actions of PEA [72][81]. However, studies reported no interaction of PEA with CB1 and CB2 [82][83]. Although CB2 involvement is controversial, multiple targets have been suggested for PEA anti-inflammatory actions, GPR55, TRPV1, and PPARα [80]. Treatment with INF-γ and TNF-α increased phosphoprotein and cytokine levels in colonic explants, while PEA significantly reduced the levels of phosphoprotein and cytokine production in colonic explants [84]. In addition, these effects of PEA were blocked by the PPARα antagonist GW6471 [84]. Furthermore, in vitro, PEA decreased inflammation-induced flux of dextrans, sensitive to PPARα [84]. PEA prevented an inflammation-induced increase in PPARα transcription and a decrease in TRPV1 [84]. Immunoreactivity of GPR55 has been observed in the lamina propria macrophages and smooth muscle cells [85]. Mouse primary macrophages were polarized toward pro-inflammatory phenotypes, and reduced N-acyl phosphatidylethanolamine phospholipase D expression and PEA bioavailability, and PEA exerted potent anti-inflammatory actions [86], implying the role of GPR55 as a target for PEA action. In addition, PEA has high affinity for PPARα, which mediate many biological effects, including anti-inflammation [3]. In a DNBS-induced colitis model, exogenous PEA administration attenuated inflammation and intestinal permeability and stimulated colonic cell proliferation [87]. This led to increased colonic levels of PEA and endocannabinoids, downregulation of GPR55 mRNA, but no changes in CB1, CB2, and PPARα [87]. Therefore, PEA exerted anti-inflammatory responses in several animal models, and its mechanism of action might be related to several targets such as PPARα, TRPV1, GPR55, and CB2 (Figure 2) [80].
3.1.3. Implications of PEA in Analgesic and Neurologic Activities
PEA showed low affinity for CB1 and CB2 receptors, yet selectively activated GPR55 receptors [3]. A possible role of GPR55 receptors in the anti-nociceptive activity of PEA has been implicated in formalin-induced pain-related behavioral modifications [88]. Furthermore, PEA actions in social interaction and neural transmission have been reported as follows.
PEA-induced activation of GPR55 in the ventral hippocampus, a critical region for cognition, recognition memory, and affective processing produced a hyperdopaminergic state in the mesolimbic dopaminergic system, which means an increased firing and bursting activity of dopaminergic neuron populations of the ventral tegmental area [89]. This resulted in strong disruptions in context-independent associative fear memory formation, social interaction, recognition memory, and spatial location memory [89].
Prenatal exposure to valproic acid in rats reduced GPR55 expression in the frontal cortex and hippocampus. In addition, levels of acylethanolamides, such as PEA, OEA, and AEA, were higher in the hippocampus of these rats immediately following social exposure, implying the involvement of GPR55 and its agonist acylethanolamides in social interaction [90].
PEA was found to enhance GABA transmission and triggered the synthesis of 2-arachidonyl glycerol as well, at the postsynaptic site in the striatum, which in turn inhibited GABA release in a retrograde manner through the stimulation of presynaptic CB1 receptors, implying complex neural networks between GABA neurons and PEA dynamics [91].
PF3845 is an inhibitor of FAAH, an acylethanolamide catabolic enzyme. Systemic administration of PF3845 increased the levels of PEA, OEA, and AEA in the hippocampus and frontal cortex of rats in vivo in TLR4-induced neuroinflammatory responses [92], implying dynamic turnover of PEA in the brain along with other acylethanolamides.
There are many reports on the production of PEA and other acylethanolamides and expression of GPR55 in the brain. These reports indicate the functions of PEA and GPR55 in analgesia, social interaction, and neural transmission.
3.1.4. Other Effects
PEA caused a concentration-dependent increase in outflow facility in an anterior segment porcine organ culture model, which was speculated to be mediated by GPR55 and PPARα [93]. PEA also inhibits vasopressor responses to exogenous noradrenaline or sympathetic stimulation, which induces hypotension. The PEA action is reported to be mediated by prejunctional and vascular CB1, TRPV1, and probably GPR55 [94].
3.2. Pharmacology of GPR55
Ryberg et al. reported GPR55 as a novel cannabinoid receptor because cannabinoid ligand CP55940 exhibits specific binding to GPR55 [6]. PEA is one of the proposed ligands including cannabidiol and abnormal cannabidiol. EC50 values of acylethanolamides, such as PEA, AEA, OEA, and 2-arachidonyl glycerol, were measured as 4, 18, 440, and 3 nM in GTPγS binding, respectively [6]. However, in other assay systems, PEA did not exhibit a statistically significant effect in GPR55-transfected cells [95][96][97]. The most potent endogenous lipid for GPR55 known is 2-arachidonyl lysophosphatidylinositol [96][97][98][99]. Phylogenetically, GPR55 belongs to a cluster of lipid receptors (GPR35, LPA4/P2Y9, LPA5/GPR92, LPA6/P2Y5, FFA1/GPR40, FFA2/GPR43, and FFA3/GPR41) [6].
The mechanism of action of PEA has been studied, and many molecular targets have been proposed, including CB2, GPR55, TRPV1, and PPARα [78]. The anti-anaphylactic, anti-inflammatory, and analgesic effects of PEA were reversed by CB2 antagonists [72][78][81], thus implicating CB2 receptor as a target of PEA. However, another putative CB-like receptor was present in the RBL-2H3 cells [78]. GPR55 was identified as a receptor that could be implicated in mast cells [6]. However, there has been no further report on this subject. Another possible mechanism suggested that the effects of PEA involve indirect regulation of microglial CB2 expression [100]. PEA was found to increase the mRNA and protein expression of CB2 through PPARα activation [100]. In addition, crosstalk between GPR55 and the cannabinoid receptors (CB1 and CB2) via heteromerization is intriguing. In the co-expression system, CB1 signaling was enhanced by GPR55 expression, and cross-antagonism between GPR55 and CB2 was observed [101][102]. Further research on the mechanism of action of PEA in mast cells via the GPR55-CB2 complex is needed.
Although GPR55 is reported to be a receptor for lipid mediators such as lysophosphatidylinositol and PEA, there have not been many studies on GPR55 being a PEA receptor. Instead, many studies have been conducted using GPR55 knockout mice and the most potent natural ligand, 2-arachidonyl lysophosphatidylinositol, or a synthetic agonist, O-1602 [103][104][105]. GPR55 is ubiquitously expressed, and its implications in anxiety, bone development, cancer, inflammation, metabolic disturbance, nociception, and synaptic transmission have been studied [6][104]. In pain, GPR55 knockout abolished mechanical hyperalgesia following inflammatory or neuropathic insults, but this was not observed in a later study [105][106]. In experimental colitis model, GPR55 knockout mice showed less severe symptoms, and CID16020046, a potent GPR55 antagonist, reduced colon inflammation [107][108]. Inhibitory effects of O-1602 on intestinal contractility and colonic motility is a GPR55-dependent process [109][110]. The role of GPR55 in memory processing, social interaction, and synaptic transmission has also been reported [92][111][112]. However, there are inconsistencies in the reports regarding the role of GPR55 given findings related to the phenotypic characterization of GPR55 knockout mice and GPR55-independent actions of O-1602 and CID1602046 [103][105][106][107][113][114][115]. So far, increasing evidence supports lysophosphatidylinositol instead of PEA as an endogenous ligand for GPR55 [6][95][96][97]. Published information on GPR55 functions is complicated by different phenotypic characterization of GPR55 knockout mice and off-target effects of O-1602 and CID1602046 [103][105][106][107][113][114][115]. Therefore, it is crucial to identify specific and selective agonists/antagonists for GPR55 in future research.
References
- Wellner, N.; Diep, T.A.; Janfelt, C.; Hansen, H.S. N-acylation of phosphatidylethanolamine and its biological functions in mammals. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 652–662.
- Aziz, M.; Wang, X.; Tripathi, A.; Bankaitis, V.A.; Chapman, K.D. Structural analysis of a plant fatty acid amide hydrolase provides insights into the evolutionary diversity of bioactive acylethanolamides. J. Biol. Chem. 2019, 294, 7419–7432.
- Godlewski, G.; Offertáler, L.; Wagner, J.A.; Kunos, G. Receptors for acylethanolamides—GPR55 and GPR119. Prostaglans Other Lipid Mediat. 2009, 89, 105–111.
- Borrelli, F.; Izzo, A.A. Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 33–49.
- Sugiura, T.; Kishimoto, S.; Oka, S.; Gokoh, M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog. Lipid Res. 2006, 45, 405–446.
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101.
- Overton, H.A.; Fyfe, M.C.; Reynet, C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br. J. Pharmacol. 2008, 153 (Suppl. 1), S76–S81.
- De Fonseca, F.R.; Navarro, M.; Gomez, R.; Escuredo, L.; Nava, F.; Fu, J.; Murillo-Rodriguez, E.; Giuffrida, A.; LoVerme, J.; Gaetani, S. An anorexic lipid mediator regulated by feeding. Nature 2001, 414, 209–212.
- Gaetani, S.; Oveisi, F.; Piomelli, D. Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamide. Neuropsychopharmacology 2003, 28, 1311–1316.
- Proulx, K.; Cota, D.; Castaneda, T.R.; Tschop, M.H.; D’Alessio, D.A.; Tso, P.; Woods, S.C.; Seeley, R.J. Mechanisms of oleoylethanolamide-induced changes in feeding behavior and motor activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R729–R737.
- Laleh, P.; Yaser, K.; Alireza, O. Oleoylethanolamide: A novel pharmaceutical agent in the management of obesity-an updated review. J. Cell Physiol. 2019, 234, 7893–7902.
- Fu, J.; Kim, J.; Oveisi, F.; Astarita, G.; Piomelli, D. Targeted enhancement of oleoylethanolamide production in proximal small intestine induces across-meal satiety in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R45–R50.
- Hansen, H.S.; Diep, T.A. N-acylethanolamines, anandamide and food intake. Biochem. Pharmacol. 2009, 78, 553–560.
- Ahern, G.P. Activation of TRPV1 by the satiety factor oleoylethanolamide. J. Biol. Chem. 2003, 278, 30429–30434.
- Wang, X.; Miyares, R.L.; Ahern, G.P. Oleoylethanolamide excites vagal sensory neurones, induces visceral pain and reduces short-term food intake in mice via capsaicin receptor TRPV1. J. Physiol. 2005, 564, 541–547.
- Fu, J.; Gaetani, S.; Oveisi, F.; Verme, J.L.; Serrano, A.; De Fonseca, F.R.; Rosengarth, A.; Luecke, H.; Di Giacomo, B.; Tarzia, G. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature 2003, 425, 90–93.
- Verme, J.L.; Gaetani, S.; Fu, J.; Oveisi, F.; Burton, K.; Piomelli, D. Regulation of food intake by oleoylethanolamide. Cell Mol. Life Sci. 2005, 62, 708.
- Overton, H.A.; Babbs, A.J.; Doel, S.M.; Fyfe, M.C.; Gardner, L.S.; Griffin, G.; Jackson, H.C.; Procter, M.J.; Rasamison, C.M.; Tang-Christensen, M.; et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006, 3, 167–175.
- Park, Y.H.; Choi, H.H.; Lee, D.H.; Chung, S.Y.; Yang, N.Y.; Kim, D.H.; Ju, M.K.; Han, T.D.; Nam, S.Y.; Kim, K.-W. YH18421, a novel GPR119 agonist exerts sustained glucose lowering and weight loss in diabetic mouse model. Arch. Pharm. Res. 2017, 40, 772–782.
- Gao, J.; Tian, L.; Weng, G.; Bhagroo, N.V.; Sorenson, R.L.; O’Brien, T.D.; Luo, J.; Guo, Z. Stimulating beta cell replication and improving islet graft function by GPR119 agonists. Transp. Int. 2011, 24, 1124–1134.
- Chu, Z.-L.; Jones, R.M.; He, H.; Carroll, C.; Gutierrez, V.; Lucman, A.; Moloney, M.; Gao, H.; Mondala, H.; Bagnol, D. A role for β-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology 2007, 148, 2601–2609.
- Lan, H.; Vassileva, G.; Corona, A.; Liu, L.; Baker, H.; Golovko, A.; Abbondanzo, S.J.; Hu, W.; Yang, S.; Ning, Y.; et al. GPR119 is required for physiological regulation of glucagon-like peptide-1 secretion but not for metabolic homeostasis. J. Endocrinol. 2009, 201, 219–230.
- Ning, Y.; O’Neill, K.; Lan, H.; Pang, L.; Shan, L.X.; Hawes, B.E.; Hedrick, J.A. Endogenous and synthetic agonists of GPR119 differ in signalling pathways and their effects on insulin secretion in MIN6c4 insulinoma cells. Br. J. Pharmacol. 2008, 155, 1056–1065.
- Gaetani, S.; Fu, J.; Cassano, T.; Dipasquale, P.; Romano, A.; Righetti, L.; Cianci, S.; Laconca, L.; Giannini, E.; Scaccianoce, S. The fat-induced satiety factor oleoylethanolamide suppresses feeding through central release of oxytocin. J. Neurosci. 2010, 30, 8096–8101.
- Kleberg, K.; Hassing, H.A.; Hansen, H.S. Classical endocannabinoid-like compounds and their regulation by nutrients. Biofactors 2014, 40, 363–372.
- Suardíaz, M.; Estivill-Torrús, G.; Goicoechea, C.; Bilbao, A.; Rodríguez de Fonseca, F. Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain 2007, 133, 99–110.
- Tough, I.R.; Forbes, S.; Herzog, H.; Jones, R.M.; Schwartz, T.W.; Cox, H.M. Bidirectional GPR119 Agonism Requires Peptide YY and Glucose for Activity in Mouse and Human Colon Mucosa. Endocrinology 2018, 159, 1704–1717.
- Grill, M.; Högenauer, C.; Blesl, A.; Haybaeck, J.; Golob-Schwarzl, N.; Ferreirós, N.; Thomas, D.; Gurke, R.; Trötzmüller, M.; Köfeler, H.C.; et al. Members of the endocannabinoid system are distinctly regulated in inflammatory bowel disease and colorectal cancer. Sci. Rep. 2019, 9, 2358.
- Payahoo, L.; Khajebishak, Y.; Alivand, M.R.; Soleimanzade, H.; Alipour, S.; Barzegari, A.; Ostadrahimi, A. Investigation the effect of oleoylethanolamide supplementation on the abundance of Akkermansia uciniphila bacterium and the dietary intakes in people with obesity: A randomized clinical trial. Appetite 2019, 141, 104301.
- Kim, H.J.; Lee, D.K.; Jin, X.; Che, X.; Choi, J.Y. Oleoylethanolamide Exhibits GPR119-Dependent Inhibition of Osteoclast Function and GPR119-Independent Promotion of Osteoclast Apoptosis. Mol. Cells 2020, 43, 340–349.
- Markovics, A.; Angyal, Á.; Tóth, K.F.; Ádám, D.; Pénzes, Z.; Magi, J.; Pór, Á.; Kovács, I.; Törőcsik, D.; Zouboulis, C.C.; et al. GPR119 Is a Potent Regulator of Human Sebocyte Biology. J. Investig. Dermatol. 2020, 140, 1909–1918.e8.
- Fredriksson, R.; Hoglund, P.J.; Gloriam, D.E.; Lagerstrom, M.C.; Schioth, H.B. Seven evolutionarily conserved human rhodopsin G protein-coupled receptors lacking close relatives. FEBS Lett 2003, 554, 381–388.
- Soga, T.; Ohishi, T.; Matsui, T.; Saito, T.; Matsumoto, M.; Takasaki, J.; Matsumoto, S.-I.; Kamohara, M.; Hiyama, H.; Yoshida, S. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2005, 326, 744–751.
- Chu, Z.-L.; Carroll, C.; Alfonso, J.; Gutierrez, V.; He, H.; Lucman, A.; Pedraza, M.; Mondala, H.; Gao, H.; Bagnol, D. A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like Peptide-1 and glucose-dependent insulinotropic Peptide release. Endocrinology 2008, 149, 2038–2047.
- Lauffer, L.M.; Iakoubov, R.; Brubaker, P.L. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes 2009, 58, 1058–1066.
- Odori, S.; Hosoda, K.; Tomita, T.; Fujikura, J.; Kusakabe, T.; Kawaguchi, Y.; Doi, R.; Takaori, K.; Ebihara, K.; Sakai, Y. GPR119 expression in normal human tissues and islet cell tumors: Evidence for its islet-gastrointestinal distribution, expression in pancreatic beta and alpha cells, and involvement in islet function. Metabolism 2013, 62, 70–78.
- Kato, T.; Harada, N.; Ikeguchi, E.; Sankoda, A.; Hatoko, T.; Lu, X.; Yasuda, T.; Yamane, S.; Inagaki, N. Gene expression of nutrient-sensing molecules in I cells of CCK reporter male mice. J. Mol. Endocrinol. 2020, 66, 11–22.
- Parker, H.; Habib, A.; Rogers, G.; Gribble, F.; Reimann, F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 2009, 52, 289.
- Sykaras, A.G.; Demenis, C.; Case, R.M.; McLaughlin, J.T.; Smith, C.P. Duodenal enteroendocrine I-cells contain mRNA transcripts encoding key endocannabinoid and fatty acid receptors. PLoS ONE 2012, 7, e42373.
- Bonini, J.A.; Borowsky, B.E.; Adham, N.; Boyle, N.; Thompson, T.O. Methods of Identifying Compounds That Bind to SNORF25 Receptors. U.S. Patent US6468756B1, 22 October 2002.
- Sakamoto, Y.; Inoue, H.; Kawakami, S.; Miyawaki, K.; Miyamoto, T.; Mizuta, K.; Itakura, M. Expression and distribution of Gpr119 in the pancreatic islets of mice and rats: Predominant localization in pancreatic polypeptide-secreting PP-cells. Biochem. Biophys. Res. Commun. 2006, 351, 474–480.
- Li, N.X.; Brown, S.; Kowalski, T.; Wu, M.; Yang, L.; Dai, G.; Petrov, A.; Ding, Y.; Dlugos, T.; Wood, H.B. GPR119 agonism increases glucagon secretion during insulin-induced hypoglycemia. Diabetes 2018, 67, 1401–1413.
- Chu, Z.-L.; Carroll, C.; Chen, R.; Alfonso, J.; Gutierrez, V.; He, H.; Lucman, A.; Xing, C.; Sebring, K.; Zhou, J. N-oleoyldopamine enhances glucose homeostasis through the activation of GPR119. Mol. Endocrinol. 2010, 24, 161–170.
- Kogure, R.; Toyama, K.; Hiyamuta, S.; Kojima, I.; Takeda, S. 5-Hydroxy-eicosapentaenoic acid is an endogenous GPR119 agonist and enhances glucose-dependent insulin secretion. Biochem. Biophys. Res. Commun. 2011, 416, 58–63.
- Hansen, K.B.; Rosenkilde, M.M.; Knop, F.K.; Wellner, N.; Diep, T.A.; Rehfeld, J.F.; Andersen, U.B.; Holst, J.J.; Hansen, H.S. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J. Clin. Endocrinol. Metab. 2011, 96, E1409–E1417.
- Hansen, H.S.; Rosenkilde, M.M.; Holst, J.J.; Schwartz, T.W. GPR119 as a fat sensor. Trends Pharmacol. Sci. 2012, 33, 374–381.
- Stone, V.M.; Dhayal, S.; Smith, D.M.; Lenaghan, C.; Brocklehurst, K.J.; Morgan, N.G. The cytoprotective effects of oleoylethanolamide in insulin-secreting cells do not require activation of GPR119. Br. J. Pharmacol. 2012, 165, 2758–2770.
- Yang, Z.; Fang, Y.; Park, H. Synthesis and biological evaluation of pyrimidine derivatives with diverse azabicyclic ether/amine as novel GPR119 agonist. Bioorg. Med. Chem. Lett. 2017, 27, 2515–2519.
- Moran, B.M.; Abdel-Wahab, Y.H.; Flatt, P.R.; McKillop, A.M. Activation of GPR119 by fatty acid agonists augments insulin release from clonal β-cells and isolated pancreatic islets and improves glucose tolerance in mice. Biol. Chem. 2014, 395, 453–464.
- Tadaki, H.; Ogawa, N.; Yamanaka, M.; Motohashi, Y.; Sasase, T.; Kawai, T.; Toriniwa, Y.; Fukuda, S.; Ogawa, N.; Harada, K.; et al. JTP-109192, a novel G protein-coupled receptor 119 agonist, prevents atherosclerosis by improving hypercholesterolaemia in congenic spontaneously hyperlipidaemic mice. Clin. Exp. Pharmacol. Physiol. 2020.
- Drzazga, A.; Kristinsson, H.; Sałaga, M.; Zatorski, H.; Koziołkiewicz, M.; Gendaszewska-Darmach, E.; Bergsten, P. Lysophosphatidylcholine and its phosphorothioate analogues potentiate insulin secretion via GPR40 (FFAR1), GPR55 and GPR119 receptors in a different manner. Mol. Cell Endocrinol. 2018, 472, 117–125.
- Fyfe, M.C.; McCormack, J.G.; Overton, H.A.; Procter, M.J.; Reynet, C. GPR119 agonists as potential new oral agents for the treatment of type 2 diabetes and obesity. Expert Opin. Drug Discov. 2008, 3, 403–413.
- Ritter, K.; Buning, C.; Halland, N.; Pöverlein, C.; Schwink, L. G protein-coupled receptor 119 (GPR119) agonists for the treatment of diabetes: Recent progress and prevailing challenges. J. Med. Chem. 2016, 59, 3579–3592.
- Jones, R.M.; Leonard, J.N.; Buzard, D.J.; Lehmann, J. GPR119 agonists for the treatment of type 2 diabetes. Expert Opin. Ther. Patents 2009, 19, 1339–1359.
- Kang, S.-U. GPR119 agonists: A promising approach for T2DM treatment? A SWOT analysis of GPR119. Drug Discov. Today 2013, 18, 1309–1315.
- Nunez, D.J.; Bush, M.A.; Collins, D.A.; McMullen, S.L.; Gillmor, D.; Apseloff, G.; Atiee, G.; Corsino, L.; Morrow, L.; Feldman, P.L. Gut hormone pharmacology of a novel GPR119 agonist (GSK1292263), metformin, and sitagliptin in type 2 diabetes mellitus: Results from two randomized studies. PLoS ONE 2014, 9, e92494.
- Semple, G.; Ren, A.; Fioravanti, B.; Pereira, G.; Calderon, I.; Choi, K.; Xiong, Y.; Shin, Y.-J.; Gharbaoui, T.; Sage, C.R. Discovery of fused bicyclic agonists of the orphan G-protein coupled receptor GPR119 with in vivo activity in rodent models of glucose control. Bioorg. Med. Chem. Lett. 2011, 21, 3134–3141.
- Hassing, H.A.; Fares, S.; Larsen, O.; Pad, H.; Hauge, M.; Jones, R.M.; Schwartz, T.W.; Hansen, H.S.; Rosenkilde, M.M. Biased signaling of lipids and allosteric actions of synthetic molecules for GPR119. Biochem. Pharmacol. 2016, 119, 66–75.
- Katz, L.; Gambale, J.; Rothenberg, P.; Vanapalli, S.; Vaccaro, N.; Xi, L.; Sarich, T.; Stein, P. Effects of JNJ-38431055, a novel GPR119 receptor agonist, in randomized, double-blind, placebo-controlled studies in subjects with type 2 diabetes. Diabetes Obes. Metab. 2012, 14, 709–716.
- Engelstoft, M.S.; Norn, C.; Hauge, M.; Holliday, N.D.; Elster, L.; Lehmann, J.; Jones, R.M.; Frimurer, T.M.; Schwartz, T.W. Structural basis for constitutive activity and agonist-induced activation of the enteroendocrine fat sensor GPR119. Br. J. Pharmacol. 2014, 171, 5774–5789.
- Han, T.; Lee, B.M.; Park, Y.H.; Lee, D.H.; Choi, H.H.; Lee, T.; Kim, H. YH18968, a novel 1, 2, 4-triazolone G-protein coupled receptor 119 agonist for the treatment of type 2 diabetes mellitus. Biomol. Ther. 2018, 26, 201.
- Matsumoto, K.; Yoshitomi, T.; Ishimoto, Y.; Tanaka, N.; Takahashi, K.; Watanabe, A.; Chiba, K. DS-8500a, an orally available G protein-coupled receptor 119 agonist, upregulates glucagon-like peptide-1 and enhances glucose-dependent insulin secretion and improves glucose homeostasis in type 2 diabetic rats. J. Pharmacol. Exp. Ther. 2018, 367, 509–517.
- Yamada, Y.; Terauchi, Y.; Watada, H.; Nakatsuka, Y.; Shiosakai, K.; Washio, T.; Taguchi, T. Efficacy and safety of GPR119 agonist DS-8500a in Japanese patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, 12-week study. Adv. Ther. 2018, 35, 367–381.
- Goodman, M.; Dow, J.; Van Vliet, A.; Pleszko, A.; Lockton, J. Orally administered GPR119 agonist PSN821 shows clinically significant glucose lowering and other potential cardiometabolic benefits in patients with type 2 diabetes. In Diabetologia; Springer: New York, NY, USA, 2011.
- Nunez, D.J.; Bush, M.A.; Collins, D.A.; Mcmullen, S.L.; Apseloff, G.; Atiee, G.; Cosino, L.; Morrow, L.; Feldman, P.L. Novel Effects on Lipids of GSK1292263, a GPR119 Agonist, in Type 2 Diabetics. In Diabetes; American Diabetes Association: Alexandria, VA, USA, 2012.
- Thabuis, C.; Destaillats, F.; Landrier, J.F.; Tissot-Favre, D.; Martin, J.C. Analysis of gene expression pattern reveals potential targets of dietary oleoylethanolamide in reducing body fat gain in C3H mice. J. Nutr. Biochem. 2010, 21, 922–928.
- Yang, J.W.; Kim, H.S.; Im, J.H.; Kim, J.W.; Jun, D.W.; Lim, S.C.; Lee, K.; Choi, J.M.; Kim, S.K.; Kang, K.W. GPR119: A promising target for nonalcoholic fatty liver disease. FASEB J. 2016, 30, 324–335.
- Ganley, O.H.; Graessle, O.E.; Robinson, H.J. Anti-inflammatory activity of compounds obtained from egg yolk, peanut oil, and soybean lecithin. J. Lab. Clin. Med. 1958, 51, 709–714.
- Ganley, O.H.; Robinson, H.J. Antianaphylactic and antiserotonin activity of a compound obtained from egg yolk, peanut oil, and soybean lecithin. J. Allergy 1959, 30, 415–419.
- Perlik, F.; Raskova, H.; Elis, J. Anti-inflammatory properties of N (2-hydroxyethyl) palmitamide. Acta Physiol. Acad. Sci. Hung. 1971, 39, 395.
- Aloe, L.; Leon, A.; Levi-Montalcini, R. A proposed autacoid mechanism controlling mastocyte behaviour. Agents Actions 1993, 39, C145–C147.
- Facci, L.; Dal Toso, R.; Romanello, S.; Buriani, A.; Skaper, S.; Leon, A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. USA 1995, 92, 3376–3380.
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; LE Fur, G.; Casellas, P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995, 232, 54–61.
- Skaper, S.D.; Buriani, A.; Dal Toso, R.; Petrelli, L.; Romanello, S.; Facci, L.; Leon, A. The ALIAmide palmitoylethanolamide and cannabinoids, but not anandamide, are protective in a delayed postglutamate paradigm of excitotoxic death in cerebellar granule neurons. Proc. Natl. Acad. Sci. USA 1996, 93, 3984–3989.
- Natarajan, V.; Reddy, P.V.; Schmid, P.C.; Schmid, H.H. N-Acylation of ethanolamine phospholipids in canine myocardium. Biochim. Biophys. Acta Lipids Lipid Metab. 1982, 712, 342–355.
- Mazzari, S.; Canella, R.; Petrelli, L.; Marcolongo, G.; Leon, A. N-(2-hydroxyethyl) hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation. Eur. J. Pharmacol. 1996, 300, 227–236.
- Roviezzo, F.; Rossi, A.; Caiazzo, E.; Orlando, P.; Riemma, M.A.; Iacono, V.M.; Guarino, A.; Ialenti, A.; Cicala, C.; Peritore, A. Palmitoylethanolamide supplementation during sensitization prevents airway allergic symptoms in the mouse. Front. Pharmacol. 2017, 8, 857.
- Lambert, D.M.; Di, V.M. The palmitoylethanolamide and oleamide enigmas: Are these two fatty acid amides cannabimimetic? Cur. Med. Chem. 1999, 6, 757–773.
- Petrosino, S.; Moriello, A.S.; Verde, R.; Allarà, M.; Imperatore, R.; Ligresti, A.; Mahmoud, A.M.; Peritore, A.F.; Iannotti, F.A.; Di Marzo, V. Palmitoylethanolamide counteracts substance P-induced mast cell activation in vitro by stimulating diacylglycerol lipase activity. J. Neuroinflamm. 2019, 16, 274.
- Showalter, V.M.; Compton, D.R.; Martin, B.R.; Abood, M.E. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): Identification of cannabinoid receptor subtype selective ligands. J. Pharmacol. Exp. Ther. 1996, 278, 989–999.
- Conti, S.; Costa, B.; Colleoni, M.; Parolaro, D.; Giagnoni, G. Antiinflammatory action of endocannabinoid palmitoylethanolamide and the synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br. J. Pharmacol. 2002, 135, 181–187.
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949.
- Felder, C.C.; Briley, E.M.; Axelrod, J.; Simpson, J.T.; Mackie, K.; Devane, W.A. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc. Natl. Acad. Sci. USA 1993, 90, 7656–7660.
- Couch, D.G.; Cook, H.; Ortori, C.; Barrett, D.; Lund, J.N.; O’Sullivan, S.E. Palmitoylethanolamide and cannabidiol prevent inflammation-induced hyperpermeability of the human gut in vitro and in vivo—A randomized, placebo-controlled, double-blind controlled trial. Inflamm. Bowel Dis. 2019, 25, 1006–1018.
- Galiazzo, G.; Giancola, F.; Stanzani, A.; Fracassi, F.; Bernardini, C.; Forni, M.; Pietra, M.; Chiocchetti, R. Localization of cannabinoid receptors CB1, CB2, GPR55, and PPARα in the canine gastrointestinal tract. Histochem. Cell Biol. 2018, 150, 187–205.
- Rinne, P.; Guillamat-Prats, R.; Rami, M.; Bindila, L.; Ring, L.; Lyytikäinen, L.-P.; Raitoharju, E.; Oksala, N.; Lehtimäki, T.; Weber, C. Palmitoylethanolamide promotes a proresolving macrophage phenotype and attenuates atherosclerotic plaque formation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2562–2575.
- Borrelli, F.; Romano, B.; Petrosino, S.; Pagano, E.; Capasso, R.; Coppola, D.; Battista, G.; Orlando, P.; Di Marzo, V.; Izzo, A.A. Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br. J. Pharmacol. 2015, 172, 142–158.
- Naderi, N.; Majidi, M.; Mousavi, Z.; Tusi, S.K.; Mansouri, Z.; Khodagholi, F. The interaction between intrathecal administration of low doses of palmitoylethanolamide and AM251 in formalin-induced pain related behavior and spinal cord IL1-β expression in rats. Neurochem. Res. 2012, 37, 778–785.
- Kramar, C.; Loureiro, M.; Renard, J.; Laviolette, S.R. Palmitoylethanolamide modulates GPR55 receptor signaling in the ventral hippocampus to regulate mesolimbic dopamine activity, social interaction, and memory processing. Cannabis Cannabinoid Res. 2017, 2, 8–20.
- Kerr, D.; Downey, L.; Conboy, M.; Finn, D.; Roche, M. Alterations in the endocannabinoid system in the rat valproic acid model of autism. Behav. Brain Res. 2013, 249, 124–132.
- Musella, A.; Fresegna, D.; Rizzo, F.; Gentile, A.; Bullitta, S.; De Vito, F.; Guadalupi, L.; Centonze, D.; Mandolesi, G. A novel crosstalk within the endocannabinoid system controls GABA transmission in the striatum. Sci. Rep. 2017, 7, 1–8.
- Henry, R.J.; Kerr, D.M.; Flannery, L.E.; Killilea, M.; Hughes, E.M.; Corcoran, L.; Finn, D.P.; Roche, M. Pharmacological inhibition of FAAH modulates TLR-induced neuroinflammation, but not sickness behaviour: An effect partially mediated by central TRPV1. Brain Behav. Immun. 2017, 62, 318–331.
- Kumar, A.; Qiao, Z.; Kumar, P.; Song, Z.-H. Effects of palmitoylethanolamide on aqueous humor outflow. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4416–4425.
- Marichal-Cancino, B.A.; González-Hernández, A.; MaassenVanDenBrink, A.; Ramírez-San Juan, E.; Villalón, C.M. Potential Mechanisms Involved in Palmitoylethanolamide-Induced Vasodepressor Effects in Rats. J. Vasc. Res. 2020, 57, 152–163.
- Kapur, A.; Zhao, P.; Sharir, H.; Bai, Y.; Caron, M.G.; Barak, L.S.; Abood, M.E. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J. Biol. Chem. 2009, 284, 29817–29827.
- Oka, S.; Nakajima, K.; Yamashita, A.; Kishimoto, S.; Sugiura, T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem. Biophys. Res. Commun. 2007, 362, 928–934.
- Lauckner, J.E.; Jensen, J.B.; Chen, H.Y.; Lu, H.C.; Hille, B.; Mackie, K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA 2008, 105, 2699–2704.
- Oka, S.; Toshida, T.; Maruyama, K.; Nakajima, K.; Yamashita, A.; Sugiura, T. 2-Arachidonoyl-sn-glycero-3-phosphoinositol: A possible natural ligand for GPR55. J. Biochem. 2009, 145, 13–20.
- Henstridge, C.M.; Balenga, N.A.; Ford, L.A.; Ross, R.A.; Waldhoer, M.; Irving, A.J. The GPR55 ligand L-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 2009, 23, 183–193.
- Guida, F.; Luongo, L.; Boccella, S.; Giordano, M.; Romano, R.; Bellini, G.; Manzo, I.; Furiano, A.; Rizzo, A.; Imperatore, R. Palmitoylethanolamide induces microglia changes associated with increased migration and phagocytic activity: Involvement of the CB2 receptor. Sci. Rep. 2017, 7, 1–11.
- Balenga, N.A.; Martinez-Pinilla, E.; Kargl, J.; Schroder, R.; Peinhaupt, M.; Platzer, W.; Balint, Z.; Zamarbide, M.; Dopeso-Reyes, I.G.; Ricobaraza, A.; et al. Heteromerization of GPR55 and cannabinoid CB2 receptors modulates signalling. Br. J. Pharmacol. 2014, 171, 5387–5406.
- Kargl, J.; Balenga, N.; Parzmair, G.P.; Brown, A.J.; Heinemann, A.; Waldhoer, M. The cannabinoid receptor CB1 modulates the signaling properties of the lysophosphatidylinositol receptor GPR55. J. Biol. Chem. 2012, 287, 44234–44248.
- Johns, D.G.; Behm, D.J.; Walker, D.J.; Ao, Z.; Shapland, E.M.; Daniels, D.A.; Riddick, M.; Dowell, S.; Staton, P.C.; Green, P.; et al. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br. J. Pharmacol. 2007, 152, 825–831.
- Alhouayek, M.; Masquelier, J.; Muccioli, G.G. Lysophosphatidylinositols, from cell membrane constituents to GPR55 ligands. Trends Pharmacol. Sci. 2018, 39, 586–604.
- Carey, L.M.; Gutierrez, T.; Deng, L.; Lee, W.-H.; Mackie, K.; Hohmann, A.G. Inflammatory and neuropathic nociception is preserved in GPR55 knockout mice. Sci. Rep. 2017, 7, 1–14.
- Staton, P.C.; Hatcher, J.P.; Walker, D.J.; Morrison, A.D.; Shapland, E.M.; Hughes, J.P.; Chong, E.; Mander, P.K.; Green, P.J.; Billinton, A.; et al. The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain 2008, 139, 225–236.
- Schicho, R.; Bashashati, M.; Bawa, M.; McHugh, D.; Saur, D.; Hu, H.M.; Zimmer, A.; Lutz, B.; Mackie, K.; Bradshaw, H.B.; et al. The atypical cannabinoid O-1602 protects against experimental colitis and inhibits neutrophil recruitment. Inflamm. Bowel Dis. 2011, 17, 1651–1664.
- Stancic, A.; Jandl, K.; Hasenohrl, C.; Reichmann, F.; Marsche, G.; Schuligoi, R.; Heinemann, A.; Storr, M.; Schicho, R. The GPR55 antagonist CID16020046 protects against intestinal inflammation. Neurogastroenterol. Motil. 2015, 27, 1432–1445.
- Ross, G.R.; Lichtman, A.; Dewey, W.L.; Akbarali, H.I. Evidence for the putative cannabinoid receptor (GPR55)-mediated inhibitory effects on intestinal contractility in mice. Pharmacology 2012, 90, 55–65.
- Li, K.; Fichna, J.; Schicho, R.; Saur, D.; Bashashati, M.; Mackie, K.; Li, Y.; Zimmer, A.; Goke, B.; Sharkey, K.A.; et al. A role for O-1602 and G protein-coupled receptor GPR55 in the control of colonic motility in mice. Neuropharmacology 2013, 71, 255–263.
- Sylantyev, S.; Jensen, T.P.; Ross, R.A.; Rusakov, D.A. Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc. Natl. Acad. Sci. USA 2013, 110, 5193–5198.
- Hurst, K.; Badgley, C.; Ellsworth, T.; Bell, S.; Friend, L.; Prince, B.; Welch, J.; Cowan, Z.; Williamson, R.; Lyon, C.; et al. A putative lysophosphatidylinositol receptor GPR55 modulates hippocampal synaptic plasticity. Hippocampus 2017, 27, 985–998.
- Okine, B.N.; Mc Laughlin, G.; Gaspar, J.C.; Harhen, B.; Roche, M.; Finn, D.P. Antinociceptive Effects of the GPR55 Antagonist CID16020046 Injected into the Rat Anterior Cingulate Cortex. Neuroscience 2020, 443, 19–29.
- Breen, C.; Brownjohn, P.W.; Ashton, J.C. The atypical cannabinoid O-1602 increases hind paw sensitisation in the chronic constriction injury model of neuropathic pain. Neurosci. Lett. 2012, 508, 119–122.
- Diaz-Arteaga, A.; Vazquez, M.J.; Vazquez-Martinez, R.; Pulido, M.R.; Suarez, J.; Velasquez, D.A.; Lopez, M.; Ross, R.A.; de Fonseca, F.R.; Bermudez-Silva, F.J.; et al. The atypical cannabinoid O-1602 stimulates food intake and adiposity in rats. Diabetes Obes. Metab. 2012, 14, 234–243.




