| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohamed Koubaa | + 1500 word(s) | 1500 | 2021-03-23 08:48:17 | | | |
| 2 | Vicky Zhou | Meta information modification | 1500 | 2021-03-25 07:03:19 | | | | |
| 3 | Vicky Zhou | Meta information modification | 1500 | 2021-03-25 07:10:51 | | | | |
| 4 | Vicky Zhou | Meta information modification | 1500 | 2021-03-25 07:11:57 | | | | |
| 5 | Vicky Zhou | Meta information modification | 1500 | 2021-03-25 07:12:29 | | | | |
| 6 | Richard G. Maroun | Meta information modification | 1500 | 2022-01-13 13:26:32 | | | | |
| 7 | Nicolas LOUKA | Meta information modification | 1500 | 2022-01-16 10:37:47 | | | | |
| 8 | Nicolas LOUKA | Meta information modification | 1500 | 2022-02-24 11:24:04 | | |
Video Upload Options
"Dyes from Microorganisms" means production of pigments using single cell microorganisms. It is gaining traction as a sustainable alternative to conventional synthesis.
1. Introduction
Microorganisms, whose functions and output can be extensively modified through genetic engineering, can be used to produce a wide variety of compounds of interest in a growing number of fields with textiles, food, and pharmaceuticals leading the charge. Their use to produce pigments, such as carotenoids for example, presents numerous advantages over alternate biomass sources such as monocrop-type agriculture. When contrasted against compound production from microbial sources, purposeful plant cultivation appears to show numerous drawbacks such as it being time-consuming, resource-intensive, vulnerable to inclement weather, highly dependent on soil composition, and marked by use of considerable surfaces of land [1][2]. Additionally, in the context of producing high value products from microbial sources, industrial byproducts such as sugarcane and sugar beet molasses can be valorized and used as alternative nutrient sources [3][4]. This contributes to a reduction of the overall cost of production, which could be driven further down through the optimization of various process parameters pertaining to both the fermentation and extraction processes.
While arguably less stable than their synthetic counterparts, microbial pigments often possess numerous bioactive properties which make them desirable in the pharmaceutical, cosmetic, and even textile sectors. These properties, coupled to their high yields and capacity to proliferate in low-cost substrates, make microorganisms prime candidates for the industrial-scale production of various pigments [5]. Indeed, the industrial-scale production of pigments such as natural carotenoids from microbial sources is already a common industry practice [6]. The extensive research which has culminated in the widespread adoption of microbial synthesis of carotenoids in particular was driven by considerable demand due to their interesting pharmaceutical and cosmetic properties in addition to increased consumer wariness of synthetic food dyes [7][8]. Efforts are currently underway to similarly produce diverse pigments such as violacein, a purple bisindole pigment with antioxidant, antimicrobial, antipyretic, analgesic, and antitumoral properties [9]. The impetus to produce biosynthetic natural pigments is animated by shifting consumer trends towards health-conscious and sustainable consumption which, in this context, entails eschewing synthetic dyes and alleviating dependence on petrol derivatives. The economic implications of contributing to this shift are also considerable, with the size of the agricultural and food colorant markets expected to reach 2.03 billion USD and 3.75 billion USD, respectively [10]. Generally, microbial pigments are produced and accumulated intracellularly and thus the cell wall must be ruptured for pigment recovery to be performed. Cell disruption techniques, such as mechanical processes or chemical methods, may be used individually or in various combinations to ensure consummate recovery of the pigments.
2. Mechanical Cell Disruption
A considerable advantage of mechanical cell disruption techniques is that, in addition to being quite effective, they can generally be scaled up with relative ease. However, a salient caveat is that they have poor selectivity, which may negatively influence the recovery of the pigments diffused into the medium. Indeed, considerable amounts of cell debris can cause a number of downstream complications [11]. Although this shortcoming could be remedied with downstream separation processes (membrane filtration, centrifugation, etc.), this recourse may contribute to some loss of pigment and result in increased energy consumption, which compounds the relatively costly mechanical cell disruption processes. Additionally, as with all cell disruption processes relying on frictional effects, temperature increases in the medium are to be expected and this constitutes a significant drawback for the extraction of thermolabile compounds. Depending on their chemical nature, these compounds should they be damaged, may undergo detrimental reactions or denaturation which result in either color attenuation or undesirable color changes.
2.1. Bead Milling
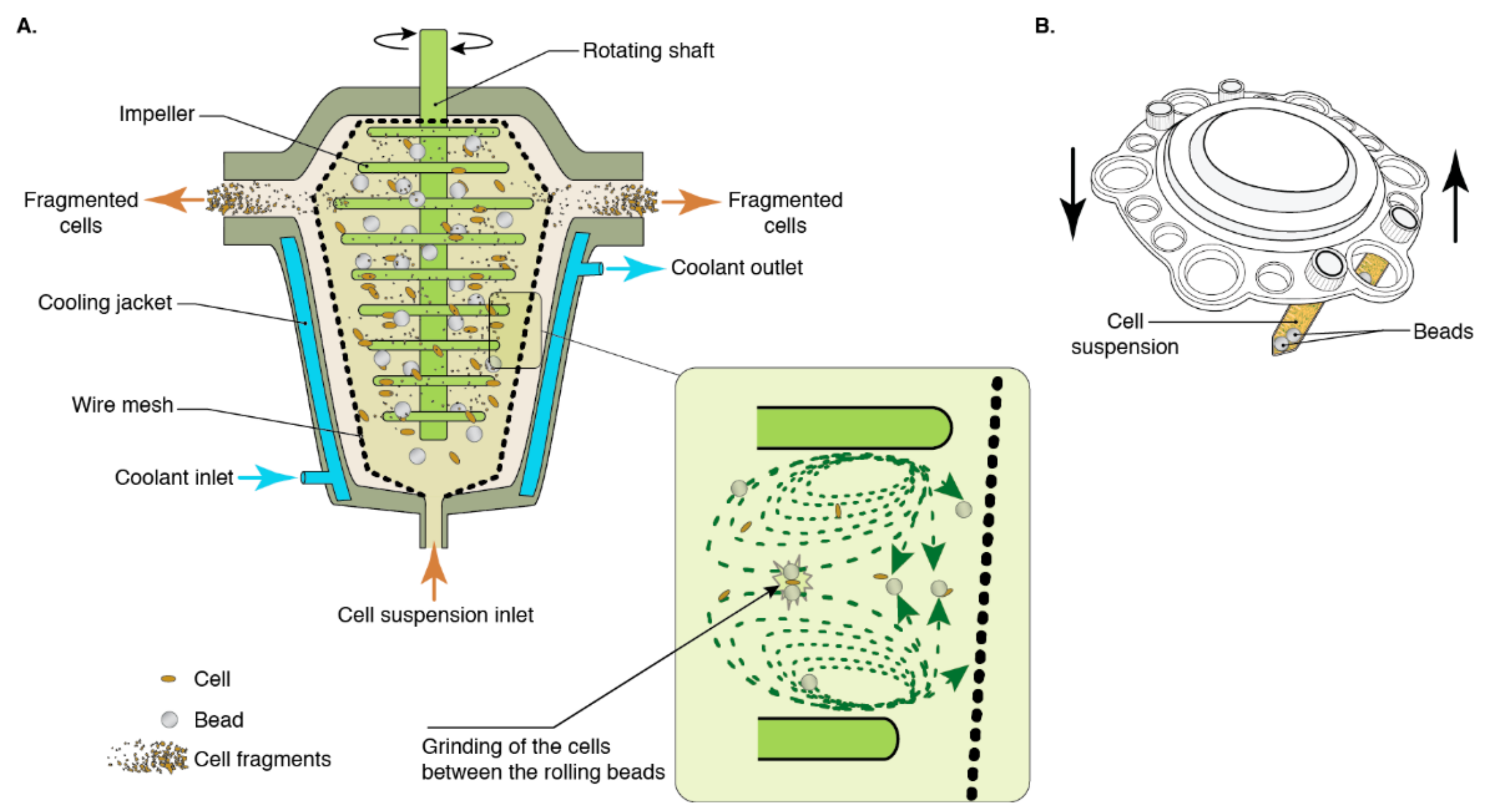
Bead milling or bead homogenization is a mechanical cell disruption process with some degree of complexity. Through this technique, cell disruption is induced through shear forces produced during the rotary movement of the cells and the beads and cell grinding between beads [12] (Figure 1). Bead-cell collisions are also purportedly implicated in the mechanism whereby bead milling disrupts cells [13]. Its parameters are bead diameter, bead density, bead filling, agitator speed, and feed rate [14]. Given that a number of components may vary depending on the make and model of the apparatus, such as the design of the grinding chamber or the agitator to name a few, some incongruencies are likely to arise should protocols be replicated with different apparatuses. Parameters like bead size, bead density, and bead filling must be optimized depending on the rigidity of the microorganism’s cell wall, the viscosity of the medium, as well as the flow rate. Optimal biomass concentration for maximum cell disruption must also be determined to enhance the yield of the overall process. There is a complex interaction between the parameters of the process, which require fine-tuning to ensure efficacy and efficiency. Indeed, inadequate parameters may lead to excessive energy consumption, which can be significantly reduced by optimization [15].
The results of bead milling can prove difficult to predict due to the complexity of parameter interactions, and experimental protocols are devised to fit the system being investigated [16].
Figure 1. Cell disruption by bead milling. (A) Continuous large-scale equipment. (B) Batch laboratory scale equipment. Insert represents the grinding mechanism between two rolling beads. Reprinted with permission from [17].
2.2. High Pressure Homogenization
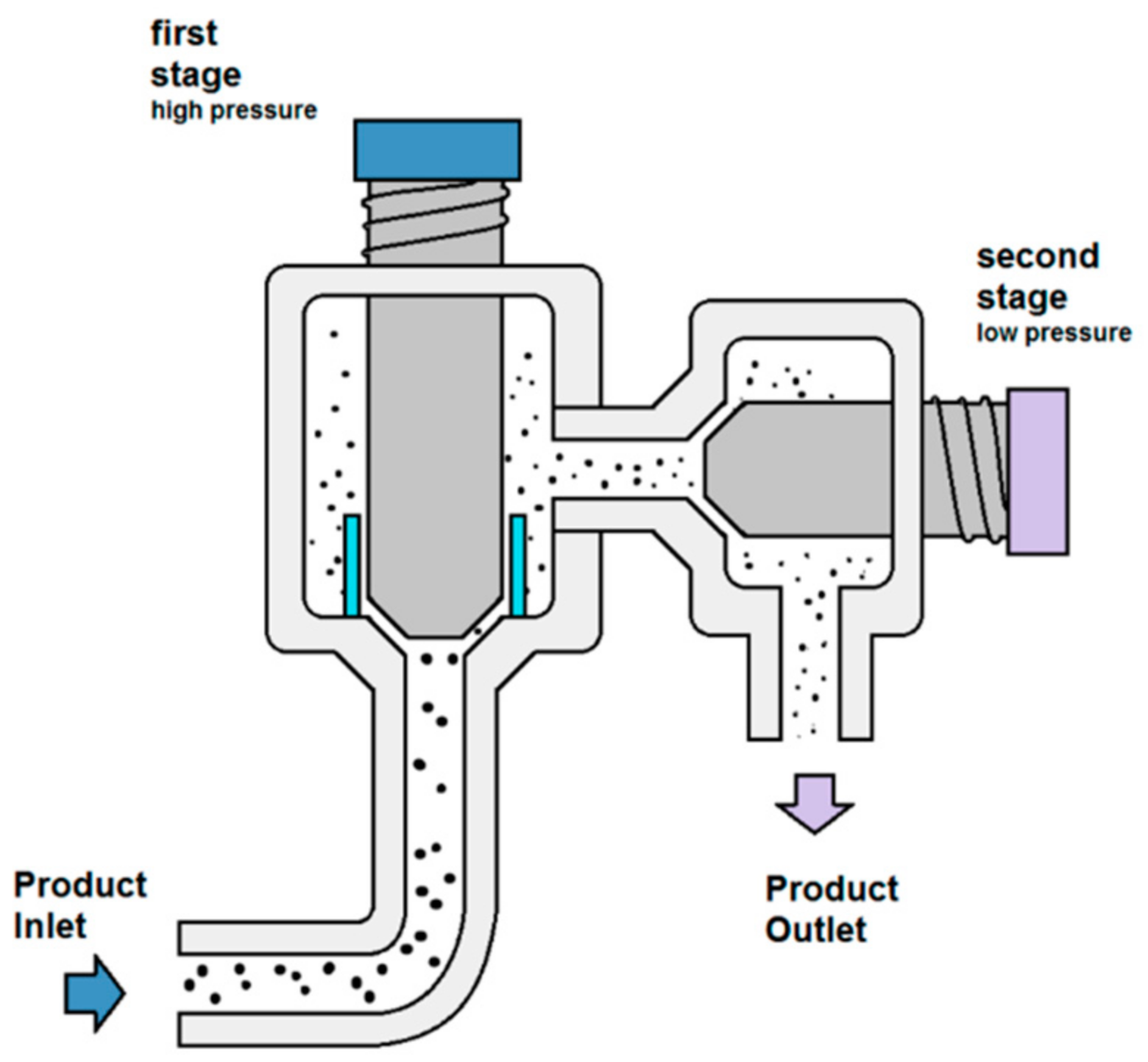
High pressure homogenization (HPH) is a highly effective and scalable method to perform cell disruption. HPH entails forcing a suspension through a strait nozzle or a valve actuated by high pressure. After passing through the valve, the suspension is released into a low-pressure chamber (Figure 2). It is chiefly suspected that cells within the suspension are subject to disruption induced by the collision between the high-speed suspension jet onto the valve surface, and shear stress induced by the pressure drop [18][19]. However, the mechanism through which cell disruption occurs via HPH is still subject for debate, and its effects are even ascribed in various sources to cavitation, turbulence, or extensional stress [20][21]. Nevertheless, empirical evidence suggests that HPH is a highly capable cell disruption method whose mettle was tested thoroughly in a variety of contexts. Homogenization effectiveness and efficiency can be enhanced by weakening the cell wall through pre-treatment via chemical or enzymatic attacks on the compounds that contribute to its overall strength [22]. Samples are usually subjected to a number of passes through a high-pressure homogenizer to yield the desired results. The method is nevertheless not without its drawbacks, with cooling being required in virtually all instances very high pressures are used, naturally resulting in temperature increases detrimental to the chemical integrity of thermolabile compounds. HPH results in the formation of cell debris, which can incur additional downstream costs to filter and eliminate these waste products. Additionally, the solutions must be pumpable. The risk of agglomeration within the low-pressure chamber or within the narrow nozzle is non-negligible, particularly when the sample is subjected to temperature increases due to high shear stress, and the apparatus is usually costly to maintain and operate [23]. These drawbacks still present challenges for the adoption of this particular technique in industrial settings.
Figure 2. Schematic representation of a two-stage high-pressure homogenizer. Reprinted from [24].
2.3. Ultrasonication
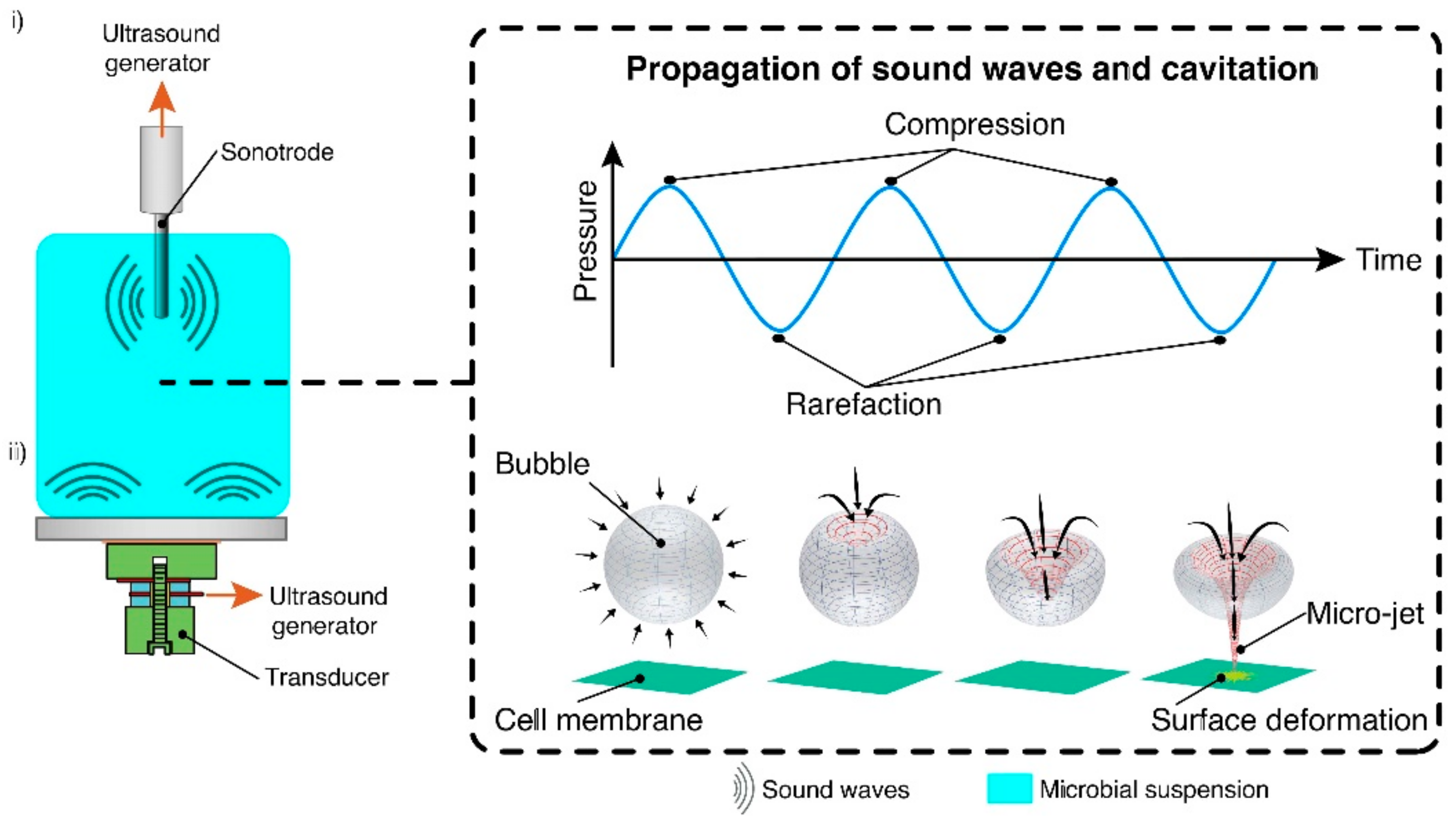
Ultrasonication is a technique whereby cavitation is created by introducing ultrasonic waves into a liquid medium via a resonance rod. It occurs when the vapor bubbles of a liquid form in a locus where the liquid’s pressure is lower than that of its vapor pressure. The bubbles distend under negative pressure and compress under positive pressure, causing a brusque and powerful collapse of these bubbles (Figure 3) [25]. Its capacity to act as a cell disruption technique is predicated on the aptness of cavitation forces at inflicting damage to the cell wall, and is this highly dependent on said cell wall’s composition. The characteristics of the cell wall considerably impact the effectiveness of this technique and microbial species with high cell wall rigidity may be inadequately processed through USN.
Figure 3. Schematic presentation of the formation of micro-jets through the collapse of acoustic cavitation bubbles. (i) Ultrasonic probe-based system, (ii) Ultrasonic transducer-based system (ultrasonic bath). Reprinted from [26].
3. Conclusions
The roster of pigments sourced from microorganisms only genuinely grows whenever successful and plentiful pigment biosynthesis is complemented by an effective and economically viable extraction method. Mechanical cell disruption techniques have exhibited considerable potential in both laboratory and pilot applications as well as industrial use at extracting various pigments from various microorganisms. Their parameters can be optimized to ensure maximum extraction yield, minimal pigment loss due to heat generation, and minimized energy consumption. One technique can possess attributes which render it a more adequate option than its counterparts at extracting pigments from defined microorganisms, and their effectiveness is highly contingent on the shape of the microorganism, the structure of its cell wall, its size, and the thermolability of its pigments among others. These attributes are further complemented by economical constraints which factor in the overall time to complete the process, the energy it consumes, its overall pigment yield, and the ease with which pigments are isolated and purified as well as industry-specific constraints such as severely discouraged solvent use in the food industry.
References
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed Electric Field-Assisted Extraction of Valuable Compounds from Microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552.
- Valduga, E.; Tatsch, P.O.; Tiggemann, L.; Treichel, H.; Toniazzo, G.; Zeni, J.; Di Luccio, M.; Fúrigo, A. Carotenoids Production: Microorganisms as Source of Natural Dyes. Quim. Nova 2009, 32, 2429–2436.
- Aksu, Z.; Tuǧba Eren, A. Carotenoids Production by the Yeast Rhodotorula Mucilaginosa: Use of Agricultural Wastes as a Carbon Source. Process Biochem. 2005, 40, 2985–2991.
- Whallans, R.C.M.; de Janaina, F.M.B. Optimization of Agroindustrial Medium for the Production of Carotenoids by Wild Yeast Sporidiobolus Pararoseus. Afr. J. Microbiol. Res. 2015, 9, 209–219.
- Buzzini, P.; Martini, A. Production of Carotenoids by Strains of Rhodotorula Glutinis Cultured in Raw Materials of Agro-Industrial Origin. Bioresour. Technol. 2000, 71, 41–44.
- Park, P.K.; Kim, E.Y.; Chu, K.H. Chemical Disruption of Yeast Cells for the Isolation of Carotenoid Pigments. Sep. Purif. Technol. 2007, 53, 148–152.
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine Carotenoids: Biological Functions and Commercial Applications. Mar. Drugs 2011, 9, 319–333.
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of Carotenoids. Mar. Drugs 2011, 9, 625–644.
- Im, H.; Choi, S.Y.; Son, S.; Mitchell, R.J. Combined Application of Bacterial Predation and Violacein to Kill Polymicrobial Pathogenic Communities. Sci. Rep. 2017, 7, 1–10.
- Venil, C.K.; Dufossé, L.; Devi, P.R. Bacterial Pigments: Sustainable Compounds With Market Potential for Pharma and Food Industry. Front. Sustain. Food Syst. 2020, 4, 1–17.
- Kholany, M.; Trébulle, P.; Martins, M.; Ventura, S.P.M.; Nicaud, J.M.; Coutinho, J.A.P. Extraction and Purification of Violacein from Yarrowia Lipolytica Cells Using Aqueous Solutions of Surfactants. J. Chem. Technol. Biotechnol. 2020, 95, 1126–1134.
- Gong, M.; Bassi, A. Carotenoids from Microalgae: A Review of Recent Developments. Biotechnol. Adv. 2016, 34, 1396–1412.
- Nooralabettu, K.P. Cell Disruption Techniques. In Downstream Process Technology: A New Horizon In Biotechnology; PHI Leaning Private Limited: New Delhi, India, 2010; pp. 101–126.
- Montalescot, V.; Rinaldi, T.; Touchard, R.; Jubeau, S.; Frappart, M.; Jaouen, P.; Bourseau, P.; Marchal, L. Optimization of Bead Milling Parameters for the Cell Disruption of Microalgae: Process Modeling and Application to Porphyridium Cruentum and Nannochloropsis Oculata. Bioresour. Technol. 2015, 196, 339–346.
- Postma, P.R.; Miron, T.L.; Olivieri, G.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M. Mild Disintegration of the Green Microalgae Chlorella Vulgaris Using Bead Milling. Bioresour. Technol. 2015, 184, 297–304.
- Burmeister, C.F.; Kwade, A. Process Engineering with Planetary Ball Millss. Chem. Soc. Rev. 2013, 42, 7660–7667.
- Koubaa, M.; Imatoukene, N.; Drévillon, L.; Vorobiev, E. Current Insights in Yeast Cell Disruption Technologies for Oil Recovery: A Review. Chem. Eng. Process. Process Intensif. 2020, 150, 107868.
- D’Hondt, E.; Martín-Juárez, J.; Bolado, S.; Kasperoviciene, J.; Koreiviene, J.; Sulcius, S.; Elst, K.; Bastiaens, L. Cell Disruption Technologies. Microalgae-Based Biofuels Bioprod. Feedstock Cultiv. End-Prod. 2017, 133–154.
- Donsì, F.; Ferrari, G.; Lenza, E.; Maresca, P. Main Factors Regulating Microbial Inactivation by High-Pressure Homogenization: Operating Parameters and Scale of Operation. Chem. Eng. Sci. 2009, 64, 520–532.
- Kleinig, A.R.; Middelberg, A.P.J. On the Mechanism of Microbial Cell Disruption in High-Pressure Homogenisation. Chem. Eng. Sci. 1998, 53, 891–898.
- Diels, A.M.J.; Michiels, C.W. High-Pressure Homogenization as a Non-Thermal Technique for the Inactivation of Microorganisms. Crit. Rev. Microbiol. 2006, 32, 201–216.
- Middelberg, A.P.J. Microbial Cell Disruption by High-Pressure Homogenization. In Downstream Processing of Proteins; Desai, M.A., Ed.; Humana Press: Totowa, NJ, USA, 2000; Volume 9, pp. 11–21.
- Hu, Y.; Bassi, A. Extraction of Biomolecules from Microalgae; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128185360.
- Comuzzo, P.; Calligaris, S. Potential Applications of High Pressure Homogenization in Winemaking: A Review. Beverages 2019, 5, 56.
- Safi, C.; Camy, S.; Frances, C.; Varela, M.M.; Badia, E.C.; Pontalier, P.Y.; Vaca-Garcia, C. Extraction of lipids and pigments of Chlorella vulgaris by supercritical carbon dioxide: Influence of bead milling on extraction performance. J. Appl. Phycol. 2014, 26, 1711–1718.
- Peng, K.; Koubaa, M.; Bals, O.; Vorobiev, E. Recent Insights in the Impact of Emerging Technologies on Lactic Acid Bacteria: A Review. Food Res. Int. 2020, 137, 109544.