
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rui Gao | + 573 word(s) | 573 | 2021-03-16 10:45:15 | | | |
| 2 | Nicole Yin | + 14 word(s) | 587 | 2021-04-06 11:42:38 | | |
Video Upload Options
Somatostatin-secreting δ-cells have aroused great attention due to their powerful roles in coordination of islet insulin and glucagon secretion and maintenance of glucose homeostasis. δ-cells exhibit neuron-like morphology with projections which enable pan-islet somatostatin paracrine regulation despite their scarcity in the islets.
1. Introduction
The islets of Langerhans are a “heterogeneous community” formed by different types of endocrine cells and nonendocrine supporting cells. In addition to glucagon-secreting α-cells and insulin-producing β-cells, somatostatin-releasing δ-cells comprise only ~5% of the islet endocrine cells [1]. δ-cells have aroused great attention in the last decade due to their powerful roles coordinating islet hormone output and the maintenance of glucose homeostasis [2].
δ-cells have been considered the intra-islet local paracrine regulator of α- and β-cells. The peptide they secrete, somatostatin, is a potent and fast inhibitor for both insulin and glucagon secretion [3][4], the two glucose-regulating islet hormones. Appropriate somatostatin secretion can therefore effectively prevent possible oversecretion of insulin and glucagon, preventing large fluctuation of plasma glucose levels. Although small in number, δ-cells are able to communicate with the majority of α- and β-cells in the same islets, a remarkable ability that is attributable to their unique neuron-like morphology: the dendrite-like process can extend typically several cell lengths, forming a pan-islet paracrine network [5]. Ablation of this network can lead to severe hypoglycemia, impaired islet function and neonatal death in rodents[6], highlighting the functional importance of δ-cells in maintaining glucose homeostasis. Despite their role in islet function, how exactly δ-cells aAblation of somatostatin cells leads to impaired pancreatic islet function and neonatal death in rodentsre regulated remains elusive. However, transcriptomic data from enriched δ-cell fractions revealed that δ-cells express a wide range of hormone and neurotransmitter receptors, including glucagon receptor (GCGR), glucagon-like peptide-1 receptor (GLP-1R), glutamate receptor 4 (GluR4) and growth hormone secretagogue receptor (GHSR)[7][8], which suggest the ability of δ-cells to sense paracrine, endocrine, neural and nutritional signals.
2. Structural Basis for δ-Cell Paracrine Regulation
In rodent islets, δ-cells are situated in the outer islet mantle closer to α-cells, while in humans they are found scattered throughout the islets [9]. However, their proximity to blood vessels is well conserved cross-species. Different from the spherical α- and β-cells, mouse δ-cells exhibit neuron-like morphology with a well-defined cell soma and filopodia-like processes (Figure 1). The elongated projections (ranging from 2 to 27 μm [5][10]) are somatostatin-release competent and can compensate for the scarcity of δ-cells, enabling pan-islet somatostatin paracrine regulation. This morphological feature is not conserved in all species. In human islets, δ-cells have fewer axon-like projections and are significantly more compact than mouse δ-cells [11]. However, human δ-cells are scattered throughout the islets and are intermingled with α- and β-cells. Therefore, their pan-islet paracrine function is conserved in human islets. It is also intriguing that these projections (or filopodia) are highly plastic, showing rapid morphological change in response to vascular endothelial growth factor A (VEGF-A) and insulin-like growth factor 1 (IGF-1) [10]. Therefore, it is possible that the plasticity of δ-cells, together with their epigenetic adaptivity [12], contributes towards the changes of islet function in response to variable metabolic environment.
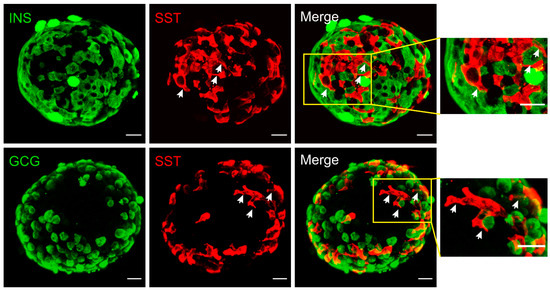
Figure 1. Immunofluorescent staining of mouse islets. Somatostatin (red), insulin (green, upper panels) or glucagon (green, lower panels). Merged panels are as indicated and areas in yellow rectangles are displayed on the right with an expanded scale. These reconstructed confocal images demonstrated the network of δ-cells and their proximity to α- and β-cells at the exterior of the islet. Note the morphology of δ-cells, which is distinctly different from α- and β-cells. The elongated projections are highlighted by arrows (white). Scale bar = 20 µm.
References
- Brissova, M.; Fowler, M.J.; Nicholson, W.E.; Chu, A.; Hirshberg, B.; Harlan, D.M.; Powers, A.C. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J. Histochem. Cytochem. 2005, 53, 1087–1097.
- Rorsman, P.; Huising, M.O. The somatostatin-secreting pancreatic delta-cell in health and disease. Nat. Rev. Endocrinol. 2018, 14, 404–414.
- Strowski, M.Z.; Parmar, R.M.; Blake, A.D.; Schaeffer, J.M. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: An in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology 2000, 141, 111–117.
- Omar-Hmeadi, M.; Lund, P.E.; Gandasi, N.R.; Tengholm, A.; Barg, S. Paracrine control of alpha-cell glucagon exocytosis is compromised in human type-2 diabetes. Nat. Commun. 2020, 11, 1896.
- Brereton, M.F.; Vergari, E.; Zhang, Q.; Clark, A. Alpha-, Delta- and PP-cells: Are They the Architectural Cornerstones of Islet Structure and Co-ordination? J. Histochem. Cytochem. 2015, 63, 575–591.
- Na Li; Zhao Yang; Qing Li; Zhen Yu; Xu Chen; Jia-Cheng Li; Bo Li; Shang-Lei Ning; Min Cui; Jin-Peng Sun; et al.Xiao Yu Ablation of somatostatin cells leads to impaired pancreatic islet function and neonatal death in rodents. Cell Death & Disease 2018, 9, 682, 10.1038/s41419-018-0741-4.
- Alice E. Adriaenssens; Berit Svendsen; Brian Y. H. Lam; Giles S. H. Yeo; Jens J. Holst; Frank Reimann; Fiona M. Gribble; Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia 2016, 59, 2156-2165, 10.1007/s00125-016-4033-1.
- Michael R. DiGruccio; Alex M. Mawla; Cynthia J. Donaldson; Glyn M. Noguchi; Joan Vaughan; Christopher Cowing-Zitron; Talitha van der Meulen; Mark O. Huising; Talitha van der Meulen; Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Molecular Metabolism 2016, 5, 449-458, 10.1016/j.molmet.2016.04.007.
- Cabrera, O.; Berman, D.M.; Kenyon, N.S.; Ricordi, C.; Berggren, P.O.; Caicedo, A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. USA 2006, 103, 2334–2339.
- Arrojo, E.D.R.; Jacob, S.; Garcia-Prieto, C.F.; Zheng, X.; Fukuda, M.; Nhu, H.T.T.; Stelmashenko, O.; Pecanha, F.L.M.; Rodriguez-Diaz, R.; Bushong, E.; et al. Structural basis for delta cell paracrine regulation in pancreatic islets. Nat. Commun. 2019, 10, 3700.
- Huising, M.O.; van der Meulen, T.; Huang, J.L.; Pourhosseinzadeh, M.S.; Noguchi, G.M. The Difference delta-Cells Make in Glucose Control. Physiology 2018, 33, 403–411.
- Li, Q.; Cui, M.; Yang, F.; Li, N.; Jiang, B.; Yu, Z.; Zhang, D.; Wang, Y.; Zhu, X.; Hu, H.; et al. A cullin 4B-RING E3 ligase complex fine-tunes pancreatic delta cell paracrine interactions. J. Clin. Investig. 2017, 127, 2631–2646.




