
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ilaria Rea | + 3815 word(s) | 3815 | 2021-03-11 03:49:19 | | | |
| 2 | Peter Tang | Meta information modification | 3815 | 2021-03-24 05:26:20 | | |
Video Upload Options
The cost-effective fabrication process, the high internal surface area, the tunable pore size, and the photonic properties made the PSi an appealing transducing substrate for biosensing purposes, with applications in different research fields. Different optical PSi biosensors are reviewed and classified into four classes, based on the different biorecognition elements immobilized on the surface of the transducing material.
1. Introduction
The term “biosensor” refers to an analytical and powerful tool made up of a bioreceptor (i.e., a biological recognition element) connected to a transducing substrate [1][2][3]. The bioreceptor is a biologically active material (i.e., enzyme, protein, antibody, oligonucleotide, and so on) responsible for the device selectivity; the transducer converts a specific biological recognition event (i.e., antibody-antigen interaction) into a measurable signal in real-time.
The first biosensor was made by Professor L.C. Clarck in 1956 for oxygen detection in the blood of patients undergoing surgery [4]. To date, incredible progress has been made both in technology and applications of biosensors, representing a very attractive research field, as demonstrated by the vast literature in the last 20 years.
It is expected that the biosensor market could reach U.S. Dollar (USD) 36.0 billion by 2027, due to the rising demand for such devices, with applications not only in biomedical diagnosis but also in environmental and food quality monitoring, industrial process control, agriculture, and others [5]. Moreover, the request for low-cost and user-friendly devices, with a fast-response time, is slowly replacing the currently available techniques for the identification of analytes, which are time-consuming, expensive, and require specialized laboratory equipment.
The design and the fabrication of the sensing system require the meticulous research of materials that have the desired transducer properties. In this context, nanostructured materials gained prominence in many applications because of their unique physicochemical characteristics over their bulk counterpart, such as high surface-to-volume ratio, small size, light absorption, optical sensitivity, and electrical and thermal conductivity [6][7]. Moreover, nanostructure-based biosensors show enhanced biosensing performances over conventional detection methods (i.e., higher sensitivity, fast response time, and low limit of detection (LoD)) [8][9][10]. Among the many available nanomaterials (e.g., quantum dots (QDs), metallic nanoparticles, carbon nanotube), porous silicon (PSi) has outstanding windows for applications in several research fields, from biosensing to drug delivery, thanks to its well-known optical and physical features [11][12][13].
Although it was accidentally discovered in 1956 by Uhlirs during an electrochemical experiment [14], this material received due attention only in 1990, when Canham discovered its intrinsic photoluminescence (PL) at room temperature [15]. Since the first experiments on PSi as a biosensor platform two decades ago, it is still an actual topic for research studies, as evidenced by the sustained number of scientific papers on PSi-based sensors published every year in peer-review journals [16].
PSi exhibits air-filled pores and a high surface area (up to 800 m2/g) [17]; these interesting characteristics, together with versatile surface chemical modification, tunable characteristic sizes, photoluminescence, biocompatibility, and biodegradability makes this material an appealing optical transducer [12][18][19][20][21][22]. Moreover, due to the high surface reactivity of PSi [23], several biomolecules can be easily immobilized within the porous matrix, by using well-established chemical approaches [13][24]. Since non-specific interactions reduce the selectivity of a biosensing platform, a blocking process of residual groups is generally performed as a final step of functionalization, by using agents like maleimide, bovine serum albumin, etc.
PSi is commonly obtained via electrochemical etching, a fabrication strategy that does not require expensive equipment, and allows a good reproducibility of the fabricated PSi substrates. This technique enables a fine control on the pore size and on the optical response of the material [25][26]. This tuning is generally not easily achievable in other porous materials, such as porous alumina [27][28] or porous titania [29][30].
Recently, PSi was also explored as a host matrix for the immobilization of several nanomaterials (i.e., QDs, graphene oxide, carbon dots) due to its high internal volume [31][32][33][34][35]. This feature made PSi more interesting than the other nanomaterials for biosensing applications. In fact, the possibility of combining different elements into the PSi matrix paved the way for the development of hybrid platforms that showed enhanced biosensing performances in terms of sensitivity, signal enhancement, signal stability, and dual-mode detection.
The optical response of PSi structures are strongly affected by their structural properties such as porosity (ratio of the fraction of voids in the layer to total volume), layer(s) thickness, and pore size and morphology [25]. Based on the International Union of Pure and Applied Chemistry (IUPAC) definition, three different PSi structures, with different pore sizes, could be distinguished—microporous Si (pore diameter d < 2 nm), mesoporous Si (pore diameter 2 < d < 50 nm), and macroporous Si (pore diameter d > 50 nm) [36]. Instead, the pore morphology is the least quantifiable aspect [25] and considers properties like shape (i.e., smooth, branched, facetted), orientation, and interconnection between pores. All these features are strongly influenced by the monocrystalline silicon and the anodizing regimes [25]. Macroporous and mesoporous Si are commonly employed as biosensing platforms to allow the attachment of biomolecules within their matrices. The capture of analytes can be monitored via reflectance, when dealing with macroporous or mesoporous structures (i.e., monolayer, Bragg mirror) [37][38], or via photoluminescence with mesoporous matrices (i.e., resonant microcavity, nanowires) [39][40]. Unfortunately, the intrinsic PL of PSi is usually unstable, since it strongly depends on the surrounding chemical environment that can influence its intensity [11]. For this reason, several works reported on the use of PSi substrates to embed emitting materials or molecules within pores, which sharpen the emission spectrum and amplify the signal [13][39][41].
Additionally, the versatile surface modification, optical tunability, low-cost fabrication, and label-free working conditions confer added value to this material. PSi is also compatible with microelectronics and MEMS fabrications systems [42][43][44]. Furthermore, the material’s biocompatibility allows the development of implantable biosensing devices that could be used for real-time detection of in vivo analytes [45].
PSi optical devices are widely used to detect different biomolecules (i.e., DNA, enzymes, cells, bacteria, antigen). Most PSi-based biosensors work in a label-free modality. The optical transduction principle relies on the change of the refractive index of the PSi layer, due to the substitution of air inside the pores with a target analyte. The change of the refractive index is detected as a wavelength shift of the corresponding reflectivity spectrum. Moreover, the intrinsic PL signal of PSi might be used as a further transduction mechanism for biosensing applications, by monitoring its variation upon a biomolecular recognition event.
2. PSi: From Fabrication to the Bioconjugation
Although different strategies were developed to fabricate PSi structures, the anodic electrochemical etching of a crystalline silicon wafer in hydrofluoric acid (HF) water solution, remains the most commonly used. In 1956, Uhlirs first observed the formation of PSi during electrochemical procedures for polishing of silicon and germanium wafers [14]. This methodology is advantageous to other fabrication techniques (i.e., metal-assisted chemical etching [46][47], etc.), since it allows us to obtain different photonic structures with a high reproducibility [48]. The electrochemical etching is performed into an etching cell in which the silicon wafer acts as an anode and metals (e.g., platinum) act as a cathode. The current flow between the two electrodes leads to the dissolution of the crystalline structure, forming pores in the silicon wafer. The pore formation process is controlled by a complex mix of electronic and chemical factors [25]. A schematization of the electrochemical cell is shown in Figure 1A. Different porous silicon structures, with specific morphological and optical properties, can be engineered by changing the type of silicon wafer conductivity, doping level, concentration of hydrofluoric acid in electrolyte, applied voltage, current density, light intensity, and temperature [49][50]. Metal-assisted chemical etching (MACE) is another technique used for the fabrication of PSi. It is less common than the above-mentioned anodic etching. In a typical MACE process, a thin layer of noble metal (i.e., Au, Pt, etc.) sputtered on the silicon surface, catalyzes the etching of the material when placed in an oxidizing solution with hydrofluoric acid (HF). This technique generates several silicon nanostructures of tailored geometry, i.e., nanopores, porous silicon layers, nanowires, nanoneedles [51][52][53]

Figure 1. (A) Electrochemical etching setup; and (B) passivation strategies to stabilize PSi surfaces.
The freshly etched PSi is composed of hydride terminated groups (Si-H, Si-H2, and Si-H3), which make PSi nanostructure highly reactive and unstable. The aging effects, responsible for the uncontrolled growth of native oxide and the degradation of PSi matrix in alkaline or aqueous environments, strongly affect this material’s physicochemical and optoelectronic properties [54]. These effects might lead to zero-point drifts in the reflectivity spectrum and reduce, as a consequence, the sensitivity of the devices [25]. In this context, a proper surface chemical modification plays an important role to stop aging and to stabilize the PSi nanostructure (Figure 1B). Among the various developed treatments, the intentional growth of an oxide layer, under controlled conditions, represents one of the main approaches used to stabilize the hydrogen-terminated PSi surface. Despite the various available strategies for oxidizing the PSi matrix (i.e., ozone oxidation, electrochemical oxidation, and oxidation in aqueous solution), thermal oxidation is the most commonly used technique, by partially or fully replacing the reactive Si-Hx species into Si-O bonds [55]. This procedure is not only used to passivate the surface but also to convert a hydrophobic surface into a hydrophilic one. The rate of passivation is highly dependent on the temperature and on the duration of the treatment. Therefore, Shtenberg et al. demonstrated that the thermal oxidation of PSi nanostructures at 800 °C for 1 h, profoundly enhanced the sensitivity of the biosensor with respect to the process conducted at lower temperatures [56]. Moreover, the high temperatures allowed the conversion of the PSi structure into a silica skeleton. Due to its simplicity, the oxidation process represents the most used method to passivate PSi.
Other methodologies, involving the substitution of Si-Hx bonds into Si-C bonds, are proposed. The greater resistance of Si-C bonds to nucleophilic attack by water or hydroxide provides more excellent protection of the PSi matrix from physiological conditions and harsh basic environments. This process can be obtained by hydrosilylation, first demonstrated by Buriak et al. [23][31][32]. This approach is carried out by reacting liquid unsaturated compounds (i.e., terminal alkenes or alkynes) with Si-Hx species present on freshly etched PSi. This reactive mechanism can be promoted by heat, light, or chemicals (Lewis acid catalysis), and requires an inert atmosphere as well as deoxygenate/dried reagents.
The thermal carbonization of PSi represents another widely used approach to achieve highly stable Si-C bonds within the PSi nanostructures. Gaseous molecules as carbon sources (i.e., acetylene or ethylene) at high temperatures, can be easily adsorbed on the PSi, thus creating complete surface carbonization. The advantage of using this technique is the fast diffusion of gas molecules in small pores and the negligible steric hindrance with respect to liquid organic compounds [57]. Therefore, the temperature, used for the treatment, strongly influences the PSi surface termination—a hydrocarbon-terminated PSi is obtained at a temperature below 650 °C, generating a hydrophobic surface, due to some Si-H bonds, which are still present in the pores (thermally hydrocarbonized PSi, THCPSi); on the contrary, a completely carbonized PSi (thermally carbonized PSi, TCPSi) is obtained at a temperature above 800 °C, resulting in a hydrophilic surface due to the complete desorbing of hydrogen atoms from the PSi matrix [58].
The stabilization of the porous material, with the above-mentioned techniques, is a crucial factor for the development of stable platforms for biosensing purposes. The selectivity of the device is another essential element, achievable by using specific bioprobes that can be or is directly grown into the PSi matrix (in situ synthesis) or is synthetized ex situ, and is then, immobilized on the surface.
After the PSi stabilization, different linkers can be used to graft biomolecules. The oxidized PSi can be readily modified via the silanization process (Figure 2A), based on the use of silane coupling agents (i.e., APTES, APDMES) characterized by different terminal motifs (i.e., NH2; SH, COOH; CHO) that act as anchorage sites for proteins, antibody, DNA and others [59]. Silane-based chemistry is one of the most exploited modifications of porous silica-based synthetic or natural materials since it offers an effortless way to attach a biological or chemical molecule to the porous surface [60][61][62]. Silanization requires the availability of hydroxyl groups on the PSi surface in order to hydrolyze the alkoxy groups of the alkyl silane molecule, thus forming Si-O-Si bonds [63]. Thiol or primary amino groups can be also used for grafting biomolecules. Their exposure on PSi surface can be obtained via a new silanization process, known as “ring-opening-click reaction”, proposed by Sailor et al., in which the heterocycles silanes, having Si-N or Si-S motifs in the ring, undergo a ring-opening reaction to modify the hydroxylated porous walls. The proposed surface chemistry, obtained in mild conditions and without the formation of by-products, does not interfere with the protein activity (Figure 2B) [64]. Although silanol chemistry is the most conventional approach for bioprobe immobilization, it is well known that Si-O-Si bonds are characterized by the lack of stability in aqueous and alkaline media, causing degradation of the coating surface, which is the main problem related to this chemistry.
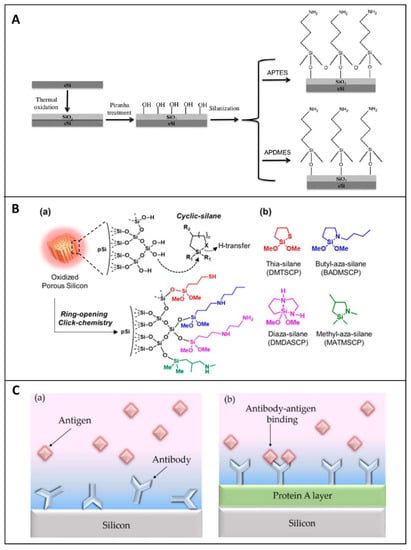
Figure 2. (A) Scheme of PSi functionalization through thermal oxidation and silanization with APTES or APDMES. Reprinted with permission from Reference [59]. (B) (a) Scheme of oxidized porous silicon modified through a ring-opening click reaction; (b) structure of reagents used for the study—thia-silane (DMTSCP, 2,2-dimethoxy-1-thia-2-silacyclopentane), butyl-aza-silane (BADMSCP, N-n-butyl-aza-2,2-dimethoxy-silacyclopentane), diaza-silane (DMDASCP, 2,2-dimethoxy-1,6-diaza-2-silacyclooctane), and methyl-aza-silane (MATMSCP, N-methyl-aza-2,2,4-trimethyl-silacyclopentane). R1 = OMe, Me. R2 = H, Me. Reprinted with permission from Reference [64] https://pubs.acs.org/doi/10.1021/jacs.6b08614 (accessed on 16 December 2020). Copyright (2016) American Chemical Society. (C) (a) Cartoons of randomly immobilized antibodies via physical adsorption and (b) properly orientated on the PSi matrix by using an intermediate layer of Protein A. Reprinted with permission from Reference [65] https://creativecommons.org/licenses/by/4.0/ (accessed on 16 December 2020).
Higher stability can be obtained through the Si-C bonds. The carboxyl acid group of undecylenic acid, as a result of the hydrosilylation process, are currently activated via 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) coupling agent for loading primary amine-containing biomolecules through direct reaction or via N-hydroxysulfosuccinimmide (NHS) [31]. Finally, the same surface chemical modification adopted for hydrosilylated-PSi can be applied for TCPSi and THCPSi. Sciacca et al. proposed a radical coupling reaction by using a radical initiator benzoyl peroxide and a dicarboxylic acid as a linker, generating a surface coated with carboxyl acid groups. This surface can be used to attach amino-terminal biomolecules via EDC/NHS chemistry [66].
The performances of a bio-device strongly depend on the right orientation of the biomolecules. This factor is particularly important for the immobilization of antibodies. Several research groups reported a valid strategy to optimize the biomolecules orientation on PSi matrix, by covering the surface with a layer of protein A. In such a design, the protein A interacts with the fragment crystallizable (FC) region of antibodies, leaving the antigen-binding fragment (Fab) region available toward the antigen (Figure 2C). This methodology allows proper anchoring of the antibody to the surface, maximizing its binding capability [65].
The stability and sensitivity of a biosensor are crucial when the target is in complex biological systems. In this context, the minimization of the unspecific adsorption of unwanted biomolecules is required. This aim can be achieved by using blocking agents to cap the available reactive sites (i.e., polyethylene glycol, maleimide, bovine serum albumin).
3. PSi Optical Transducer for Biosensors Development
The PSi structure is by far one of the most fascinating materials for the construction of a variety of biosensors, due to its optical, electrical, and chemical properties. To date, optical PSi biosensors are the most used label-free devices in biosensing, due to the low-cost production, high sensitivity, and real-time analysis. The label-free sensing mechanism of PSi optical structures is studied by interferometric reflectance spectroscopy (IRS), based on the monitoring of the refractive index (RI) changes on the photonic structure, induced by a specific bio-recognition event [67]. In the IRS set-up, an incident white light is reflected from the two typical interfaces of the PSi structure (air/PSi and PSi/Si bulk), generating a fringe pattern in the reflectance spectrum, known as Fabry-Pérot, which depends on the effective optical thickness (EOT = 2nL, where n is the average refractive index and L is the thickness of PSi layer). The Fabry-Pérot relationship is described by Equation (1):
in which m is an integer and λ the wavelength of the incident light [68]. Therefore, the EOT is recorded by Fast Fourier Transform (FFT) of the reflectivity spectrum (Figure 3a–d). PSi is defined as a mixture of air and silicon in which the effective refractive index is dependent on the content of air inside the pores. When the air in the porous matrix is replaced with an analyte, an increase in the RI occurs, producing a shift of the reflectivity spectrum to longer wavelengths (red-shift). Moreover, a decrease in the average RI of the material causes a shift to shorter wavelengths (blue-shift); this phenomenon is generally ascribable to the oxidation/corrosion of the PSi skeleton [38][69].
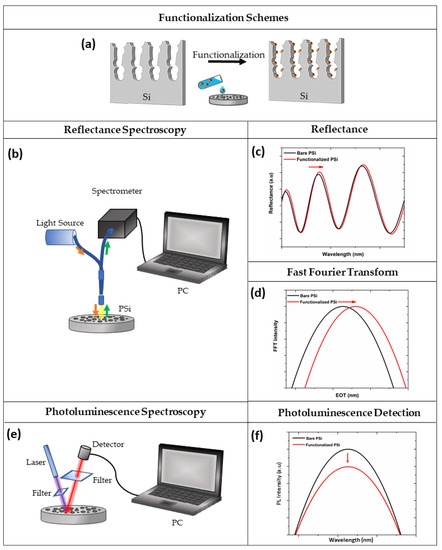
Figure 3. (a) Functionalization scheme of PSi; (b) optical setup for spectroscopy reflectometry, (c) reflectivity, and (d) FFT spectra before (black line) and after (red line) PSi functionalization; (e) optical setup for photoluminescence spectroscopy, and (f) corresponding spectra before (black line) and after (red line) the functionalization procedure.
Although all PSi devices are able to detect the presence of analytes within the pores, the optical response of the material can be finely tuned by changing the layers porosity, thickness, and number. The PSi monolayer is the simplest photonic structure used in biosensing, whose spectrum shows a periodic behaviour typical of Fabry-Pérot interferometer. However, more complex multi-layered structures with different porosities (i.e., Bragg mirror, microcavity, PSi rugate filter) can be fabricated, showing enhanced optical properties. For example, the reflectivity spectra of the Bragg mirror show a wide range of wavelengths having high reflectance (i.e., stopbands) whose maximum reflectance value is dependent on the number of layers in the structure. Moreover, to detect the infiltration of analytes into the pores, the spectral position of one edge of the stopband is monitored [70][71]. The typical narrow spectral feature of a microcavity makes this photonic structure easy to control upon molecular infiltration, with respect to the Bragg mirror. Moreover, as previously reported, PSi exhibits an intrinsic PL signal at room temperature. Therefore, the modulation of this signal, caused by a biological recognition event, might be employed for biosensing purposes. A scheme of the optical setup for PL measurements is shown in Figure 3e,f.
The main drawback of label-free, PSi-based biosensors is a low sensitivity, in the micromolar range, due to the slow and limited mass-diffusion inside the matrix. This limit is overcome through the fabrication of more complex and multi-layered optical structures (i.e., Bragg mirror, rugate filters, microcavities, ring resonators). An example is provided by the open-ended PSi microcavity membrane that allows a fast response time and a more favourable interaction between the analyte and the inner surface [72][73]. Moreover, the sensitivity of PSi-based biosensors can be greatly improved by optimizing the experimental platform engineering [74][75], data processing methodologies [76][77], as well as signal amplification mechanisms [78]. Moreover, the PSi matrix can be integrated with inorganic materials (i.e., quantum dots, graphene oxide, titanium dioxide) allowing the realization of hybrid devices with advanced properties, and improving the biosensing performances (i.e., sensitivity, stability, the limit of detection). In the following sections, the realization of PSi-based devices are reported, highlighting the enormous versatility of PSi as a transducer surface for the development of different types of biosensors.
4. PSi Biosensors for Analytes Detection in Complex Matrices
Many studies highlight the ability of biosensors to discriminate between target and non-target sequences, reaching a high sensitivity level. Although most of these analyses were carried out in clean buffers, the detection of analytes in complex media is still a challenge. The cross-reactivity and the non-specific attachment of biomolecules must be avoided in order to obtain a reliable result. This issue could be achieved by treating the surfaces with blocking agents (i.e., Tris, BSA, PEG). In some cases, additional steps, including pre-treatment or sample separation through suitable methodologies, are required to improve the specificity. Involving PSi as a transducer material for the detection of analytes in complex matrices, not only requires a proper surface stabilization, but also the appropriate tailoring of the porous matrix. The most common real samples are blood or serum, saliva, water, bacterial lysates, and human isles of Langerhans [78][79][80][81].
Bonanno et al. developed a PSi Bragg-mirror-competitive test to detect opioids in urine specimens. They demonstrated that, by varying the device chemical surface and volume of urine sample added to the porous matrix, sensitivity and specificity of the assay could be improved. Additionally, the fabrication of PSi layers, with different porosity, improved the infiltration of macromolecules through the pores. Good results were obtained by using a device that was previously oxidized, amino-silanized, and subsequently, exposed to bovine serum albumin (BSA). Finally, a morphine analogue (M3G) was covalently bound to lysine groups exposed on the BSA-PSi platform. The free drugs, contained in urine samples and analogues, bound to the matrix, competed for the binding site of the antibodies. An indirect methodology was used to detect the free drug, measuring an LoD of 0.018 µM and making the platform appealing for POC applications [82][83].
The foodborne diseases represent a public health problem, caused by the contamination of food with several types of microorganisms or mycotoxins that could occur during the several steps of food production. The ingestion of contaminated food is the cause of serious complications. For this reason, the rapid detection of bacterial contamination in the complex food industry water is crucial to prevent foodborne diseases. This issue was pursued by Massad-Ivanir et al. They designed a PSi-based biosensor for the real-time detection of E. coli in complex water samples. The silanized-PSi was modified with GA followed by exposure to SA. Finally, a biotinylated E. coli antibody was bound to the device. In the biosensing experiment, the interaction between E. coli cells-antibody was monitored as a decrease in the intensity of the reflected light, demonstrating an LoD of 103 cells mL−1 in the food industry water, without pre-enrichment of the sample. Such a device could be considered a promising tool to detect contaminations during product processing, reducing the risk of the spread of pathogens [81].
A huge step forward was taken by the Voelcker group, which investigated the use of PSi optical rugate filter like an in vivo biosensor. First, the stability and the biocompatibility of thermally oxidized and thermal hydrocarbonized devices were compared in in vitro studies. Data obtained demonstrated that not only did the THC-PSi maintain its structural and optical integrity, but its pre-incubation in cell medium (DMEM) for 10 days made the material not cytotoxic. As a result, the PITHC-PSi, subcutaneously implanted in a murine model, revealed that the optical signal was recorded through the skin of the mice 1 week after implantation, emphasizing how the stabilization of the surface is important to minimize the degradation of the device in physiological media [45].
References
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159.
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8.
- Thvenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification (Technical Report). Pure Appl. Chem. 1999, 71, 2333–2348.
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 2006, 102, 29–45.
- Biosensors Market Size Worth $36.0 Billion By 2027|CAGR: 7.9%. Available online: (accessed on 16 August 2020).
- Kreyling, W.G.; Semmler-Behnke, M.; Chaudhry, Q. A complementary definition of nanomaterial. Nano Today 2010, 5, 165–168.
- Nasrollahzadeh, M.; Issaabadi, Z.; Sajjadi, M.; Sajadi, S.M.; Atarod, M. Types of Nanostructures. In Interface Science and Technology; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 28, pp. 29–80.
- Su, H.; Li, S.; Jin, Y.; Xian, Z.; Yang, D.; Zhou, W.; Mangaran, F.; Leung, F.; Sithamparanathan, G.; Kerman, K. Nanomaterial-based biosensors for biological detections. Adv. Health Care Technol. 2017, 3, 19–29.
- Pirzada, M.; Altintas, Z. Nanomaterials for Healthcare Biosensing Applications. Sensors 2019, 19, 5311.
- Malhotra, B.D.; Ali, M.A. Nanomaterials in Biosensors. In Nanomaterials for Biosensors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–74.
- Tieu, T.; Alba, M.; Elnathan, R.; Cifuentes-Rius, A.; Voelcker, N.H. Advances in Porous Silicon-Based Nanomaterials for Diagnostic and Therapeutic Applications. Adv. Ther. 2019, 2, 1800095.
- Li, W.; Liu, Z.; Fontana, F.; Ding, Y.; Liu, D.; Hirvonen, J.T.; Santos, H.A. Tailoring Porous Silicon for Biomedical Applications: From Drug Delivery to Cancer Immunotherapy. Adv. Mater. 2018, 30, 1703740.
- Arshavsky-Graham, S.; Massad-Ivanir, N.; Segal, E.; Weiss, S. Porous Silicon-Based Photonic Biosensors: Current Status and Emerging Applications. Anal. Chem. 2019, 91, 441–467.
- Uhlir, A. Electrolytic Shaping of Germanium and Silicon. Bell Syst. Tech. J. 1956, 35, 333–347.
- Canham, L.T. Silicon quantum wire array fabrication by electrochemical and chemical dissolution of wafers. Appl. Phys. Lett. 1990, 57, 1046–1048.
- Available online: (accessed on 25 January 2021).
- Herino, R.; Bomchil, G.; Barla, K.; Bertrand, C.; Ginoux, J.L. Porosity and Pore Size Distributions of Porous Silicon Layers. J. Electrochem. Soc. 1987, 134, 1994–2000.
- Lee, S.; Kang, J.; Kim, D. A Mini Review: Recent Advances in Surface Modification of Porous Silicon. Materials 2018, 11, 2557.
- Santos, H.A. Porous Silicon for Biomedical Applications; Woodhead Publishing: Cham, Switzerland, 2014; ISBN 978-0-85709-715-6.
- Low, S.P.; Voelcker, N.H. Biocompatibility of Porous Silicon. In Handbook of Porous Silicon; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–13.
- Terracciano, M.; Rea, I.; Borbone, N.; Moretta, R.; Oliviero, G.; Piccialli, G.; De Stefano, L. Porous Silicon-Based Aptasensors: The Next Generation of Label-Free Devices for Health Monitoring. Molecules 2019, 24, 2216.
- Torres-Costa, V.; Agulló-Rueda, F.; Martín-Palma, R.J.; Martínez-Duart, J.M. Porous silicon optical devices for sensing applications. Opt. Mater. 2005, 27, 1084–1087.
- Buriak, J.M.; Allen, M.J. Lewis Acid Mediated Functionalization of Porous Silicon with Substituted Alkenes and Alkynes. J. Am. Chem. Soc. 1998, 120, 1339–1340.
- Dhanekar, S.; Jain, S. Porous silicon biosensor: Current status. Biosens. Bioelectron. 2013, 41, 54–64.
- Sailor, M.J. Porous Silicon in Practice: Preparation, Characterization and Applications; Wiley-VCH: Weinheim, Germany, 2012; ISBN 9783527313785.
- Canham, L. Tunable properties of Porous Silicon. In Handbook of Porous Silicon; Springer International Publishing: Cham, Switzerland, 2014; pp. 201–206. ISBN 9783319057446.
- Md Jani, A.M.; Losic, D.; Voelcker, N.H. Nanoporous anodic aluminium oxide: Advances in surface engineering and emerging applications. Prog. Mater. Sci. 2013, 58, 636–704.
- Alvarez, S.D.; Li, C.P.; Chiang, C.E.; Schuller, I.K.; Sailor, M.J. A label-free porous alumina interferometric immunosensor. ACS Nano 2009, 3, 3301–3307.
- Mun, K.S.; Alvarez, S.D.; Choi, W.Y.; Sailor, M.J. A stable, label-free optical interferometric biosensor based on TiO2 Nanotube Arrays. ACS Nano 2010, 4, 2070–2076.
- Song, Y.Y.; Schmuki, P. Modulated TiO2 nanotube stacks and their use in interference sensors. Electrochem. Commun. 2010, 12, 579–582.
- Moretta, R.; Terracciano, M.; Dardano, P.; Casalino, M.; De Stefano, L.; Schiattarella, C.; Rea, I. Toward multi-parametric porous silicon transducers based on covalent grafting of Graphene oxide for biosensing applications. Front. Chem. 2018, 6, 583.
- Moretta, R.; Terracciano, M.; Dardano, P.; Casalino, M.; Rea, I.; De Stefano, L. Covalent grafting of graphene oxide on functionalized macroporous silicon. Open Mater. Sci. 2018, 4, 15–22.
- Gammoudi, H.; Belkhiria, F.; Sahlaoui, K.; Zaghdoudi, W.; Daoudi, M.; Helali, S.; Morote, F.; Saadaoui, H.; Amlouk, M.; Jonusauskas, G.; et al. Enhancement of the photoluminescence property of hybrid structures using single-walled carbon nanotubes/pyramidal porous silicon surface. J. Alloys Compd. 2018, 731, 978–984.
- Massad-Ivanir, N.; Bhunia, S.K.; Raz, N.; Segal, E.; Jelinek, R. Synthesis and characterization of a nanostructured porous silicon/carbon dot-hybrid for orthogonal molecular detection. NPG Asia Mater. 2018, 10, e463.
- Li, Y.; Jia, Z.; Lv, G.; Wen, H.; Li, P.; Zhang, H.; Wang, J. Detection of Echinococcus granulosus antigen by a quantum dot/porous silicon optical biosensor. Biomed. Opt. Express 2017, 8, 3458.
- Harraz, F.A. Porous silicon chemical sensors and biosensors: A review. Sens. Actuators B Chem. 2014, 202, 897–912.
- Moretta, R.; Terracciano, M.; Borbone, N.; Oliviero, G.; Schiattarella, C.; Piccialli, G.; Falanga, A.P.; Marzano, M.; Dardano, P.; De Stefano, L.; et al. PNA-Based Graphene Oxide/Porous Silicon Hybrid Biosensor: Towards a Label-Free Optical Assay for Brugada Syndrome. Nanomaterials 2020, 10, 2233.
- Terracciano, M.; Rea, I.; Stefano, L.; Rendina, I.; Oliviero, G.; Nici, F.; D’Errico, S.; Piccialli, G.; Borbone, N. Synthesis of mixed-sequence oligonucleotides on mesoporous silicon: Chemical strategies and material stability. Nanoscale Res. Lett. 2014, 9, 317.
- Krismastuti, F.S.H.; Cavallaro, A.; Prieto-Simon, B.; Voelcker, N.H. Toward Multiplexing Detection of Wound Healing Biomarkers on Porous Silicon Resonant Microcavities. Adv. Sci. 2016, 3, 1500383.
- Ghosh, R.; Das, R.; Giri, P.K. Label-free glucose detection over a wide dynamic range by mesoporous Si nanowires based on anomalous photoluminescence enhancement. Sens. Actuators B Chem. 2018, 260, 693–704.
- Sciacca, B.; Frascella, F.; Venturello, A.; Rivolo, P.; Descrovi, E.; Giorgis, F.; Geobaldo, F. Doubly resonant porous silicon microcavities for enhanced detection of fluorescent organic molecules. Sens. Actuators B Chem. 2009, 137, 467–470.
- Benecke, W.; Splinter, A. MEMS applications of porous silicon. In Proceedings of the Device and Process Technologies for MEMS and Microelectronics II; Chiao, J.-C., Faraone, L., Harrison, H.B., Shkel, A.M., Eds.; SPIE: Bellingham, WA, USA, 2001; Volume 4592, p. 76.
- Gautier, G.; Defforge, T.; Desplobain, S.; Billoue, J.; Capelle, M.; Poveda, P.; Vanga, K.; Lu, B.; Bardet, B.; Lascaud, J.; et al. Porous Silicon in Microelectronics: From Academic Studies to Industry. ECS Trans. 2015, 69, 123–134.
- Gautier, G.; Leduc, P. Porous silicon for electrical isolation in radio frequency devices: A review. Appl. Phys. Rev. 2014, 1, 11101.
- Tong, W.Y.; Sweetman, M.J.; Marzouk, E.R.; Fraser, C.; Kuchel, T.; Voelcker, N.H. Towards a subcutaneous optical biosensor based on thermally hydrocarbonised porous silicon. Biomaterials 2016, 74, 217–230.
- Balderas-Valadez, R.F.; Agarwal, V.; Pacholski, C. Fabrication of porous silicon-based optical sensors using metal-assisted chemical etching. RSC Adv. 2016, 6, 21430–21434.
- Huang, Z.; Geyer, N.; Werner, P.; De Boor, J.; Gösele, U. Metal-assisted chemical etching of silicon: A review. Adv. Mater. 2011, 23, 285–308.
- Maniya, N.H.; Patel, S.R.; Murthy, Z.V.P. Electrochemical preparation of microstructured porous silicon layers for drug delivery applications. Superlattices Microstruct. 2013, 55, 144–150.
- Smith, R.L.; Collins, S.D. Porous silicon formation mechanisms. J. Appl. Phys. 1992, 71, R1–R22.
- Rendina, I.; Rea, I.; Rotiroti, L.; De Stefano, L. Porous silicon-based optical biosensors and biochips. Phys. E 2007, 38, 188–192.
- Chiappini, C.; Liu, X.; Fakhoury, J.R.; Ferrari, M. Biodegradable porous silicon barcode nanowires with defined geometry. Adv. Funct. Mater. 2010, 20, 2231–2239.
- Chiappini, C.; Martinez, J.O.; De Rosa, E.; Almeida, C.S.; Tasciotti, E.; Stevens, M.M. Biodegradable nanoneedles for localized delivery of nanoparticles in vivo: Exploring the biointerface. ACS Nano 2015, 9, 5500–5509.
- Mikhael, B.; Elise, B.; Xavier, M.; Sebastian, S.; Johann, M.; Laetitia, P. New silicon architectures by gold-assisted chemical etching. ACS Appl. Mater. Interfaces 2011, 3, 3866–3873.
- Shabir, Q.; Webb, K.; Nadarassan, D.K.; Loni, A.; Canham, L.T.; Terracciano, M.; De Stefano, L.; Rea, I. Quantification and Reduction of the Residual Chemical Reactivity of Passivated Biodegradable Porous Silicon for Drug Delivery Applications. Silicon 2017, 10, 1–11.
- Pap, A.E.; Kordás, K.; Tóth, G.; Levoska, J.; Uusimäki, A.; Vähäkangas, J.; Leppävuori, S.; George, T.F. Thermal oxidation of porous silicon: Study on structure. Appl. Phys. Lett. 2005, 86, 041501.
- Shtenberg, G.; Massad-Ivanir, N.; Fruk, L.; Segal, E. Nanostructured Porous Si Optical Biosensors: Effect of Thermal Oxidation on Their Performance and Properties. ACS Appl. Mater. Interfaces 2014, 6, 16049–16055.
- Layouni, R.; Choudhury, M.H.; Laibinis, P.E.; Weiss, S.M. Thermally Carbonized Porous Silicon for Robust Label-Free DNA Optical Sensing. ACS Appl. Bio Mater. 2020, 3, 622–627.
- Riikonen, J.; Rigolet, S.; Marichal, C.; Aussenac, F.; Lalevée, J.; Morlet-Savary, F.; Fioux, P.; Dietlin, C.; Bonne, M.; Lebeau, B.; et al. Endogenous Stable Radicals for Characterization of Thermally Carbonized Porous Silicon by Solid-State Dynamic Nuclear Polarization 13C NMR. J. Phys. Chem. C 2015, 119, 19272–19278.
- Terracciano, M.; Rea, I.; Politi, J.; De Stefano, L. Optical characterization of aminosilane-modified silicon dioxide surface for biosensing. J. Eur. Opt. Soc. 2013, 8, 13075.
- Tramontano, C.; Chianese, G.; Terracciano, M.; de Stefano, L.; Rea, I. Nanostructured Biosilica of Diatoms: From Water World to Biomedical Applications. Appl. Sci. 2020, 10, 6811.
- Terracciano, M.; Shahbazi, M.A.; Correia, A.; Rea, I.; Lamberti, A.; De Stefano, L.; Santos, H.A. Surface bioengineering of diatomite based nanovectors for efficient intracellular uptake and drug delivery. Nanoscale 2015, 7, 20063–20074.
- Terracciano, M.; De Stefano, L.; Rea, I. Diatoms green nanotechnology for biosilica-based drug delivery systems. Pharmaceutics 2018, 10, 242.
- Gooding, J.J.; Ciampi, S. The molecular level modification of surfaces: From self-assembled monolayers to complex molecular assemblies. Chem. Soc. Rev. 2011, 40, 2704–2718.
- Kim, D.; Zuidema, J.M.; Kang, J.; Pan, Y.; Wu, L.; Warther, D.; Arkles, B.; Sailor, M.J. Facile Surface Modification of Hydroxylated Silicon Nanostructures Using Heterocyclic Silanes. J. Am. Chem. Soc. 2016, 138, 15106–15109.
- Caroselli, R.; García Castelló, J.; Escorihuela, J.; Bañuls, M.; Maquieira, Á.; García-Rupérez, J. Experimental Study of the Oriented Immobilization of Antibodies on Photonic Sensing Structures by Using Protein A as an Intermediate Layer. Sensors 2018, 18, 1012.
- Sciacca, B.; Alvarez, S.D.; Geobaldo, F.; Sailor, M.J. Bioconjugate functionalization of thermally carbonized porous silicon using a radical coupling reaction. Dalton Trans. 2010, 39, 10847–10853.
- Pacholski, C.; Sartor, M.; Sailor, M.J.; Cunin, F.; Miskelly, G.M. Biosensing Using Porous Silicon Double-Layer Interferometers: Reflective Interferometric Fourier Transform Spectroscopy. J. Am. Chem. Soc. 2005, 127, 11636–11645.
- Lin, V.S.Y.; Motesharei, K.; Dancil, K.P.S.; Sailor, M.J.; Ghadiri, M.R. A porous silicon-based optical interferometric biosensor. Science 1997, 278, 840–843.
- Chapron, J.; Alekseev, S.A.; Lysenko, V.; Zaitsev, V.N.; Barbier, D. Analysis of interaction between chemical agents and porous Si nanostructures using optical sensing properties of infra-red Rugate filters. Sens. Actuators B Chem. 2007, 120, 706–711.
- Zhang, H.; Lv, J.; Jia, Z. Detection of ammonia-oxidizing bacteria (AOB) using a porous silicon optical biosensor based on a multilayered double bragg mirror structure. Sensors 2018, 18, 105.
- Rea, I.; Iodice, M.; Coppola, G.; Rendina, I.; Marino, A.; De Stefano, L. A porous silicon-based Bragg grating waveguide sensor for chemical monitoring. Sens. Actuators B Chem. 2009, 139, 39–43.
- Zhao, Y.; Gaur, G.; Mernaugh, R.L.; Laibinis, P.E.; Weiss, S.M. Comparative Kinetic Analysis of Closed-Ended and Open-Ended Porous Sensors. Nanoscale Res. Lett. 2016, 11, 1–9.
- Zhao, Y.; Gaur, G.; Retterer, S.T.; Laibinis, P.E.; Weiss, S.M. Flow-Through Porous Silicon Membranes for Real-Time Label-Free Biosensing. Anal. Chem. 2016, 88, 10940–10948.
- Rea, I.; Orabona, E.; Lamberti, A.; Rendina, I.; De Stefano, L. A microfluidics assisted porous silicon array for optical label-free biochemical sensing. Biomicrofluidics 2011, 5, 034120.
- De Stefano, L.; Orabona, E.; Lamberti, A.; Rea, I.; Rendina, I. Microfluidics assisted biosensors for label-free optical monitoring of molecular interactions. Sens. Actuators B Chem. 2013, 179, 157–162.
- Mariani, S.; Pino, L.; Strambini, L.M.; Tedeschi, L.; Barillaro, G. 10 000-Fold Improvement in Protein Detection Using Nanostructured Porous Silicon Interferometric Aptasensors. ACS Sens. 2016, 1, 1471–1479.
- Mariani, S.; Strambini, M.; Barillaro, G. Femtomole Detection of Proteins Using a Label-Free Nanostructured Porous Silicon Interferometer for Perspective Ultrasensitive Biosensing. Anal. Chem. 2016, 88, 8.
- Arshavsky-Graham, S.; Massad-Ivanir, N.; Paratore, F.; Scheper, T.; Bercovici, M.; Segal, E. On Chip Protein Pre-Concentration for Enhancing the Sensitivity of Porous Silicon Biosensors. ACS Sens. 2017, 2, 1767–1773.
- Chhasatia, R.; Sweetman, M.J.; Prieto-Simon, B.; Voelcker, N.H. Performance optimisation of porous silicon rugate filter biosensor for the detection of insulin. Sens. Actuators B Chem. 2018, 273, 1313–1322.
- Chhasatia, R.; Sweetman, M.J.; Harding, F.J.; Waibel, M.; Kay, T.; Thomas, H.; Loudovaris, T.; Voelcker, N.H. Non-invasive, in vitro analysis of islet insulin production enabled by an optical porous silicon biosensor. Biosens. Bioelectron. 2017, 91, 515–522.
- Massad-Ivanir, N.; Shtenberg, G.; Raz, N.; Gazenbeek, C.; Budding, D.; Bos, M.P.; Segal, E. Porous Silicon-Based Biosensors: Towards Real-Time Optical Detection of Target Bacteria in the Food Industry. Sci. Rep. 2016, 6, 1–12.
- Bonanno, L.M.; DeLouise, L.A. Tunable detection sensitivity of opiates in urine via a label-free porous silicon competitive inhibition immunosensor. Anal. Chem. 2010, 82, 714–722.
- Bonanno, L.M.; Kwong, T.C.; DeLouise, L.A. Label-free porous silicon immunosensor for broad detection of opiates in a blind clinical study and results comparison to commercial analytical chemistry techniques. Anal. Chem. 2010, 82, 9711–9718.





