
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ilaria Rea | + 1123 word(s) | 1123 | 2021-03-17 10:11:11 | | | |
| 2 | Vivi Li | Meta information modification | 1123 | 2021-03-24 02:32:12 | | | | |
| 3 | Vivi Li | Meta information modification | 1123 | 2021-03-24 11:17:05 | | |
Video Upload Options
Porous materials showing some useful transducing features, i.e., any changes in their physical or chemical properties as a consequence of molecular interaction, are very attractive in the realization of sensors and biosensors. Diatom frustules have been gaining support for biosensors since they are made of nanostructured amorphous silica, but do not require any nano-fabrication step; their surface can be easily functionalized and customized for specific application; diatom frustules are photoluminescent, and they can be found in almost every pond of water on the Earth, thus assuring large and low-cost availability.
1. Introduction
Sometimes the term biosensor is improperly used to describe a molecular system that detects a physical or chemical interaction between a probe and its target analyte, such as the antibody (the probe) antigen (the target analyte) couple. A biosensor is instead a working device that must give a numerical value when used to selectively quantify the amount of the target analyte in a complex mixture, just like any other commercial sensor used in the industry or everyday life [1]. A biosensor is made of a transducer element, the true sensitive part of the device, and a receptor that interacts with the target analyte. The advent of nanostructured materials as high-quality transducer supports has strongly improved the performance of biosensors, thus increasing the interest of the academic and industrial world. Among several nanostructured materials engineered in the research laboratories, the porous ones have been deeply investigated since they offered a specific feature with respect to their bulk or planar counterparts and, that is, a huge specific surface, available to sense the molecular probe-target interaction. This is the case, for example, of the porous silicon which is the nanostructured analog of crystalline silicon [2][3]. The porous silicon not only has a sponge-like morphology with a specific area that can be of hundreds of m2/g but it could also be photoluminescent. Porous silicon changes its physical properties on exposure to gases or liquids, whereas the crystalline silicon is almost inert and it cannot emit light [4]. Even if the nanostructured porous transducers show superior performances in sensing applications, they require specialized fabrication processes that could be expensive and complex. These are the main reasons why researchers considered some naturally-derived or biomimetic alternatives to man-made materials. Diatoms are microalgae diffused in marine and freshwater all over the planet, largely studied in biology concerning oxygen production and, also, as markers of environmental pollution [5]. Diatoms are constituted by a single cell enclosed in a nanostructured skeleton, called frustule, made of hydrogenated amorphous silica, which has dimensions ranging from few microns up to a millimeter. The frustule consists of two valves joined by girdle bands. The valves are pierced from side to side, as it shown in Figure 1 where images of the diatom Coscinodiscus wailesii by scanning electron microscope are reported. A very detailed description on diatom structure can be found in a paper of Losic et al. [6], where scanning electron microscopy and atomic force microscopy results are presented. From the biologic point of view, the frustule provides mechanical protection of the living cell, molecular sieving of nutrients, and light managing.
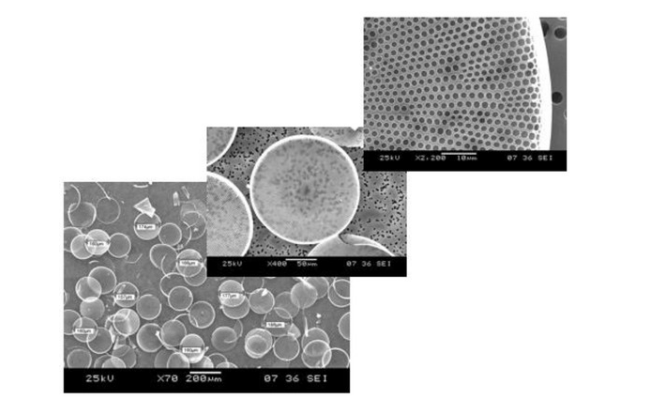
Figure 1. Scanning electron microscope images of the Coscinodiscus wailesii diatom valves at different magnifications (Images courtesy of Prof. M. De Stefano, University of Campania “Vanvitelli”, Italy).
Frustules show a hierarchical distribution of pores from tenths to hundreds of nanometers in size [7]. Frustules size, pore arrangement, and dimensions are species-specific as can be seen in Figure 2. From the material point of view, once the organic part is removed with acid or basic wet treatments, the diatom frustules are very similar to man-made porous silica, except for the fact that they can be found in nature and do not require any complex fabrication process. Moreover, just like the porous silicon, the diatom frustules have remarkable optical properties that change on exposure to chemical substances [8][9][10][11][12][13][14][15][16]; their surface can be functionalized [17], and in conclusion, the diatom frustules have been considered as bio-derived transducers for biosensing applications [18]. In the last years, due to their unique characteristics, an increasing enthusiasm about the use of diatoms in nanotechnology has been registered in many important fields, from nanomedicine to environment monitoring [19][20][21][22][23][24][25][26][27][28][29][30][31]. Two books have been recently published in the area of diatoms, fixing the state of art in emerging applications [32][33]. Moreover, a recent review summarized the results on the use of diatoms in biosensing until 2015 [34]. In this paper, the new significant papers published in peer-reviewed scientific journals in the last four years will be commented, and some future perspectives about the evolution of diatom-based biosensors will be given.

Figure 2. Different shapes, sizes, and morphologies of diatom frustules in an optical image (125× magnification). Frustule dimensions could range from few microns up to a millimeter (Copyright to http://golubcollection.berkeley.edu/diatoms/modern.html).
2. Diatom-Based Biosensors
The photoluminescence features of diatom frustules are still under discussion [35]. Three main emission contributions are classified. The first one is related to the excitation and emission in the ultraviolet region between 250 and 300 nm. This activity is normally attributed to the organic fraction entrapped in the biosilica structure. The second type is associated with the emission in the blue region of the visible spectrum, and the third type of photoluminescence activity is characterized by strong emission in the green at wavelength 523 nm. The origin of visible emission in the blue and green regions is due to the different defect states, such as oxygen defect centers as non-bridging oxygen hole centers or neutral oxygen vacancy and self-trapped excitons. By using the photoluminescence modulation in antibody functionalized diatom frustules of Amphora sp., Viji et al. detected BSA protein at mM level, while Selvaraj et al., using the same species, selectively detected Salmonella typhi with a detection limit of 10 pg [36][37].
The hierarchical, quasi ordered disposition of porous nanostructures in diatom frustules has been exploited by the Rorrer group for demonstrating a photonic crystal enhanced fluorescence imaging immunoassay able to detect target analytes for cardiovascular disease and IgG molecules [38][39]. The pores of the diatom Pinnularia sp. frustules used were under 100 nm in size, arranged in such a way that the optical fields were enhanced by the Purcell effect. As can be noted in Figure 3, the functionalized frustules could be simply imaged by fluorescence microscopy and the image rapidly analyzed to get quantitative information. The coupling between the fluorophore emission and the optical field form the diatom surface allowed a limit of detection of the mouse IgG down to 10−16 M (14 fg/mL) and of a few pg/mL of the hormone N-terminal pro-B-type natriuretic peptide. These results were 100× and 2× better than others in the literature, respectively.
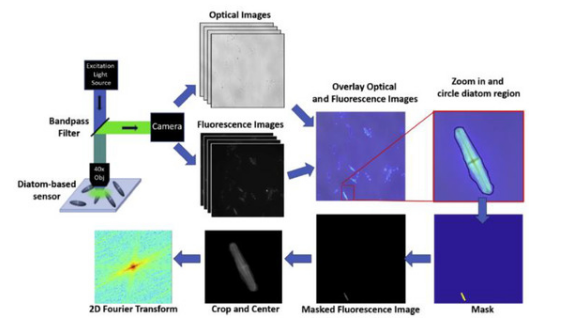
Figure 3. Schematic of quantification of the signal from imaged diatom frustules. Reprinted with permission from [40]. Copyright 2019 Elsevier.
Lim et al. used fluorescence molecularly imprinted polymer based on Navicula sp. frustules for lysozyme sensing. The authors demonstrated a limit of detection equal to 1.5 µg/mL [40].
References
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131.
- Lin, V.S.Y.; Motesharei, K.; Dancil, K.P.S.; Sailor, M.J.; Ghadiri, M.R. A porous silicon-based optical interferometric biosensor. Science 1997, 278, 840–843.
- De Stefano, L.; Rossi, M.; Staiano, M.; Mamone, G.; Parracino, A.; Rotiroti, L.; Rendina, I.; Rossi, M.; D’Auria, S. Glutamine-binding protein from Escherichia coli specifically binds a wheat gliadin peptide allowing the design of a new porous silicon-based optical biosensor. J. Proteome Res. 2006, 5, 1241–1245.
- Cullis, A.G.; Canham, L.T.; Calcott, P.D.J. The structural and luminescence properties of porous silicon. J. Appl. Phys. 1997, 82, 909–965.
- Round, F.E.; Crawford, R.M.; Mann, D.G. Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990.
- Losic, D.; Mitchell, J.G.; Voelcker, N.H. Diatomaceous Lessons in Nanotechnology and Advanced Materials. Adv. Mater. 2009, 21, 2947–2958.
- De Stefano, M.; De Stefano, L. Nanostructures in diatom frustules: Functional morphology of valvocopulae in Cocconeidacean monoraphid taxa. J. Nanosci. Nanotechnol. 2005, 5, 15–24.
- De Stefano, L.; De Stefano, M.; Bismuto, A.; Maddalena, P.; Rendina, I. Marine diatoms as optical chemical sensors. Appl. Phys. Lett. 2005, 87, 233902.
- Setaro, A.; Lettieri, S.; Maddalena, P.; De Stefano, L. Highly sensitive optochemical gas detection by luminescent marine diatoms. Appl. Phys. Lett. 2007, 91, 051921.
- Bismuto, A.; Setaro, A.; Maddalena, P.; De Stefano, L.; De Stefano, M. Marine diatoms as optical chemical sensors: A time resolved study. Sens. Actuators B Chem. 2008, 130, 396–399.
- Lettieri, S.; Setaro, A.; De Stefano, L.; De Stefano, M.; Maddalena, P. The Gas-Detection Properties of Light-Emitting Diatoms. Adv. Funct. Mater. 2008, 18, 1257–1264.
- De Stefano, L.; Rea, I.; Rendina, I.; De Stefano, M.; Moretti, L. Lensless light focusing with the centric marine diatom Coscinodiscus walesii. Opt. Exp. 2007, 15, 18082–18088.
- De Stefano, L.; Maddalena, P.; Moretti, L.; Rea, I.; Rendina, I.; De Tommasi, E.; Mocella, V.; De Stefano, M. Nano-biosilica from marine diatoms: A brand new material for photonic applications. Superlattices Microstruct. 2009, 46, 84–89.
- De Tommasi, E.; Rea, I.; Mocella, V.; Moretti, L.; De Stefano, M.; Rendina, I.; De Stefano, L. Multi-wavelength study of light transmitted through a single marine centric diatom. Opt. Exp. 2010, 18, 12203–12212.
- Di Caprio, G.; Coppola, G.; De Stefano, L.; De Stefano, M.; Antonucci, A.; Congestri, R.; De Tommasi, E. Shedding Light on Diatom Photonics by means of Digital Holography. J. Biophotonics 2014, 7, 341–350.
- Ferrara, M.A.; Dardano, P.; De Stefano, L.; Rea, I.; Coppola, G.; Rendina, I.; Congestri, R.; Antonucci, A.; De Stefano, M.; De Tommasi, E. Optical Properties of Diatom Nanostructured Biosilica in Arachnoidiscus sp: Micro-Optics from Mother Nature. PLoS ONE 2014, 9, e103750.
- De Stefano, L.; Lamberti, A.; Rotiroti, L.; De Stefano, M. Interfacing the nanostructured biosilica microshells of the marine diatom Coscinodiscus wailesii with biological matter. Acta Biomater. 2008, 4, 126–130.
- De Stefano, L.; Rotiroti, L.; Lamberti, A.; Lettieri, S.; Setaro, A.; De Stefano, M.; Maddalena, P. Marine Diatoms as Optical Biosensors. Biosens. Bioelectron. 2009, 24, 1580–1584.
- De Stefano, L.; De Stefano, M.; De Tommasi, E.; Rea, I.; Rendina, I. A natural source of porous biosilica for nanotech applications: The diatoms microalgae. Phys. Status Solidi C 2011, 8, 1820–1825.
- Ruggiero, I.; Terracciano, M.; Martucci, N.M.; De Stefano, L.; Migliaccio, N.; Tatè, R.; Rendina, I.; Arcari, P.; Lamberti, A.; Rea, I. Diatomite Silica Nanoparticles for Drug Delivery. Nanoscale Res. Lett. 2014, 9, 329.
- Rea, I.; Martucci, N.M.; De Stefano, L.; Ruggiero, I.; Terracciano, M.; Dardano, P.; Migliaccio, N.; Arcari, P.; Tatè, R.; Rendina, I.; et al. Diatomite biosilica nanocarriers for siRNA transport inside cancer cells. Biochim. Biophys. Acta 2014, 1840, 3393–3403.
- Terracciano, M.; Shahbazi, M.-A.; Correia, A.; Rea, I.; Lamberti, A.; De Stefano, L.; Santos, H.A. Surface bioengineering of diatomite based nanovectors for efficient intracellular uptake and drug delivery. Nanoscale 2015, 7, 20063–20074.
- Ferrara, M.A.; De Tommasi, E.; Coppola, G.; De Stefano, L.; Rea, I.; Dardano, P. Diatom valve three-dimensional representation: A new imaging method based on combined microscopies. Int. J. Mol. Sci. 2016, 17, 1645.
- Pannico, M.; Rea, I.; Chandrasekaran, S.; Musto, P.; Voelcker, N.H.; De Stefano, L. Electroless gold-modified diatoms as surface-enhanced Raman scattering supports. Nanoscale Res. Lett. 2016, 11, 315.
- Rea, I.; Terracciano, M.; Chandrasekaran, S.; Voelcker, N.H.; Dardano, P.; Martucci, N.M.; Lamberti, A.; De Stefano, L. Bioengineered silicon diatoms: Adding photonic features to a nanostructured semiconductive material for biomolecular sensing. Nanoscale Res. Lett. 2016, 11, 405.
- Rea, I.; Terracciano, M.; De Stefano, L. Synthetic vs Natural: Diatom bioderived porous materials for next generation of healthcare nanodevices. Adv. Healthc. Mater. 2017, 6, 1601125.
- Managò, S.; Migliaccio, N.; Terracciano, M.; Napolitano, M.; Martucci, N.M.; De Stefano, L.; Rendina, I.; De Luca, A.C.; Lamberti, A.; Rea, I. Internalization kinetics and cytoplasmic localization of functionalized diatomite nanoparticles in cancer cells by Raman imaging. J. Biophotonics 2017, 11, e201870137.
- Terracciano, M.; De Stefano, L.; Rea, I. Diatoms green nanotechnology for biosilica-based drug delivery systems. Pharmaceutics 2018, 10, 242.
- Terracciano, M.; De Stefano, L.; Tortiglione, C.; Tino, A.; Rea, I. In vivo toxicity assessment of hybrid diatomite nanovectors using Hydra vulgaris as model system. Adv. Biosyst. 2019, 3, 1800247.
- Ragni, R.; Cicco, S.R.; Vona, D.; Farinola, G.M. Multiple routes to smart nanostructured materials from diatom microalgae: A chemical perspective. Adv. Mater. 2018, 30, 1704289.
- Panwar, V.; Dutta, T. Diatom Biogenic Silica as a Felicitous Platform for Biochemical Engineering: Expanding Frontiers. ACS Appl. Bio Mater. 2019, 2, 2295–2316.
- Diatom Nanotechnology: Progress and Emerging Applications. Editor: Dusan Losic. Available online: (accessed on 4 November 2019).
- Seckbach, J.; Gordon, R. (Eds.) Diatoms: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2019; ISBN 978-1-119-37021-5.
- Leonardo, S.; Prieto-Simon, B.; Campas, M. Past, present and future of diatoms in biosensing. Trac-Trends Anal. Chem. 2016, 79, 276–285.
- Sprynskyy, M.; Pomastowski, P.; Hornowska, M.; Król, A.; Rafińska, K.; Buszewski, B. Naturally organic functionalized 3D biosilica from diatom microalgae. Mater. Des. 2017, 132, 22–29.
- Viji, S.; Anbazhagi, M.; Ponpandian, N.; Mangalaraj, D.; Jeyanthi, S.; Santhanam, P.; Shenbaga Devi, A.; Viswanathan, C. Diatom-Based Label-Free Optical Biosensor for Biomolecules. Appl. Biochem. Biotechnol. 2014, 174, 1166–1173.
- Selvaraj, V.; Muthukumar, A.; Nagamony, P. Detection of typhoid fever by diatom-based optical biosensor. Env. Sci. Pollut. Res. 2018, 25, 20385–20390.
- Squire, K.; Kong, X.; LeDuff, P.; Rorrer, G.L.; Wang, A.X. Photonic crystal enhanced fluorescence immunoassay on diatom biosilica. J. Biophotonics 2018, 11, e201800009.
- Squire, K.J.; Zhao, Y.; Tan, A.; Sivashanmugan, K.; Kraai, J.A.; Rorrer, G.L.; Wang, A.X. Photonic crystal-enhanced fluorescence imaging immunoassay for cardiovascular disease biomarker screening with machine learning analysis. Sens. Actuators B Chem. 2019, 290, 118–124.
- Lim, G.W.; Lim, J.K.; Ahmad, A.L.; Chan, D.J.C. Fluorescent molecularly imprinted polymer based on Navicula sp. Frustules for optical detection of lysozyme. Anal. Bioanal. Chem. 2016, 408, 2083–2093.




