
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Florus C de Jong | + 3136 word(s) | 3136 | 2021-03-15 09:08:30 | | | |
| 2 | Vivi Li | Meta information modification | 3136 | 2021-03-24 02:06:42 | | | | |
| 3 | Vivi Li | + 1 word(s) | 3137 | 2021-03-24 02:07:14 | | |
Video Upload Options
In high-risk non-muscle invasive bladder cancer (HR-NMIBC), patient outcome is negatively affected by lack of response to Bacillus-Calmette Guérin (BCG) treatment. Lack of response to cisplatin-based neoadjuvant chemotherapy and cisplatin ineligibility reduces successful treatment outcomes in muscle-invasive bladder cancer (MIBC) patients. The effectiveness of PD-1/PD-L1 immune checkpoint inhibitors (ICI) in metastatic disease has stimulated its evaluation as a treatment option in HR-NMIBC and MIBC patients. However, the observed responses, immune-related adverse events and high costs associated with ICI have provided impetus for the development of methods to improve patient stratification, enhance anti-tumorigenic effects and reduce toxicity.
1. Introduction
Bladder cancer (BC) is among the ten most common cancers worldwide with 550k new cases yearly and 200k deaths in 2018 [1]. BC is classified into non-muscle invasive (NMIBC) and muscle invasive bladder cancer (MIBC) [2][3]. Based on molecular [3] and clinical-pathological features, a subgroup of NMIBC patients are defined as having high-risk disease (HR-NMIBC), which indicates a high risk of disease recurrence and progression to MIBC [3]. To lower this risk in HR-NMIBC, patients are treated with a transurethral resection followed by intravesical instillations with Bacillus Calmette-Guérin (BCG) immunotherapy [4]. Despite this approach, ~35% of HR-NMIBC patients develop high-grade (HG) recurrences and ~20% develop disease progression to MIBC [5][6]. These so called “BCG-unresponsive” patients are exposed to unnecessary BCG toxicity without any benefit, and for these patients the recommended treatment is surgical removal of the bladder (radical cystectomy (RC)) with a urinary diversion [2][3][7]. Yet some patients have comorbidities that preclude them from a RC, a surgical procedure associated with significant morbidity and mortality, while others have a strong desire for bladder-sparing treatments [2][6][8][9][10]. In addition, as some BCG-unresponsive patients recur without progression to MIBC, a RC may be considered as overtreatment. In summary, both HR-NMIBC and MIBC patients have an urgent need for better treatments.
In recent years, anti-PD-1/anti-PD-L1 immune checkpoint inhibition (ICI) has been proposed as new treatment modality in both BCG-unresponsive HR-NMIBC and MIBC. However, as in metastatic MIBC, clinical response rates to ICI seem unsatisfying. Here, we discuss opportunities on how to improve the response rate to PD-1/PD-L1 ICI using synergistic treatment regimens.
Evidence Acquisition
A literature search was conducted using ClinicalTrials.gov for ongoing trials on PD-1/PD-L1 combination therapy in localized BC until 1 February 2021. The search strategy was as follows: Bladder Cancer (Condition or Disease) and PD-1 or PD-L1 or any FDA approved PD-1/PD-L1 inhibitors, namely pembrolizumab, atezolizumab, nivolumab, durvalumab, avelumab or cemiplimab (Other Terms). The search resulted in 350 registered trials, six additional trials were identified from other review articles [11][12][13]. After removal of duplicates and terminated trials, 191 trials were assessed for eligibility. Trials with PD-1/PD-L1 ICI combined with chemotherapy, radiotherapy, other immune checkpoint inhibitors or targeted treatments were included. Other non-targeted treatments (e.g., BCG) and studies involving metastatic BC patients were excluded. A total of 37 clinical trials were included in this systematic review (Figure 1). NCT number, population, timing, phase, antibody, additional treatment and primary endpoints were collected for each trial.

Figure 1. PRISMA flow diagram of evidence acquisition.
2. Opportunities to Improve Efficacy of PD-1/PD-L1 Inhibition
2.1. Combined Treatment with Platinum-Based Chemotherapy
Combining PD-1/PD-L1 inhibitors with platinum-based chemotherapy (PBC) may increase tumor immunogenicity [14]. PBC causes DNA damage and induces cell death, thereby attracting antigen presenting cells (APC) [15]. PBC also increases TMB, and tumor-specific neoantigens are presented by MHC-1 and cause cytotoxic T cell activation [16]. While MHC-1 is often downregulated in cancer, in vitro experiments have shown that PBC induces MHC-1 on tumor cells [14][17][18]. IL-12 is essential for antigen presentation; in vivo knockout experiments showed that PBC increases dendritic cell (DC) maturation and leads to an increased ability of DCs to present antigens in an IL-12 dependent manner, resulting in the hypothesis that PBC sensitizes tumors for immune recognition [19]. Experiments in a murine model revealed that T cell costimulatory molecules such as CD80/CD86 are increased in tumor infiltrating immune cells after cisplatin treatment, suggesting that CD80/CD86 expression can be modulated by cisplatin treatment [20]. In vitro experiments showed that PBC induces PD-L1, making PD-L1 an interesting target to inhibit after PBC [17][21][22][23]. PBC may also decrease PD-L2 expression via modulation of the transcriptional regulator STAT6 [24]. As PD-L2 competes with PD-L1 to bind PD-1, decreased expression of PD-L2 after PBC results in enhanced affinity of PD-L1 to PD-1, and increases the relevance of PD-L1 for ICI [25]. The beneficial effects of the addition of PBC to PD-1/PD-L1 blockade is summarized in Figure 2A.
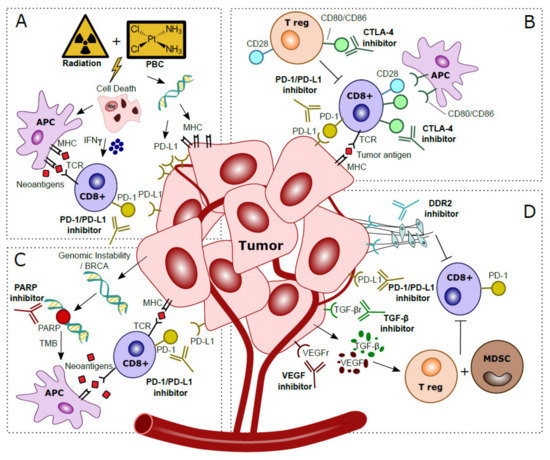
Figure 2. Hypothesized mechanisms of combination treatments to improve clinical response to anti-PD-1/PD-L1 treatments in localized bladder cancer patients. (A) Platinum-Based Chemotherapy (PBC) and/or radiation prompts tumor cell death. This process attracts Antigen Presenting Cells (APC), which upregulate presentation of tumor-specific neoantigens to cytotoxic T cells. Activation via IFN-γ released in the tumor microenvironment stimulates anti-tumor immunity. Direct effects of DNA damage by PBC and radiation cause upregulated expression of PD-L1 and MHC in tumor cells. (B) CTLA-4 checkpoint inhibitors block CTLA-4 on CD8+ T cells and T regulatory (T reg), thereby further stimulating CD28–CD80/CD86 T cell co-stimulatory responses and anti-tumor immunity. (C) Genomic instability is observed in tumor cells, especially in patients with BRCA alterations. The inability to restore DNA damage with blockade of PARP, normally used for DNA repair, leads to more somatic mutations, a higher Tumor Mutational Burden (TMB) and more tumor-specific neoantigens. Neoantigens are presented by Antigen Presenting Cells (APC) to provoke additional cytotoxic T cell responses. (D) Blockade of VEGF and TGF-β excreted by the tumor prevents activation of T regulatory cells (T reg) and Myeloid Derived Suppressor Cells (MDSC) that caused an immunosuppressive microenvironment. Inhibition of DDR2 prevents development of Cancer Associated Fibroblasts (CAFs), which stimulated metastases and yielded additional immune suppressive effects and increased CD8+ T cell infiltration.
The favorable effects of combined ICI and PBC have been shown in numerous studies [26]. In an MB49 BC subcutaneous model, anti-PD-L1 combined with PBC was more effective than either therapy alone [27]. In an MBT-2 subcutaneous model, anti-PD-L1 combined with PBC was more effective than PBC alone, but counterintuitively, anti-PD-L1 monotherapy was most effective, suggesting that combined treatment results are model-dependent [27]. Immune profiling of baseline tumors by flow cytometry did not demonstrate differences of tumor immune cell populations, and both MB49 and MBT-2 tumors were highly enriched with myeloid-derived suppressor cells (MDSCs). However, expression of the TIGIT checkpoint proteins on immune cells were decreased after PBC treatment in the MB49 vs. MBT-2 model, and the authors suggest that TIGIT may play a role in the diverse anti-tumor responses between models.
Multiple clinical trials evaluate a combination of PBC with PD-1/PD-L1 ICI in MIBC (Table 1) [13]. Neoadjuvant pembrolizumab plus gemcitabine/cisplatin showed a partial response rate to NMIBC at RC of 61% (n = 36) (NCT02365766) [28]. In BLASST-1, a multicenter Phase II trial, PBC combination therapy with nivolumab demonstrated a very high pCR rate of 65.8% (n = 27) [29]. Gemcitabine combined with pembrolizumab for cisplatin-ineligible patients yielded results comparable to pembrolizumab alone in NCT02365766, a Phase 1b/II study: 45.2% achieved pCR, whereas 51.6% reached <pT1N0 [30]. Interim analysis of SAKK06/17 showed pCR in 30% (n = 9) after treatment with gemcitabine and durvalumab pre- and post-operatively, whereas 20% of patients reached non-muscle invasive disease (pT1/pTis) [31]. Preclinical studies and interim study reports are encouraging and suggest a synergistic effect of chemotherapy with PD-L1 blockade. However, pCR rates are a surrogate marker for actual clinical outcome, thus long-term results analyzing disease progression are necessary. More information on treatment sequence and timing of PBC with ICI is also needed before these alternative treatment paradigms can be implemented.
Table 1. Studies investigating anti-PD-1/PD-L1 combined with chemo- and/or radiotherapy in localized bladder cancer.
| NCT Number | Population | Timing | Phase | PD-1/PD-L1 | Addition | Endpoint(s) |
|---|---|---|---|---|---|---|
| NCT03294304 | T2-T4a N0 M0 MIBC | Neoadjuvant | 2 | Nivolumab | Gem/cis | pRR |
| NCT02690558 | T2-T4a N0 M0 MIBC | Neoadjuvant | 2 | Pembrolizumab | Gem/cis | pDS to < pT2 |
| NCT03406650 | T2-T4a N0-1 M0 | Neoadjuvant + adjuvant | 2 | Durvalumab | Gem/cis | EFS |
| NCT03661320 | T2-T4a N0 M0 | Neoadjuvant | 3 | Nivolumab | Gem/cis | pCRR, EFS |
| NCT03924856 | T2-T4a N0 M0 or T1-T4a N1 M0 | Neoadjuvant | 3 | Pembrolizumab | Gem/cis | pCRR, EFS |
| NCT03732677 | T2-T4a N0-1 M0 | Neoadjuvant + adjuvant | 3 | Durvalumab | Gem/cis | pCRR, EFS |
| NCT03558087 | T2-T4a N0 M0 | Neoadjuvant | 2 | Nivolumab | Gem/cis | CRR, pCRR |
| NCT04099589 | T2-T4a N0 M0 | Neoadjuvant | 2 | Toripalimab | Gem or cis | pCRR |
| NCT03674424 | T2-T4a Nx M0 | Neoadjuvant | 2 | Avelumab | Gem/cis or ddMVAC or paclitaxel + gemcitabine | pCRR |
| NCT02365766 | T2-T4a N0 M0 | Neoadjuvant | 1/2 | Pembrolizumab | Gem/cis or gemcitabine | AE, PalR |
| NCT04164082 | BCG-unresponsive | Neoadjuvant | 2 | Pembrolizumab | Gemcitabine hydrochloride | CRR, EFS |
| NCT02560636 | T2-T4a N0-3 M0-1 | Neoadjuvant | 1 | Pembrolizumab | Radiotherapy | MTD, AE |
| NCT02891161 | T2-T4a N0-2 M0 | Neoadjuvant | 2 | Durvalumab | Radiotherapy | DLT, PFS, DCR |
| NCT03950362 | BCG-unresponsive | Ineligible/refusal of RC | 2 | Avelumab | Radiotherapy | RFS |
| NCT03317158 | BCG-unresponsive | Neoadjuvant | 1/2 | Durvalumab | EBRT + BCG | Recommended dose, RFS |
| NCT03775265 | T2-4a N0 M0 | Bladder sparing | 3 | Atezolizumab | Gem or CIS or fluorouracil + MMC + radiotherapy | EFS |
| NCT02662062 | T2-T4a Nx M0 | Ineligible/refusal of RC | 2 | Pembrolizumab | Cisplatin + radiotherapy | AE (grade 3–4) |
| NCT02621151 | T2-T4a N0 M0 | Ineligible/refusal of RC | 2 | Pembrolizumab | Gemcitabine + radiotherapy | DFS |
| NCT03617913 | T2-T4a N0 M0 | Neoadjuvant | 2 | Avelumab | Flourouracil + MMC or cisplatin and radiotherapy | CRR |
| NCT03702179 | T2-T4a | Neoadjuvant | 2 | Durvalumab | Tremelimumab + radiotherapy | pRR |
| NCT03601455 | T2-T4a or N+/M+ | Ineligible for RC | 2 | Durvalumab | Tremelimumab + EBRT | AE, PFS |
| NCT03549715 | T2-T4a N0-1 M0 | Neoadjuvant | 1/2 | Durvalumab | Tremelimumab + ddMVAC | AE, pCRR |
AE = adverse events, DCR = disease control rate, ddMVAC = dose dense methotrexate vinblastine doxorubicin cisplatin, DFS = disease-free survival, DLT = dose limiting toxicity, EFS = event-free survival, EBRT = External Beam Radiotherapy, Gem/cis = gemcitabine/cisplatin, MMC = mitomycine C, MTD = maximum tolerated dose, PalR = pathologic muscle invasive response rate, p(C)RR = pathological (complete) response rate, pDS = pathological downstaging, PFS = progression-free survival, RFS = recurrence-free survival.
2.2. Combined Treatment with Radiotherapy
Ionizing radiation induces double-stranded DNA breaks and produces reactive oxygen species (ROS) leading to cell cycle arrest, apoptosis and cell death [32][33]. Radiation elicits immunogenic effects within the tumor microenvironment (TME) by upregulating and activating the complement pathway and stimulation of IFN-γ release, both implicated in tumor eradication [34][35]. Radiotherapy enhances the adaptive immune system by upregulation of MHC-I, increasing cytotoxic T cell activity. Combining radiotherapy with immune-modulation may inhibit cancer via APC cross-presentation and improvement of T cell priming [36][37][38][39]. By inducing genotoxic stress, radiation prompts NK-mediated innate immune responses [40]. Interestingly, radiation also elicits chemotactic signals that attract MDSCs, instigating T-cell suppression [41][42]. Immunosuppressive effects can also be induced via activation of TGF-β, leading to upregulation of PD-L1 on tumor cells [43]. Combined radiotherapy with PD-L1 inhibition led to synergistic anti-tumor effects in vivo, thus providing the rationale for combined regimens with anti-PD-1/PD-L1 drugs (mechanism depicted in Figure 2A) [44][45][46][47]. Anti-PD-L1 therapy started one day prior and continued until two weeks after local irradiation (12Gy) in an HT1197/MB49 mouse model led to significant tumor growth delay compared to monotherapy [48].
Radiation may have immune-mediated distant effects, termed the abscopal effect, which refers to regression of non-irradiated lesions located elsewhere than the primary radiated site [40]. The abscopal effect is more often detected in mice treated with a combination of radiotherapy and PD-1/PD-L1 than radiation alone [43][49][50]. The underlying mechanism remains unclear, but it is hypothesized that anti-tumor immune responses are amplified. Taken together, radiation therapy stimulates tumor immunogenicity and suggests higher PD-1/PD-L1 susceptibility, hence combined regimes are appealing for investigation in patients with localized BC who prefer a bladder-sparing treatment [37][38][51]. Issues that need to be resolved include determining the optimal fractioning of radiotherapy, timing of ICI and an appropriate balance with regard to side effects associated with each modality [52][53]. Currently, multiple trials investigating radiotherapy and PD-1/PD-L1 blockade in BC are underway but none have been published so far (Table 1) [11].
2.3. Combined Treatment with Anti-CTLA-4
For optimal cytotoxic T cell-mediated tumor response, co-stimulation by CD80/CD86 binding to CD28 is required. CD28-CD80/CD86 interaction leads to increased T cell proliferation, differentiation and survival [54]. CTLA-4, a CD28 homolog, is another checkpoint-inhibitor expressed by T cells. CTLA-4 binds competitively with CD80/CD86 on APC’s, resulting in downregulation of T cell proliferation and response. Hence, CTLA-4 blockade can facilitate T cell recognition and increases eradication of malignant cells, thus strengthening the immunological response (illustrated in Figure 2B) [55][56].
In vivo mouse models studying melanoma, ovarian and colon carcinoma showed higher tumor rejection rates and increased tumor induced lymphocyte activity and proliferation after combined therapy with CTLA-4 and PD-1 compared to monotherapy [57][58]. Combining CTLA-4 and PD-L1 inhibitors yields increased CD8+ and CD4+ counts, reduced CD4+ Tregs, increased expression of pro-inflammatory cytokines and a polarization shift from M2 to M1 macrophages [59]. In another study, MB49 BC cells were injected subcutaneously in C57Bl/6 female mice to investigate the response to anti-PD-1 vs. anti-CTLA-4 alone or combined treatment and found that the combination showed increased circulating CD8+ T cells and superior overall survival compared to each monotherapy. In a control group, BCG treatment had no effect on tumor growth [60]. These results were confirmed in a second study that investigated a combination of anti-PD-1/anti-CTLA-4 in an orthotopic mouse model [61].
The NABUCCO-trial recently demonstrated a 46% pCR rate after treatment with ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1) prior to RC in cisplatin-ineligible stage III BC patients [62]. Of 24 patients, 14 (58%) reached either non-muscle invasive disease or pCR. Biomarker analysis revealed that tumors from pCR had higher frequencies of DDR alterations compared to non-pCR tumors (p = 0.03), and trended towards a higher TMB (p = 0.056) [62]. An increased TGF-β expression signature was associated with non-response, while response was independent of baseline CD8+ presence or T-effector signatures. However, upregulation of B cell-related genes at baseline correlated with non-response. Non-responding tumors exhibited more stromal B cells than responding tumors (p = 0.043) whereas the density of B cells in the tumor compartment was higher (p = 0.07) [62]. Overall, NABUCCO indicates that combination regimens with anti-CTLA-4 blockade should be investigated further, as the pCR results are promising. Other studies investigating CTLA-4 plus PD-1/PD-L1 combinations are illustrated in Table 1 and Table 2. No combination regimes have reported data in HR-NMIBC, but considering toxicity profiles, ICI combinations might also prove useful in BCG-unresponsive patients. The extent to which auto-immune symptoms will develop and whether they are manageable will likely determine if combination therapies are feasible and safe [63].
Table 2. Studies investigating anti-PD-1/PD-L1 combined with immune checkpoint inhibitors and targeted treatments.
| NCT Number | Population | Timing | Phase | PD-1/PD-L1 Antibody | Additional Treatment | Primary Endpoint(s) |
|---|---|---|---|---|---|---|
| NCT03472274 | T2-T4a N0-1 M0 | Neoadjuvant | 2 | Durvalumab | Tremelimumab | pCRR |
| NCT02812420 | T2-T3a N0 M0 | Neoadjuvant | 1 | Durvalumab | Tremelimumab | AE |
| NCT03520491 | T2-T4a N0 M0 | Neoadjuvant | 2 | Nivolumab | Ipilimumab | Patients proceeding to RC |
| NCT03387761 | T3-T4a N0 M0 or T1-4a N1-3 M0 | Neoadjuvant | 1 | Nivolumab | Ipilimumab | Patients proceeding to RC |
| NCT04430036 | T2-T4a N0-1 M0 | Neoadjuvant | 2 | Balstilimab | Zalifrelimab + gem/cis | pDS to pT0 |
| NCT04579133 | T2-T4a N0 M0 or T1-T4a N1 M0 | Neoadjuvant | 2 | Durvalumab | Olaparib | pCRR |
| NCT03534492 | T2-T4a N0 M0 | Neoadjuvant | 2 | Durvalumab | Olaparib | pCRR |
| NCT03832673 | T2-T3a N0 M0 TCC | Neoadjuvant | 2 | Pembrolizumab | Epacadostat | pCRR |
| NCT02845323 | T2-T4a N0-N2 M0 | Neoadjuvant | 2 | Nivolumab | Urelumab | CD8 density |
| NCT04209114 | T2-T4a N0 M0 | Neoadjuvant + adjuvant | 3 | Nivolumab | Bempeg | pCRR, EFS |
| NCT03924895 | T2-T4a N0 M0 or T1-T4aN1M0 | Neoadjuvant | 3 | Pembrolizumab | Enfortumab vedotin | pCRR, EFS |
| NCT03258593 | BCG-unresponsive | Neoadjuvant | 1 | Durvalumab | Vicinium | AE |
| NCT03978624 | T2-T4a N0-x M0 | Neoadjuvant | 2 | Pembrolizumab | Entinostat | Change in CD8 immune signature |
| NCT03773666 | T2-T4a N0 M0 | Neoadjuvant | 1 | Durvalumab | Oleclumab | Patients receiving a single dose followed by surgery |
| NCT03532451 | T2-T4a N0-1 M0 | Neoadjuvant | 1B | Nivolumab | Lirilumab | AE |
AE = adverse events, DFS = disease free survival, DLT = dose limiting toxicity, EFS = event-free survival, pCRR = pathological complete response rate, RR = response rate.
2.4. Enhancing Anti-PD-1/PD-L1 Treatment with Targeted Therapies
Poly (ADP-ribose) polymerase (PARP) is involved in DDR. PARP inhibitors are synthetically lethal against tumors with BRCA1/2 mutations, as these cancer cells are the result of genomic instability [64]. Secondly, PARP inhibitor induced genomic instability leads to more somatic mutations and a higher TMB, which in turn may increase the neoantigen load and immunogenicity, which can be exploited using ICI (Figure 2C) [65][66]. In mice, PARP increased expression of PD-L1, while dual blockade of both PARP and PD-L1 may lead to synergistic effects and increased tumor cell death [67]. In a Phase II study in MIBC, combined inhibition of PARP with olaparib and anti-PD-L1 resulted in a systemic immune response demonstrated by release of chemokines, TNF-α, angiogenic factors, tumor infiltrating lymphocytes (TIL) and increased IFN-y expression [68]. Preliminary results showed a pCR of 44.5% (n = 28) after treatment with anti-PDL1 and olaparib [69].
TGF-β is overexpressed in many malignancies and has long been implicated as a stimulator of cancer progression by regulation of apoptosis, cell proliferation and initiation of epithelial mesenchymal transition (EMT) [70]. An RNA-based TGF-β signature was associated with non-response to anti-PD-1 therapy in locally advanced BC and anti-PD-L1 therapy in metastatic BC [62][71]. Murine models showed that blocking of TGF-β reduces TGF-β signaling in stromal cells, facilitated T-cell penetration and provoked strong anti-tumor immunity and tumor regression [71]. Interestingly, a bi-functional fusion protein (Bintrafusp-α) targeting anti-PD-L1 and TGF-β has recently been developed, and it is expected to be tested in localized BC [72]. Vascular endothelial growth factor (VEGF) impairs hematopoietic differentiation and like TGF- β causes an immunosuppressive microenvironment in BC, thereby facilitating evasion of the immune response [73]. Anti-VEGF therapy may eliminate immunosuppressive effects, decreases tumor vasculature, and increases perfusion and influx of T cells [74]. Several trials tested anti-VEGF with concurrent chemotherapy, but this did not result in improved clinical outcome for metastatic BC patients [75]. Thus far, no trials have reported on ICI combinations with anti-VEGF in localized BC yet, but in other cancer entities synergistic effects have been identified [76]. The collagen discoidin domain receptor (DDR2), a kinase, is recognized as a metastases promoter [66]. DDR2 depletion increases sensitivity to anti-PD-1 treatment in vivo in BC and multiple other murine models of cancer [77]. Treatment with the anti-tyrosine kinase inhibitor dasatinib (anti-DDR2) and anti-PD-1 resulted in increased CD8+ tumor lymphocytes compared to monotherapy and a reduced tumor load [77]. Thus, a combination treatment of ICI with dasatinib appears to be worth exploring in localized BC. In summary, the synergistic mechanisms of anti-TGF-β, anti-VEGF and anti-DDR2 with PD-1/PD-L1 blockade are depicted in Figure 2D.
Finally, Toll-like receptor (TLR) agonists are being investigated in localized BC. Treatment with BCG in vitro triggers TLRs and prevents deprivation of MHC-II expression, thereby promoting immunogenicity [78][79]. Treatment with the TLR-4 agonist tasquinimod had weak anti-tumorigenic effects in AY-27 rats and MBT-2 mice models [80]. Combination treatment was tested only in mice, but anti-PD-L1 modulated M1/M2 tumor-infiltrating myeloid cells and increased activity and proliferation of CD8+ T-cells were observed, resulting in tumor regression [80]. To summarize, targeted therapies seem promising when combined with anti-PD-1/PD-L1 treatment, though clinical studies are now needed to assess whether these combination strategies truly enhance clinical efficacy of ICI. An overview of currently ongoing combination trials using targeted treatment combinations with PD-1/PD-L1 blockade are included in Table 2.
References
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904.
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.; Cowan, N.C.; Gakies, G.; Hernández, V.; Lorch, A.; Ribal, M.J.; Thalmann, G.N.; et al. Eau Guidelines on Muscle Invasive and Metastatic Bladder Cancer. In Proceedings of the EAU Annual Congress Barcelona, Barcelona, Spain, 17–21 July 2020.
- Tran, L.; Xiao, J.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Scientific Advances Transforming Bladder Cancer Care. Nat. Rev. Cancer 2020, in press.
- Malmström, P.-U.; Sylvester, R.J.; Crawford, D.E.; Friedrich, M.; Krege, S.; Rintala, E.; Solsona, E.; Di Stasi, S.M.; Witjes, J.A. An Individual Patient Data Meta-Analysis of the Long-Term Outcome of Randomised Studies Comparing Intravesical Mitomycin C versus Bacillus Calmette-Guérin for Non-Muscle-Invasive Bladder Cancer. Eur. Urol. 2009, 56, 247–256.
- Witjes, J.A. Management of BCG Failures in Superficial Bladder Cancer: A Review. Eur. Urol. 2006, 49, 790–797.
- de Jong, F.C.; Hoedemaeker, R.F.; Kvikstad, V.; Mensink, J.T.M.; de Jong, J.J.; Boevé, E.R.; van der Schoot, D.K.E.; Zwarthoff, E.C.; Boormans, J.L.; Zuiverloon, T.C.M. T1-Substaging of Non-Muscle Invasive Bladder Cancer Is Associated with BCG-Failure and Improves Patient Stratification at Diagnosis. J. Urol. 2020, 205, 701–708.
- Li, R.; Tabayoyong, W.B.; Guo, C.C.; González, G.M.N.; Navai, N.; Grossman, H.B.; Dinney, C.P.; Kamat, A.M. Prognostic Implication of the United States Food and Drug Administration-defined BCG-unresponsive Disease. Eur. Urol. 2019, 75, 8–10.
- Aziz, A.; May, M.; Burger, M.; Palisaar, R.-J.; Trinh, Q.-D.; Fritsche, H.-M.; Rink, M.; Chun, F.; Martini, T.; Bolenz, C.; et al. Prediction of 90-day Mortality After Radical Cystectomy for Bladder Cancer in a Prospective European Multicenter Cohort. Eur. Urol. 2014, 66, 156–163.
- Stein, J.P.; Skinner, D.G. Radical cystectomy for invasive bladder cancer: Long-term results of a standard procedure. World J. Urol. 2006, 24, 296–304.
- James, N.D.; Hussain, S.A.; Hall, E.; Jenkins, P.; Tremlett, J.; Rawlings, C.; Crundwell, M.; Sizer, B.; Sreenivasan, T.; Hendron, C.; et al. Radiotherapy with or without Chemotherapy in Muscle-Invasive Bladder Cancer. N. Engl. J. Med. 2012, 366, 1477–1488.
- Daro-Faye, M.; Kassouf, W.; Souhami, L.; Marcq, G.; Cury, F.; Niazi, T.; Sargos, P. Combined radiotherapy and immunotherapy in urothelial bladder cancer: Harnessing the full potential of the anti-tumor immune response. World J. Urol. 2020, 1–13.
- Hahn, N.M.; Necchi, A.; Loriot, Y.; Powles, T.; Plimack, E.R.; Sonpavde, G.; Roupret, M.; Kamat, A.M. Role of Checkpoint Inhibition in Localized Bladder Cancer. Eur. Urol. Oncol. 2018, 1, 190–198.
- Rouanne, M.; Bajorin, D.F.; Hannan, R.; Galsky, M.D.; Williams, S.B.; Necchi, A.; Sharma, P.; Powles, T. Rationale and Outcomes for Neoadjuvant Immunotherapy in Urothelial Carcinoma of the Bladder. Eur. Urol. Oncol. 2020, 3, 728–738.
- Hato, S.V.; Khong, A.; De Vries, I.J.M.; Lesterhuis, W.J. Molecular Pathways: The Immunogenic Effects of Platinum-Based Chemotherapeutics. Clin. Cancer Res. 2014, 20, 2831–2837.
- Emens, L.A.; Middleton, G. The Interplay of Immunotherapy and Chemotherapy: Harnessing Potential Synergies. Cancer Immunol. Res. 2015, 3, 436–443.
- Grimaldi, A.; Cammarata, I.; Martire, C.; Focaccetti, C.; Piconese, S.; Buccilli, M.; Mancone, C.; Buzzacchino, F.; Berrios, J.R.G.; D’Alessandris, N.; et al. Combination of chemotherapy and PD-1 blockade induces T cell responses to tumor non-mutated neoantigens. Commun. Biol. 2020, 3, 1–13.
- Grabosch, S.; Bulatovic, M.; Zeng, F.; Ma, T.; Zhang, L.; Ross, M.; Brozick, J.; Fang, Y.; Tseng, G.; Kim, E.; et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene 2019, 38, 2380–2393.
- Ohtsukasa, S.; Okabe, S.; Yamashita, H.; Iwai, T.; Sugihara, K. Increased expression of CEA and MHC class I in colorectal cancer cell lines exposed to chemotherapy drugs. J. Cancer Res. Clin. Oncol. 2003, 129, 719–726.
- Shurin, G.V.; Tourkova, I.L.; Kaneno, R.; Shurin, M.R. Chemotherapeutic Agents in Noncytotoxic Concentrations Increase Antigen Presentation by Dendritic Cells via an IL-12-Dependent Mechanism. J. Immunol. 2009, 183, 137–144.
- Beyranvand Nejad, E.; van der Sluis, T.C.; van Duikeren, S.; Yagita, H.; Janssen, G.M.; van Veelen, P.A.; Melief, C.J.; van der Burg, S.H.; Arens, R. Tumor Eradication by Cisplatin Is Sustained by Cd80/86-Mediated Costimulation of Cd8+ T Cells. Cancer Res. 2016, 76, 6017–6029.
- Li, S.; Ji, J.; Zhang, Z.; Peng, Q.; Hao, L.; Guo, Y.; Zhou, W.; Cui, Q.; Shi, X. Cisplatin promotes the expression level of PD-L1 in the microenvironment of hepatocellular carcinoma through YAP1. Mol. Cell. Biochem. 2020, 475, 79–91.
- Tran, L.; Allen, C.T.; Xiao, R.; Moore, E.; Davis, R.; Park, S.-J.; Spielbauer, K.; Van Waes, C.; Schmitt, N.C. Cisplatin Alters Antitumor Immunity and Synergizes with PD-1/PD-L1 Inhibition in Head and Neck Squamous Cell Carcinoma. Cancer Immunol. Res. 2017, 5, 1141–1151.
- Tsai, T.-F.; Lin, J.-F.; Lin, Y.-C.; Chou, K.-Y.; Chen, H.-E.; Ho, C.-Y.; Chen, P.-C.; Sheng Hwang, T.I. Cisplatin Contributes to Programmed Death-Ligand 1 Expression in Bladder Cancer through Erk1/2-Ap-1 Signaling Pathway. Biosci. Rep. 2019, 39, BSR20190362.
- Ghiotto, M.; Gauthier, L.; Serriari, N.; Pastor, S.; Truneh, A.; Nunès, J.A.; Olive, D. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. Int. Immunol. 2010, 22, 651–660.
- Lesterhuis, W.J.; Punt, C.J.A.; Hato, S.V.; Eleveld-Trancikova, D.; Jansen, B.J.H.; Nierkens, S.; Schreibelt, G.; De Boer, A.; Van Herpen, C.M.L.; Kaanders, J.H.; et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J. Clin. Investig. 2011, 121, 3100–3108.
- Xue, Y.; Gao, S.; Gou, J.; Yin, T.; He, H.; Wang, Y.; Zhang, Y.; Tang, X.; Wu, R. Platinum-based chemotherapy in combination with PD-1/PD-L1 inhibitors: Preclinical and clinical studies and mechanism of action. Expert Opin. Drug Deliv. 2021, 18, 187–203.
- Grasselly, C.; Denis, M.; Bourguignon, A.; Talhi, N.; Mathe, D.; Tourette, A.; Serre, L.; Jordheim, L.P.; Matera, E.L.; Dumontet, C. The Antitumor Activity of Combinations of Cytotoxic Chemotherapy and Immune Checkpoint Inhibitors Is Model-Dependent. Front. Immunol. 2018, 9, 2100.
- Kaimakliotis, H.; Albany, C.; Hoffman-Censits, J.; Trabulsi, E.; Kelly, W.K.; Picus, J.; Adra, N.; Cary, C.; Koch, M.; Fleming, M.; et al. PD52-03 a multicenter phase 1B/2 study of neoadjuvant pembrolizumab and cisplatin chemotherapy for muscle invasive urothelial cancer. J. Urol. 2019, 201, 924.
- Gupta, S.; Sonpavde, G.; Weight, C.J.; McGregor, B.A.; Gupta, S.; Maughan, B.L.; Wei, X.X.; Gibb, E.; Thyagarajan, B.; Einstein, D.J.; et al. Results from BLASST-1 (Bladder Cancer Signal Seeking Trial) of nivolumab, gemcitabine, and cisplatin in muscle invasive bladder cancer (MIBC) undergoing cystectomy. J. Clin. Oncol. 2020, 38, 439.
- Kaimakliotis, H.Z.; Adra, N.; Kelly, W.K.; Trabulsi, E.J.; Lauer, R.C.; Picus, J.; Smith, Z.L.; Walling, R.; Masterson, T.A.; Calaway, A.C.; et al. Phase II neoadjuvant (N-) gemcitabine (G) and pembrolizumab (P) for locally advanced urothelial cancer (laUC): Interim results from the cisplatin (C)-ineligible cohort of GU14-188. J. Clin. Oncol. 2020, 38, 5019.
- Cathomas, R.; Petrausch, U.; Hayoz, S.; Schneider, M.; Schardt, J.A.; Seiler, R.; Erdmann, A.; Rothschild, S.; Aeppli, S.; Mach, N.; et al. Perioperative Chemoimmunotherapy with Durvalumab (Durva) in Combination with Cisplatin/Gemcitabine (Cis/Gem) for Operable Muscle-Invasive Urothelial Carcinoma (Miuc): Preplanned Interim Analysis of a Single-Arm Phase Ii Trial (Sakk 06/17). J. Clin. Oncol. 2020, 38, 499.
- Iliakis, G.; Wang, Y.; Guan, J.; Wang, H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene 2003, 22, 5834–5847.
- Mikkelsen, R.B.; Wardman, P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene 2003, 22, 5734–5754.
- Burnette, B.C.; Liang, H.; Lee, Y.; Chlewicki, L.; Khodarev, N.N.; Weichselbaum, R.R.; Fu, Y.-X.; Auh, S.L. The Efficacy of Radiotherapy Relies upon Induction of Type I Interferon—Dependent Innate and Adaptive Immunity. Cancer Res. 2011, 71, 2488–2496.
- Fuertes, M.B.; Kacha, A.K.; Kline, J.; Woo, S.-R.; Kranz, D.M.; Murphy, K.M.; Gajewski, T.F. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J. Exp. Med. 2011, 208, 2005–2016.
- Lugade, A.A.; Moran, J.P.; Gerber, S.A.; Rose, R.C.; Frelinger, J.G.; Lord, E.M. Local Radiation Therapy of B16 Melanoma Tumors Increases the Generation of Tumor Antigen-Specific Effector Cells That Traffic to the Tumor. J. Immunol. 2005, 174, 7516–7523.
- Weichselbaum, R.R.; Liang, H.; Deng, L.; Fu, Y.-X. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379.
- Formenti, S.C.; DeMaria, S. Combining Radiotherapy and Cancer Immunotherapy: A Paradigm Shift. J. Natl. Cancer Inst. 2013, 105, 256–265.
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; De Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271.
- Gasser, S.; Orsulic, S.; Brown, E.J.; Raulet, D.H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005, 436, 1186–1190.
- Chiang, C.-S.; Fu, S.Y.; Wang, S.-C.; Yu, C.-F.; Chen, F.-H.; Lin, C.-M.; Hong, J.-H. Irradiation Promotes an M2 Macrophage Phenotype in Tumor Hypoxia. Front. Oncol. 2012, 2, 89.
- Bunt, S.K.; Yang, L.; Sinha, P.; Clements, V.K.; Leips, J.; Ostrand-Rosenberg, S. Reduced Inflammation in the Tumor Microenvironment Delays the Accumulation of Myeloid-Derived Suppressor Cells and Limits Tumor Progression. Cancer Res. 2007, 67, 10019–10026.
- Vanpouille-Box, C.; Diamond, J.M.; Pilones, K.A.; Zavadil, J.; Babb, J.S.; Formenti, S.C.; Barcellos-Hoff, M.H.; DeMaria, S. TGFβ Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res. 2015, 75, 2232–2242.
- Victor, C.T.-S.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377.
- Derer, A.; Frey, B.; Fietkau, R.; Gaipl, U.S. Immune-modulating properties of ionizing radiation: Rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol. Immunother. 2016, 65, 779–786.
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.-X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695.
- Sharabi, A.B.; Nirschl, C.J.; Kochel, C.M.; Nirschl, T.R.; Francisca, B.J.; Velarde, E.; Deweese, T.L.; Drake, C.G. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1–Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol. Res. 2015, 3, 345–355.
- Wu, C.-T.; Chen, W.-C.; Chang, Y.-H.; Lin, W.-Y.; Chen, M.-F. The role of PD-L1 in the radiation response and clinical outcome for bladder cancer. Sci. Rep. 2016, 6, 19740.
- Liu, Y.; Dong, Y.; Kong, L.; Shi, F.; Zhu, H.; Yu, J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 1–15.
- Park, S.S.; Dong, H.; Liu, X.; Harrington, S.M.; Krco, C.J.; Grams, M.P.; Mansfield, A.S.; Furutani, K.M.; Olivier, K.R.; Kwon, E.D. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol. Res. 2015, 3, 610–619.
- Solanki, A.A.; Bossi, A.; Efstathiou, J.A.; Lock, D.; Mondini, M.; Ramapriyan, R.; Welsh, J.; Kang, J. Combining Immunotherapy with Radiotherapy for the Treatment of Genitourinary Malignancies. Eur. Urol. Oncol. 2019, 2, 79–87.
- Walshaw, R.C.; Honeychurch, J.; Illidge, T.M.; Choudhury, A. The anti-PD-1 era—An opportunity to enhance radiotherapy for patients with bladder cancer. Nat. Rev. Urol. 2018, 15, 251–259.
- Tholomier, C.; Marcq, G.; Shinde-Jadhav, S.; Ayoub, M.; Huang, J.M.; Kool, R.; Skowronski, R.; Brimo, F.; Mansure, J.J.; Kassouf, W. Optimizing Sequence of PD-L1 Immune-Checkpoint Inhibitors and Radiation Therapy in Bladder Cancer. Bladder Cancer 2020, 6, 295–302.
- Alegre, M.-L.; Frauwirth, K.A.; Thompson, C.B. T-cell regulation by CD28 and CTLA-4. Nat. Rev. Immunol. 2001, 1, 220–228.
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106.
- Webb, E.S.; Liu, P.; Baleeiro, R.; Lemoine, N.R.; Yuan, M.; Wang, Y.-H. Immune checkpoint inhibitors in cancer therapy. J. Biomed. Res. 2018, 32, 317–326.
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280.
- Duraiswamy, J.; Kaluza, K.M.; Freeman, G.J.; Coukos, G. Dual Blockade of PD-1 and CTLA-4 Combined with Tumor Vaccine Effectively Restores T-Cell Rejection Function in Tumors. Cancer Res. 2013, 73, 3591–3603.
- Fiegle, E.; Doleschel, D.; Koletnik, S.; Rix, A.; Weiskirchen, R.; Borkham-Kamphorst, E.; Kiessling, F.; Lederle, W. Dual CTLA-4 and PD-L1 Blockade Inhibits Tumor Growth and Liver Metastasis in a Highly Aggressive Orthotopic Mouse Model of Colon Cancer. Neoplasia 2019, 21, 932–944.
- Mangsbo, S.M.; Sandin, L.C.; Anger, K.; Korman, A.J.; Loskog, A.; Tötterman, T.H. Enhanced Tumor Eradication by Combining CTLA-4 or PD-1 Blockade with CpG Therapy. J. Immunother. 2010, 33, 225–235.
- Van Hooren, L.; Sandin, L.C.; Moskalev, I.; Ellmark, P.; Dimberg, A.; Black, P.; Tötterman, T.H.; Mangsbo, S.M. Local checkpoint inhibition of CTLA-4 as a monotherapy or in combination with anti-PD1 prevents the growth of murine bladder cancer. Eur. J. Immunol. 2017, 47, 385–393.
- Van Dijk, N.; Gil-Jimenez, A.; Silina, K.; Hendricksen, K.; Smit, L.A.; De Feijter, J.M.; Van Montfoort, M.L.; Van Rooijen, C.; Peters, D.; Broeks, A.; et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: The NABUCCO trial. Nat. Med. 2020, 26, 1839–1844.
- Postow, M.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168.
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.D.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921.
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294.
- Criscuolo, D.; Morra, F.; Giannella, R.; Visconti, R.; Cerrato, A.; Celetti, A. New combinatorial strategies to improve the PARP inhibitors efficacy in the urothelial bladder Cancer treatment. J. Exp. Clin. Cancer Res. 2019, 38, 1–9.
- Shiping, J.; Xia, W.; Yamaguchi, H.; Wei, Y.; Chen, M.-K.; Hsu, J.-M.; Hsu, J.L.; Yu, W.-H.; Du, Y.; Lee, H.-H.; et al. Parp Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin. Cancer Res. 2017, 23, 3711–3720.
- Lampert, E.J.; Zimmer, A.S.; Padget, M.R.; Cimino-Mathews, A.; Nair, J.R.; Liu, Y.; Swisher, E.M.; Hodge, J.W.; Nixon, A.B.; Nichols, E.; et al. Combination of PARP Inhibitor Olaparib, and PD-L1 Inhibitor Durvalumab, in Recurrent Ovarian Cancer: A Proof-of-Concept Phase II Study. Clin. Cancer Res. 2020, 26, 4268–4279.
- Rodriguez-Moreno, J.F.; de Velasco, G.; Fernandez, I.B.; Alvarez-Fernandez, C.; Fernandez, R.; Vazquez-Estevez, S.; Virizuela, J.A.; Gajate, P.; Font, A.; Lainez, N.; et al. Impact of the Combination of Durvalumab (Medi4736) Plus Olaparib (Azd2281) Administered Prior to Surgery in the Molecular Profile of Resectable Urothelial Bladder Cancer: Neodurvarib Trial. J. Clin. Oncol. 2019, 38, 542.
- Pickup, M.W.; Novitskiy, S.V.; Moses, H.L. The roles of TGFβ in the tumour microenvironment. Nat. Rev. Cancer 2013, 13, 788–799.
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ Attenuates Tumour Response to PD-L1 Blockade by Contributing to Exclusion of T Cells. Nature 2018, 554, 544–548.
- Ravi, R.; Noonan, K.A.; Pham, V.; Bedi, R.; Zhavoronkov, A.; Ozerov, I.V.; Makarev, E.; Artemov, A.V.; Wysocki, P.T.; Mehra, R.; et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy. Nat. Commun. 2018, 9, 1–14.
- Ohm, J.E.; Carbone, D.P. VEGF as a Mediator of Tumor-Associated Immunodeficiency. Immunol. Res. 2001, 23, 263–272.
- Farsaci, B.; Donahue, R.N.; Coplin, M.A.; Grenga, I.; Lepone, L.M.; Molinolo, A.A.; Hodge, J.W. Immune Consequences of Decreasing Tumor Vasculature with Antiangiogenic Tyrosine Kinase Inhibitors in Combination with Therapeutic Vaccines. Cancer Immunol. Res. 2014, 2, 1090–1102.
- Ghafouri, S.; Burkenroad, A.; Pantuck, M.; Almomani, B.; Stefanoudakis, D.; Shen, J.; Drakaki, A. VEGF inhibition in urothelial cancer: The past, present and future. World J. Urol. 2020, 1–9.
- Molife, C.; Hess, L.M.; Cui, Z.L.; Li, X.I.; Beyrer, J.; Mahoui, M.; Oton, A.B. Sequential therapy with ramucirumab and/or checkpoint inhibitors for non-small-cell lung cancer in routine practice. Futur. Oncol. 2019, 15, 2915–2931.
- Tu, M.M.; Lee, F.Y.F.; Jones, R.T.; Kimball, A.K.; Saravia, E.; Graziano, R.F.; Coleman, B.; Menard, K.; Yan, J.; Michaud, E.; et al. Targeting DDR2 enhances tumor response to anti-PD-1 immunotherapy. Sci. Adv. 2019, 5, eaav2437.
- Bakhru, P.; Sirisaengtaksin, N.; Soudani, E.; Mukherjee, S.; Khan, A.; Jagannath, C. BCG vaccine mediated reduction in the MHC-II expression of macrophages and dendritic cells is reversed by activation of Toll-like receptors 7 and 9. Cell. Immunol. 2014, 287, 53–61.
- Wang, Y.-H.; Cao, Y.-W.; Yang, X.-C.; Niu, H.-T.; Sun, L.-J.; Wang, X.-S.; Liu, J. Effect of TLR4 and B7-H1 on Immune Escape of Urothelial Bladder Cancer and its Clinical Significance. Asian Pac. J. Cancer Prev. 2014, 15, 1321–1326.
- Nakhlé, J.; Pierron, V.; Bauchet, A.-L.; Plas, P.; Thiongane, A.; Meyer-Losic, F.; Schmidlin, F. Tasquinimod modulates tumor-infiltrating myeloid cells and improves the antitumor immune response to PD-L1 blockade in bladder cancer. OncoImmunology 2016, 5, e1145333.




