| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sang-Hee Park | + 3713 word(s) | 3713 | 2021-03-16 05:08:48 | | | |
| 2 | Bruce Ren | Meta information modification | 3713 | 2021-03-23 06:35:08 | | | | |
| 3 | Bruce Ren | Meta information modification | 3713 | 2021-03-23 10:22:30 | | |
Video Upload Options
separation system owing to its excellent cost and energy saving efficiency, easy scale-up in the narrow area and mild operation conditions. Membrane properties are the key part in terms of determining the separation efficiency in the OSN system. In this entry, the recently reported OSN thin-film composite (TFC) membranes were investigated to understand insight of membrane materials and performance.
1. Introduction
Organic solvent nanofiltration (OSN) is a pressure-driven separation process, applicable for the solvent recycle and exchange, concentrations, and purification of chemicals and pharmaceuticals in organic solvent environments, which is also called as solvent resistant nanofiltration (SRNF) [1][2]. In general, OSN is an emerging platform to effectively separate solutes with molecular weights ranging from 100 to 1000 g mol−1 from polar or non-polar solvent solution, which could replace the conventional separation process such as the adsorption, extraction, distillation, evaporation, and chromatography [3].
With respect to the sustainability, the membrane-based OSN separation process has been recognized as a best separation technology due to its low energy requirements, low solid waste generation, low carbon footprint, low labor intensity, straightforward scale-up, stability in harsh environments (pH, high temperature, and solvents), mild operating conditions (room temperature and low pressure), easy solvent swap from high to low boiling point solvents, and simultaneous removal of solutes from different chemical environments [4][5].
For last a few decades, polymeric membranes have been intensively explored to apply for diverse OSN separation processes due to their huge merits including the large variety of available polymers, relatively low price, simple fabrication process, and ease of upscaling [6]. Two kinds of main polymeric membranes are integrally skinned asymmetric (ISA) and thin-film composite (TFC) membranes [7]. ISA membranes are usually prepared by the phase inversion technique, resulting in the formation of dense selective layer with a few hundred nanometers in thickness on highly porous sublayer with several microns in thickness [8]. Despite of their advantages including easy fabrication process and high mechanical strength, the permeance reduction by its physical aging and compaction over time in operation is a critical issue to be solved [9].
Thin-film composite (TFC) membranes are commonly prepared by diverse coating methods, which are composed of an ultra-thin selective layer on top of an ISA-type porous support membrane [10]. Compared with ISA membrane, the selective layer was easily controlled, leading to better separation performance.
2. Materials for TFC Membrane
TFC membranes consists of an ultra-thin selective layer on top of a highly porous asymmetric support membrane. The selective layer can be usually formed via the interfacial polymerization (IP) of two monomers of coating polymers or prepolymers. In the OSN applications, the selective layer mainly determines the solvent permeance and selectivity between solvent and solutes, the support membrane should have an excellent chemical stability against diverse organic solvents and the high mechanical and thermal stabilities. Thus, many studies have focused on the design for the selective layer and the support membrane independently to obtain the optimum performance. In this section, the recently reported organic monomers and polymers to prepare TFC OSN membranes are described.
2.1. Support Membranes
The asymmetric support membranes are commonly fabricated by a non-solvent induced phase inversion separation (NIPS) technique that includes three steps as follows:
-
Preparation of the polymer dope solution using polar aprotic solvents such as dimethylformamide (DMF), N-methyl-2-pyrrolidone (NMP), and dimethyl sulfoxide (DMSO) and degassing
-
Casting the polymer film on the highly porous non-woven fabric using a casting knife
-
Immersing the cast film in the non-solvent (usually water) bath
When the cast film is immersed in non-solvent bath, the solidification of the polymer immediately occurs through the solvent exchange between the polar aprotic solvent and non-solvent, leading to the formation of the asymmetric porous support membrane. This is the simple and most powerful technique to generally prepare the ultrafiltration (UF)-grade and nanofiltration (NF)-grade asymmetric membranes. It has been usually used in water purification applications. It is also called as an ISA membrane in OSN applications. During the phase inversion separation step, the dense skin layer is rapidly formed by the fast rate of solvent exchanging, while the porous sublayer with large pores is slowly formed due to the relatively slow rate of solvent exchanging. Therefore, the asymmetric porous membrane is formed, which is composed of a thin and dense skin layer and a thick and more porous sublayer.
It is a key point to control the thermodynamic stability of the phase inversion system and the speed of solvent exchanging, in terms of the formation of membrane morphology (i.e., asymmetric structures, thickness, and porosity of the skin layer) and the membrane performance. In 2011, Livingston research group demonstrated the main membrane formation parameters, including polymer/solvent/non-solvent system, evaporation step, and the role of a co-solvent, and influence of polymer characteristics on membrane performance during the phase inversion process [11][12][13].
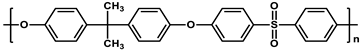
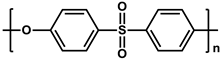
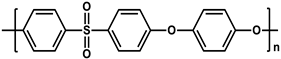
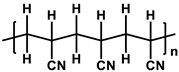
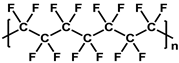
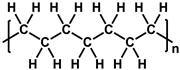
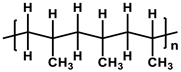
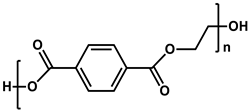
To prepare the porous support membrane, various materials have been commonly used such as polysulfone (PSF) [14][15][16][17][18][19], polyethersulfone (PES) [20][21], poly(ether ether ketone) (PEEK) [22][23][24], polyacrylonitrile (PAN) [25][26], poly(vinylidene fluoride) (PVDF) [27][28][29], polyethylene (PE) [30], polypropylene (PP) [31][32][33], the recycled polyethylene terephthalate (PET) [34], and cellulose [35] as shown in Table 1. Most of the porous support membranes are prepared by the phase inversion method, while the common polyolefin membranes including PE and PP are manufactured by sequential steps consisting of the melt extrusion and mechanical stretching [36]. Porous PP and PE membranes are the strong candidate as a support membrane for OSN applications owing to their great mechanical and thermal properties and excellent resistance against most common polar and non-polar solvents. However, the common polymeric membranes such as PSF and PES shows the poor solvent resistance for strong polar solvents including DMF and DMSO etc., since it is fabricated from the polymeric dope solution of the same strong polar solvents, obstructing the expansion of their OSN application areas without a further cross-linking step.
Table 1. Chemical names and structures of common support membranes.
Here, there are the representative cross-linked porous supports that are prepared through the reaction with multi-functional cross-linking agent after forming the porous structures by the phase inversion separation technique as shown in Figure 1. One is a cross-linked polybenzimidazole (PBI) membrane, which can be prepared via the cross-linking reaction with multi-functional alkyl halides [37][38][39], epoxies [40], and acyl chlorides [41]. These reactions lead to the formation of covalent carbon-nitrogen bonds, resulting in showing the excellent solvent resistance of the porous support membrane. Other cases are cross-linked P84 and Materimid polyimide (PI) membranes. These polymers can form amide bonds through the cross-linking reaction with multi-functional amines [42][43]. To date, the cross-linking approach is the best method to prepare the OSN membrane with the great solvent resistance, while these materials are so expensive and brittle [36]. In particular, the cross-linking step of PI support membranes is time consuming, which takes more than 16 h, contributing to the enhancement of manufacturing cost and the production of chemical wastes.
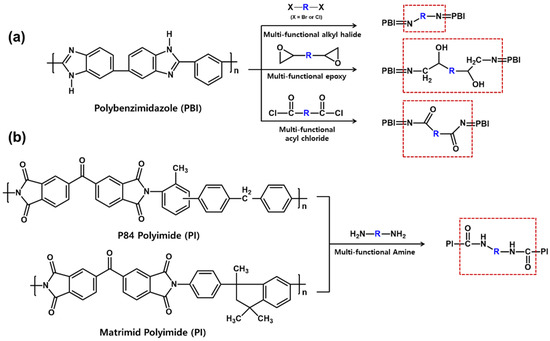
Figure 1. Cross-linking reaction scheme of (a) polybenzimidazole (PBI) and (b) polyimides (PI).
Recently, some different cross-linking methods have been reported to obtain solvent-stable porous supports. For example, Zhao et al. [44] reported the interpenetrating polymer networking (IPN) concept using PBI and polydopamine (PDA). PDA polymerization is conducted by immersing in the NIO4 and Tris buffer solution for several days after the phase inversion step of the PBI/PDA dope solution. The prepared porous membrane showed excellent thermal stability and solvent resistance. In addition, Lu et al. [45] and Kim et al. [46] have prepared polyamic acid (PAA) and hydroxy polyimide (HPI) precursor polymers, respectively. Both PI and HPI nanofiber mats are prepared by an electrospinning of each solution, which revealed great solvent resistance after the heat-treatment at 300~400 °C.
2.2. Selective Layers
As mentioned above, a selective layer of TFC membranes determines the final membrane performance, which can be fabricated by interfacial polymerization [47], coating [8][48], 3D printing [49][50], and self-assembly layer-by-layer [51][52] techniques on the surface of a porous support membrane. In general, polyamide (PA)-based selective layer is prepared via interfacial polymerization of m-phenylenediamine (MPD) and trimesoyl chloride (TMC), where two monomers rapidly react at the interface between two immiscible water and organic solvent phases [53]. The three-dimensional (3D) cross-linked PA-selective layer is formed by the reaction combination between two amine groups of MPD and three acid chloride groups of TMC. In addition, the dense and highly cross-linked PA-selective layer is formed in a few seconds due to the high reactivity of acyl chlorides with amines. The whole interfacial polymerization process contains multisteps, but it is so easy and short as follows:
-
Preparation of two immiscible monomer solutions (MPD and TMC commonly dissolved in water and n-hexane, respectively)
-
First step: immersing the porous support membrane in MPD aqueous solution for a few minutes (3~30 min)
-
Second step: contacting MPD-immersed support membrane in TMC organic solution for a few minutes (1~5 min) after removing excess MPD solution on the porous support membrane using a gummous roller.
-
Final step: cleaning the prepared PA TFC membrane using a pure n-hexane to stop the reaction and then drying.
Thus, the simple and cost-effective interfacial polymerization technique has been widely used for fabricating TFC membranes in both the academic world and industrial applications like as nanofiltration (NF) and reverse osmosis (RO) process.
2.2.1. Conventional MPD-based Selective Layer
Since Solomon et al. have first introduced PA TFC membrane as a OSN membrane, many researchers have reported PA TFC membranes prepared using multi-functional amines and acid chlorides on various types of support membranes [10]. They prepared the selective layer using MPD/piperazine (PIP) and TMC on the cross-linked PI support membrane to obtain the stable membrane against DMF. Compared to the conventional ISA OSN membranes (first generation membrane), the developed TFC membranes showed excellent solvent permeance without sacrificing selectivity, which has been considered as a strong candidate of the second generation OSN membrane.
In 2015, the ultra-thin selective layer with only 8 nm thickness was successfully prepared via interfacial polymerization of MPD and TMC as shown in Figure 2 [54]. PIP and 4-(aminomethyl)piperidine (AMP) were also used as a monomer in an aqueous phase instead of MPD, leading to the formation of MPD-based and/or PIP-based polyamide selective layer. The ultra-thin polyamide selective layer with the flat surface morphology was fabricated by introducing the highly porous cadmium hydroxide (Cd(OH)2) nanostrand as a middle layer on top of porous cross-linked PI or alumina. Performance of the developed TFC membranes was systemically investigated with different MPD and TMC concentrations, reaction times, and types of amine monomers. It is interesting to note that the significantly enhanced permeance of the MPD-based selective layer was observed after DMF activation without the noticeable decline of rejection but was not revealed at the selective layers prepared with other monomers (PIP or 4-(aminomethyl)piperidine (AMP)) [54].
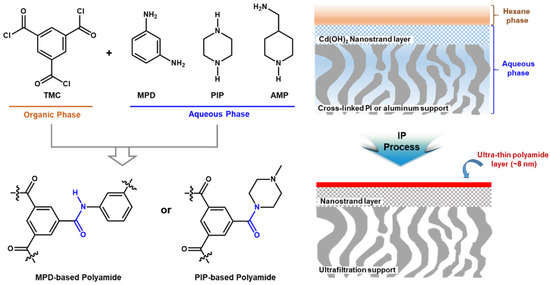
Recently, it was experimentally demonstrated that unreacted monomers and small PA fragments are extracted from PA network during the solvent activation [55]. Therefore, it can be reasonably postulated that the high permeable pathways inside PA networks are formed by removing the small PA fragments. Nevertheless, this solvent activation effect of a PA-selective layer should be carefully studied, because its chemical structures and swelling effects can be dramatically changed by the monomer kinds and concentrations.
For the MPD-based PA-selective layer, it should be noted that the use of a surfactant dramatically increases in membrane performance even if it also increases the thickness and thus transport resistance of the selective layer. Park et al. have reported the TFC OSN membrane composed of a very thick selective layer on top of PE porous support membrane using sodium dodecyl sulfate (SDS) as shown in Figure 3 [56]. To successfully prepare the MPD-based selective layer on the hydrophobic PE support membrane (contact angle = 120°), the PE support membrane was O2 plasma-treated to provide the hydrophilic nature. In addition, the surfactant (SDS) as an additive was added in a MPD aqueous solution, resulting in the improvement of the wettability of the support with a MPD aqueous solution and the formation of the stable reaction interface between a MPD aqueous solution and a TMC organic solution.
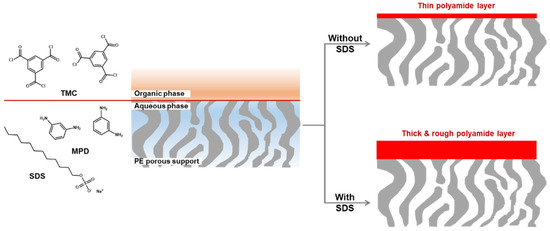
Compared to the pristine selective layer without SDS, the thickness and roughness of the selective layer increased more than 6 and 2.5 times, respectively. In general, the thick selective layer has the high transport resistance, leading to the decline of the solvent permeance [56][57]. Nevertheless, acetone permeance of the TFC membrane prepared by the assistance of 0.2 wt% SDS was significantly enhanced more than 6 times and the styrene oligomer rejection dramatically increased. The enhanced acetone permeance is ascribed to the increased surface roughness that overwhelms the increased thickness. In addition, the observed high rejection indicates the formation of the highly cross-linked PA-selective layer.
Thus, the use of the proper amount of a surfactant and/or some additives could help to the formation of the highly cross-linked and more permeable PA-selective layer. In terms of solvent permeance, it should be mentioned that the developed TFC OSN membranes prepared by MPD and TMC showed great permeance enhancement in polar solvents, such as acetonitrile, acetone, and methanol, but not in non-polar solvents, such as toluene, n-hexane, and n-heptane. This is because, these TMC-based TFC membranes have the relatively hydrophilic selective layer (contact angle = 60~70°) due to a lot of carboxylic acid groups, which are donated by the hydrolysis of TMC [58][59]. Therefore, new design of a selective layer is necessary to obtain high permeable TFC membrane for non-polar solvents.
2.2.2. Selective Layer with Enhanced Microporosity
Here, some highly permeable and selective membranes for non-polar solvents have been introduced. Among them, Solomon et al. [60] have reported the defect-free and high cross-linked polyester selective layer prepared by interfacial polymerization of TMC and diverse contorted phenols, instead of MPD on top of a cross-linked PI or an alumina support as shown in Figure 4. It was their hypothesis that the contorted monomers can provide non-coplanar orientations in the film networks, increasing interconnectivity of intermolecular voids, which are beneficial for enhancing membrane performance.
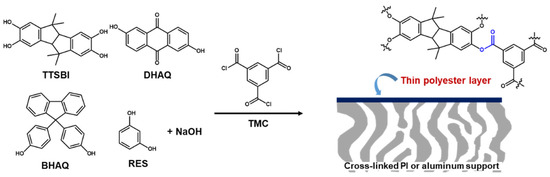
To demonstrate the hypothesis, diverse phenols, which are contorted monomers of spiro-structured 5,5′,6,6′-tetrahydroxy-3,3,3′,3′-tetra- methylspirobisindane (TTSBI) and cardo-structured 9,9-bis(4-hydroxyphenyl) fluorene (BHPF) and non-contorted monomers of dihydroxyanthraquinone (DHAQ), and 1,3-benzenediol (RES), were used to react with TMC. Compared to amine groups, the relatively slow reactivity of hydroxyl groups donated from a phenol was greatly promoted by adding sodium hydroxide (NaOH) with molar ratio of 4:1 (NaOH to monomer). The prepared BHPF- and TTSBI-based polyester contorted films showed much higher solvent permeance in both polar and non-polar solvents than that of the DHAQ- and RES-based polyester non-contorted films. Indeed, much more interconnected voids and the enhanced microporosity were formed by the contorted structures of BHPF and TTSBI, which was demonstrated through the three-dimensional (3D) molecular modeling study.
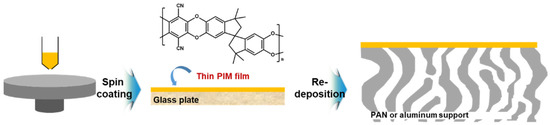
Furthermore, polymers of intrinsic microporosity (PIMs) have been explored to fabricate the high permeable OSN membranes. It has a great attractive merit as a selective layer with the high free volume donated from its continuous network of interconnected intermolecular voids like the polyester contorted film mentioned above [8]. PIM-based selective layers have been usually prepared on porous support membranes by dip coating [61][62], roll-to-roll dip coating [63][64], or spin coating [8][48].
Gorgojo et al. [8] have reported that ultra-thin PIM film with intrinsic microporosity and 35 nm in thickness is prepared on a glass substrate by a spin coating method, subsequently transferred to a porous PAN or an alumina support as shown in Figure 5. Thickness of the PIM-selective layer was systemically controlled by the PIM concentrations dissolved in chloroform (CF). The prepared PIM-based TFC membrane showed excellent non-polar solvent (n-heptane) permeance with a rejection for 90% of hexaphenylbenzene.

The boron-based COF thin film is synthesized by the self-condensation of boronic esters or the condensation reaction of boronic acid and catechol groups. It is a good candidate for the selective layer for OSN application, but the hydrolysis property of its ester-based chemical structures is the significant weak point to use as a selective layer, compared to other COF thin films. On the other hand, imine-based COF thin film is prepared by the condensation reaction between amine and aldehyde groups, which is less susceptible to the hydrolysis than boron-based one. The outstanding solvent resistance against diverse organic solvents has been demonstrated by other pioneer researchers [68][69][70]. Additionally, the triazine-based COF also showed lower crystallinity and higher stability than boron-based COF, which is stable in diverse organic solvents [71].
COF-based membranes have been fabricated by the solution casting, assembly of COF nanosheets, solvothermal synthesis, mechanochemical synthesis, and interfacial polymerization techniques [72][73]. Matsumoto et al. reported crystalline and the free-standing COF thin film prepared through the interfacial polymerization of polyfunctional amines and aldehydes [74]. They used scandium(ΙΙΙ) triflate (Sc(OTf)3) as a Lewis-acid catalyst to promote imine reaction, resulting in the formation of the continuous imine-based COF thin film. The film thickness was controlled by the monomer concentrations. Especially, the ultra-thin film with 2.5 nm thickness was successfully fabricated at low monomer concentrations. The results are most valuable in terms of that the interfacial polymerization system using Sc(OTf)3 catalyst would help to easily fabricate new class of COF-based OSN TFC membranes.
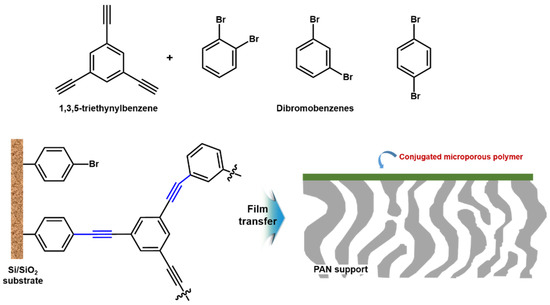
In addition, Liang et al. [75] recently fabricated the conjugated microporous polymers (CMP) membrane with about 40 nm thickness via surface-initiated Sonogashira–Hagihara polymerization between 1,3,5-triethylnylbenzene and dibromobenzenes on the surface of the bromobenzene-functionalized silicon wafer (Si/SiO2 substrate) as shown in Figure 7. It was transferred to the top of porous PAN support to test membrane performance. As a result, the prepared CMP membrane showed ultra-fast n-hexane permeance (32 L m−2 h−1 bar−1) including dye rejection with molecule-weight cutoff (MWCO) of ~560 g mol−1. Therefore, above two cases could be the excellent evidence to provide new opportunity to fabricate electronically active and ultra-thin selective layer for OSN applications.
2.2.3. Selective Layer Prepared by Sustainable Sources
For preparing green TFC membranes, many researchers have tried to alternate the traditional petroleum-based monomers to the diverse sustainable sources. Figure 8 shows the promising candidates of the sustainable sources for preparing the eco-friendly selective layer, which are extracted from natural materials such as mussels, red onion, guava, starch, chitin shells, corns, vanillin bean, sumac leaves, etc.
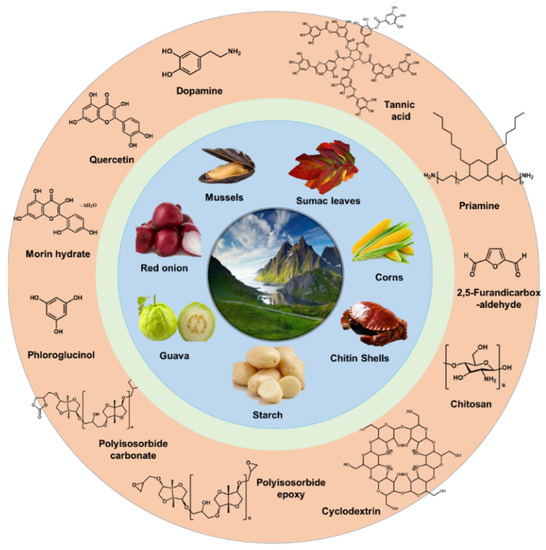
Among them, tannic acid [76][77][78][79][80], quercetin [81], morin hydrate [82], cyclodextrin [83][84][85], chitosan [86], and vanillin [87] played a well role as a reactive material, resulting in the formation of the stable selective layer having great performance. However, most of approaches have changed only one monomer. For example, cyclodextrins with different chemical structures were reacted with the petroleum-based chlorides including TMC or terephthaloyl chloride (TPC) [83]. In addition, they have still used toxic organic solvents such as n-hexane and toluene.
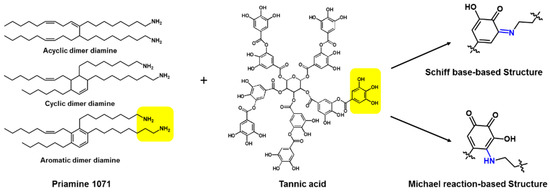
To achieve the real green TFC membrane with solving the drawbacks, Park et al. recently reported that the eco-friendly selective layer with 30 nm thickness was prepared with the new green monomer combination of tannic acid and Priamine using less toxic organic solvent of p-cymene instead of n-hexane as shown in Figure 9 [76]. The reaction between tannic acid and Priamine lead to the formation of C=N and C-N covalent bonds via Schiff base-based and Michael reaction-based reactions, respectively. Additionally, the recycled PET porous membrane was used as a support membrane. Thus, the eco-friendly selective layer, recycled support, layer and less toxic solvents are enough to meet the requirements of the next-generation green TFC membrane. Moreover, it is the most promising candidate to replace the traditional petroleum-based selective layer prepared by MPD and TMC due to its rapid reaction time at room temperature. Priamine having long aliphatic chains endowed high contact angle values around 100°, leading to the outstanding permeance in non-polar solvent.
Membrane materials for OSN TFC membranes and their performance against non-polar solvents including toluene, n-hexane, and n-heptane are summarized in Table 2. The TFC membranes are usually prepared using different methods, solvent systems, diverse monomers, and some additives like a nanomaterial including zeolites, silicalites, quantum dots (QD), and graphene oxides (GO). Performance test is conducted under a cross-flow or dead-end configuration system with diverse parameters such as pressure, temperature, agitation rate, and different concentration of solutes in various solvents [88]. Performance in the RO system is easy to compare each other since the fixed concentration of sodium chloride in water and operation conditions have been used [88]. However, it is very difficult to fairly compare membrane performance owing to more complex testing system with diverse solvent–solute combinations and operating conditions. Therefore, the standard protocol for performance testing is required.
| Membrane No. |
Fabrication | Performance | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Materials (Selective Layer/ Support) |
Method | Solvent System |
Solvent | Permeance (L m−2 h−1 bar−1) |
Solute | Solute MW (g mol−1) |
Rejection (%) |
||
| 1 | Nanoparticle/PI | Coating | Methanol | Toluene | 0.6 | Styrene dimers | 220 | 90 | [89] |
| 2 | Zeolite-filled PDMS/PAN | Coating | Hexane/water | 0.58 | Wilkinson catalyst | 925 | >97 | [90] | |
| 3 | Silicalite-filled PDMS/PI | Coating | Hexane | 0.9 | Bromothymol blue | 624 | 80 | [91] | |
| 4 | Fluoro-functional PA/PEEK | IP (1) | Hexane/water | 2.0 | Styrene dimers | 236 | 98 | [92] | |
| 5 | PIM/PAN | Dip coating | Chloroform | 7.1 | Polystyrene | 800 | 90 | [63] | |
| 6 | QD-based PA/PAN | IP | Hexane/water | 2.5 | Acid yellow 14 | 450 | 90 | [93] | |
| 7 | TA-based polyimine/PET | IP | p-cymene/water | 3.5 | Styrene dimers | 235 | 75 | [76] | |
| 8 | CMP/PAN | Grafting | Toluene/triethylamine | n-Hexane | 31.7 | Protoporphyrin IX | 562 | 90 | [75] |
| 9 | GO-filled PA | IP | Hexane/water | 0.1 | Rhodamine B | 475 | 95 | [94] | |
| 10 | QD-based PA/PAN | IP | Hexane/water | 51 | AY79 Dyes | 1280 | 99 | [93] | |
| 11 | PIM/PAN | Spin coating | Chloroform | n-Heptane | 18 | Hexaphenylbenzene | 535 | 86~90 | [8] |
| 12 | Ti3C2Tx-filled PA/PAN | IP | Hexane/water | 1.8 | PEG | 200 | 92 | [95] | |
| 13 | PIM/PAN | Dip coating | Chloroform | 2.5 | polystyrene | 900 | 90 | [63] | |
| 14 | TA-based polyimine/PET | IP process | p-cymene/water | 2.5 | Styrene dimers | 235 | 91 | [76] | |
References
- Marchetti, P.; Solomon, M.F.J.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806.
- Farahani, M.H.D.A.; Ma, D.; Ardakani, P.N. Nanocomposite membranes for organic solvent nanofiltration. Sep. Purif. Rev. 2018, 49, 177–206.
- Marchetti, P.; Peeva, L.; Livingston, A. The Selectivity Challenge in Organic Solvent Nanofiltration: Membrane and Process Solutions. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 473–497.
- Szekely, S.; Solomon, M.F.J.; Marchetti, P.; Kim, J.F.; Livingston, A.G. Sustainability assessment of organic solvent nanofiltration: From fabrication to application. Green Chem. 2014, 16, 4431–4606.
- Tashvigh, A.A.; Feng, Y.; Weber, M.; Maletzko, C.; Chung, T.S. 110th Anniversary: Selection of Cross-Linkers and Cross-Linking Procedures for the Fabrication of Solvent-Resistant Nanofiltration Membranes: A Review. Ind. End. Chem. Res. 2019, 58, 10678–10691.
- Hermans, S.; Marien, H.; Goethem, C.V.; Vankelecom, I.F.J. Recent developments in thin film (nano)composite membranes for solvent resistant nanofiltration. Curr. Opin. Chem. Eng. 2015, 8, 45–54.
- Vandezande, P.; Gevers, L.E.M.; Vankelecom, I.F.J. Solvent resistant nanofiltration: Separating on a molecular level. Chem. Soc. Rev. 2008, 37, 365–405.
- Gorgojo, P.; Karan, S.; Wong, H.C.; Solomon, M.F.J.; Cabral, J.T.; Livingston, A.G. Ultrathin Polymer Films with Intrinsic Microporosity: Anomalous Solvent Permeation and High Flux Membranes. Adv. Funct. Mater. 2014, 24, 4729–4737.
- Soroko, I.; Livingston, A. Impact of TiO2 nanoparticles on morphology and performance of crosslinked polyimide organic solvent nanofiltration (OSN) membranes. J. Membr. Sci. 2009, 343, 189–198.
- Solomon, M.F.J.; Bhole, Y.; Livingston, A.G. High flux membranes for organic solvent nanofiltration (OSN)—Interfacial polymerization with solvent activation. J. Membr. Sci. 2012, 423–424, 371–382.
- Soroko, I.; Lopes, M.P.; Livingston, A. The effect of membrane formation parameters on performance of polyimide membranes for organic solvent nanofiltration (OSN): Part A. Effect of polymer/solvent/non-solvent system choice. J. Membr. Sci. 2011, 381, 152–162.
- Soroko, I.; Makowski, M.; Spill, F.; Livingston, A. The effect of membrane formation parameters on performance of polyimide membranes for organic solvent nanofiltration (OSN). Part B: Analysis of evaporation step and the role of a co-solvent. J. Membr. Sci. 2011, 381, 163–171.
- Soroko, I.; Sairam, M.; Livingston, A.G. The effect of membrane formation parameters on performance of polyimide membranes for organic solvent nanofiltration (OSN). Part C. Effect of polyimide characteristics. J. Membr. Sci. 2011, 381, 172–182.
- Darvishmanesh, S.; Tasselli, F.; Jansen, J.C.; Tocci, E.; Bazzarelli, F.; Bernado, P.; Luis, P.; Degrève, J.; Drioli, E.; Van der Bruggen, B.J. Preparation of solvent stable polyphenylsulfone hollow fiber nanofiltration membranes. J. Membr. Sci. 2011, 384, 89–96.
- Darvishmanesh, S.; Jansen, J.C.; Tasselli, F.; Tocci, E.; Luis, P.; Degrève, J.; Drioli, E.; Van der Bruggen, B.J. Novel polyphenylsulfone membrane for potential use in solvent nanofiltration. J. Membr. Sci. 2011, 379, 60–68.
- Holda, A.K.; Aernouts, B.; Saeys, W.; Vankelecom, I.F.J.J. Study of polymer concentration and evaporation time as phase inversion parameters for polysulfone-based SRNF membranes. J. Membr. Sci. 2013, 442, 196–205.
- Holda, A.K.; De Roeck, M.; Hendrix, K.; Vankelecom, I.F.J.J. The influence of polymer purity and molecular weight on the synthesis of integrally skinned polysulfone membranes. J. Membr. Sci. 2013, 446, 113–120.
- Holda, A.K.; Vankelecom, I.F.J.J. Integrally skinned PSf-based SRNF-membranes prepared via phase inversion—Part A: Influence of high molecular weight additives. J. Membr. Sci. 2014, 450, 512–521.
- Holda, A.K.; Vankelecom, I.F.J.J. Integrally skinned PSf-based SRNF-membranes prepared via phase inversion—Part B: Influence of low molecular weight additives. J. Membr. Sci. 2014, 450, 499–511.
- White, L.S.; Nitsch, A.R. Solvent recovery from lube oil filtrates with a polyimide membrane. J. Membr. Sci. 2000, 179, 267–274.
- Van der Bruggen, B.; Greens, J.; Vandecasteele, C. Fluxes and rejections for nanofiltration with solvent stable polymeric membranes in water, ethanol and n-hexane. Chem. Eng. Sci. 2002, 57, 2511–2518.
- Da Silva Burgal, J.; Peeva, L.; Livingston, A. Negligible ageing in poly(ether-ether-ketone) membranes widens application range for solvent processing. J. Membr. Sci. 2017, 525, 48–56.
- Da Silva Burgal, J.; Peeva, L.G.; Kumbharkar, S.; Livingston, A. Organic solvent resistant poly(ether-ether-ketone) nanofiltration membranes. J. Membr. Sci. 2015, 479, 105–116.
- Da Silva Burgal, J.; Peeva, L.; Marchetti, P.; Livingston, A. Controlling molecular weight cut-off of PEEK nanofiltration membranes using a drying method. J. Membr. Sci. 2015, 493, 524–538.
- Dalwani, M.; Benes, N.E.; Bargeman, G.; Stamatialis, D.; Wessling, M. Effect of pH on the performance of polyamide/polyacrylonitrile based thin film composite membranes. J. Membr. Sci. 2011, 372, 228–238.
- Ren, J.; McCutcheon, J.R. Polyacrylonitrile supported thin film composite hollow fiber membranes for forward osmosis. Desalination 2015, 372, 67–74.
- Kim, E.S.; Kim, Y.J.; Yu, Q.; Deng, B. Preparation and characterization of polyamide thin-film composite (TFC) membranes on plasma-modified polyvinylidene fluoride (PVDF). J. Membr. Sci. 2009, 344, 71–81.
- Lee, J.S.; Seo, J.A.; Lee, H.H.; Jeong, S.K.; Park, H.S.; Min, B.R. Simple method for preparing thin film composite polyamide nanofiltration membrane based on hydrophobic polyvinylidene fluoride support membrane. Thin Solid Films 2017, 624, 136–143.
- Mertens, M.; Goethem, C.V.; Thijs, M.; Koeckelberghs, G.; Vankelecom, I.F.J. Crosslinked PVDF-membranes for solvent resistant nanofiltration. J. Membr. Sci. 2018, 566, 223–230.
- Hua, D.; Chung, T.S. Polyelectrolyte functionalized lamellar graphene oxide membranes on polypropylene support for organic solvent nanofiltration. Carbon 2017, 122, 604–613.
- Kim, H.I.; Kim, S.S. Plasma treatment of polypropylene and polysulfone supports for thin film composite reverse osmosis membrane. J. Membr. Sci. 2006, 286, 193–201.
- Korikov, A.P.; Kosaraju, P.B.; Sirkar, K.K. Interfacially polymerized hydrophilic microporous thin film composite membranes on porous polypropylene hollow fibers and flat films. J. Membr. Sci. 2006, 279, 588–600.
- Kosaraju, P.B.; Sirkar, K.K. Interfacially polymerized thin film composite membranes on microporous polypropylene supports for solvent-resistant nanofiltration. J. Membr. Sci. 2008, 321, 155–161.
- Pulido, B.A.; Habboub, O.S.; Aristizabal, S.L.; Szekely, G.; Nunes, S.P. Recycled Poly(ethylene terephthalate) for High Temperature Solvent Resistant Membranes. ACS Appl. Polym. Mater. 2019, 1, 2379–2387.
- Abdellah, M.H.; Perez-Manriquez, L.; Puspasari, T.; Scholes, C.A.; Kentish, S.E.; Peinemann, K.-V. A catechin/cellulose composite membrane for organic solvent nanofiltration. J. Membr. Sci. 2018, 567, 139–145.
- Park, S.H.; Kwon, S.J.; Shin, M.G.; Park, M.S.; Lee, J.S.; Park, C.H.; Park, H.; Lee, J.H. Polyethylene-supported high performance reverse osmosis membranes with enhanced mechanical and chemical durability. Desalination 2018, 436, 28–38.
- Valtcheva, I.B.; Marchetti, P.; Livingston, A.G. Crosslinked polybenzimidazole membranes for organic solvent nanofiltration (OSN): Analysis of crosslinking reaction mechanism and effects of reaction parameters. J. Membr. Sci. 2015, 493, 568–579.
- Tashvigh, A.A.; Chung, T.S. Facile fabrication of solvent resistant thin film composite membranes by interfacial crosslinking reaction between polyethylenimine and dibromo-pxylene on polybenzimidazole substrates. J. Membr. Sci. 2018, 560, 115–124.
- Yang, J.; Jiang, H.; Gao, L.; Wang, J.; Xu, Y.; He, R. Fabrication of crosslinked polybenzimidazole membranes by trifunctional crosslinkers for high temperature proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2018, 43, 3299–3307.
- Lin, H.L.; Chou, Y.C.; Yu, T.L.; Lai, S.W. Poly(benzimidazole)-epoxide crosslink membranes for high temperature proton exchange membrane fuel cells. J. Hydrog. Energy 2012, 37, 383–392.
- Farahani, M.H.D.A.; Chung, T.S. A novel crosslinking technique towards the fabrication of high-flux polybenzimidazole (PBI) membranes for organic solvent nanofiltration (OSN). Sep. Purif. Technol. 2019, 209, 182–192.
- Vanherck, K.; Odena, A.C.; Koeckelberghs, G.; Dedroog, T.; Vankelecom, I. A simplified diamine crosslinking method for PI nanofiltration membranes. J. Membr. Sci. 2010, 353, 135–143.
- Soroko, I.; Bhole, Y.; Livingston, A.G. Environmentally friendly route for the preparation of solvent resistant polyimide nanofiltration membranes. Green Chem. 2011, 13, 162–168.
- Zhao, D.; Kim, J.F.; Ignacz, G.; Pogany, P.; Lee, Y.M.; Szekely, G. Bio-Inspired Robust Membranes Nanoengineered from Interpenetrating Polymer Networks of Polybenzimidazole/Polydopamine. ACS Nano 2019, 13, 125–133.
- Lu, T.D.; Zhao, L.L.; Yong, W.F.; Wang, Q.; Duan, L.; Sun, S.P. Highly solvent-durable thin-film molecular sieve membranes with insoluble polyimide nanofibrous substrate. Chem. Eng. J. 2021, 409, 128206–128215.
- Kim, J.H.; Moon, S.J.; Park, S.H.; Cook, M.; Livingston, A.G.; Lee, Y.M. A robust thin film composite membrane incorporating thermally rearranged polymer support for organic solvent nanofiltration and pressure retarded osmosis. J. Membr. Sci. 2018, 550, 322–331.
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717.
- Gao, J.; Japip, S.; Chung, T.S. Organic solvent resistant membranes made from a cross-linked functionalized polymer with intrinsic microporosity (PIM) containing thioamide groups. Chem. Eng. J. 2018, 353, 689–698.
- Chowdhury, M.R.; Steffes, J.; Huey, B.D.; McCutcheon, J.R. 3D printed polyamide membranes for desalination. Science 2018, 361, 682–686.
- Tijing, L.D.; Dizon, J.R.C.; Ibrahim, I.; Nisay, A.R.N.; Shon, H.K.; Advincula, R.C. 3D printing for membrane separation, desalination and water treatment. Appl. Mater. Today 2020, 18, 1–19.
- Choi, W.; Gu, J.E.; Park, S.H.; Kim, S.; Bang, J.; Baek, K.Y.; Park, B.; Lee, J.S.; Chan, E.P.; Lee, J.H. Tailor-Made Polyamide Membranes for Water Desalination. ACS Nano 2015, 9, 345–355.
- Gu, J.E.; Stafford, C.M.; Lee, J.S.; Choi, W.; Kim, B.Y.; Beak, K.Y.; Chan, E.P.; Chung, J.Y.; Bang, J.; Lee, J.H. Molecular Layer-by-Layer Assembled Thin-Film Composite Membranes for Water Desalination. Adv. Mater. 2013, 25, 4778–4782.
- Dai, R.; Li, J.; Wang, Z. Constructing interlayer to tailor structure and performance of thin-film composite polyamide membranes: A review. Adv. Colloid Interface Sci. 2020, 282, 102204.
- Karan, S.; Jiang, Z.; Livingston, A.G. Sub–10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351.
- Shin, M.G.; Park, S.H.; Kwon, S.J.; Kwon, H.E.; Park, J.B.; Lee, J.H. Facile performance enhancement of reverse osmosis membranes via solvent activation with benzyl alcohol. J. Membr. Sci. 2019, 578, 220–229.
- Park, S.H.; Kim, Y.J.; Kwon, S.J.; Shin, M.G.; Nam, S.E.; Cho, Y.H.; Park, Y.I.; Kim, J.F.; Lee, J.H. Polyethylene Battery Separator as a Porous Support for Thin Film Composite Organic Solvent Nanofiltration Membranes. ACS Appl. Mater. Interfaces 2018, 10, 44050–44058.
- Xie, W.; Geise, G.M.; Freeman, B.D.; Lee, H.S.; Byun, G.; McGrath, J.E. Polyamide interfacial composite membranes prepared from m-phenylene diamine, trimesoyl chloride and a new disulfonated diamine. J. Membr. Sci. 2012, 403–404, 152–161.
- Jeon, S.; Park, C.H.; Park, S.H.; Shin, M.G.; Kim, H.J.; Baek, K.Y.; Chan, E.P.; Bang, J.; Lee, J.H. Star polymer-assembled thin film composite membranes with high separation performance and low fouling. J. Membr. Sci. 2018, 555, 369–378.
- Soroush, A.; Barzin, J.; Barikani, M.; Fathizadeh, M. Interfacially polymerized polyamide thin film composite membranes: Preparation, characterization and performance evaluation. J. Membr. Sci. 2012, 287, 310–316.
- Solomon, M.F.J.; Song, Q.; Jelfs, K.E.; Ibanez, M.M.; Livingston, A.G. Polymer nanofilms with enhanced microporosity by interfacial polymerization. Nat. Mater. 2016, 15, 760–767.
- Fritsch, D.; Merten, P.; Heinrich, K.; Lazar, M.; Priske, M. Ultrathin Polymer Films with Intrinsic Microporosity: Anomalous Solvent Permeation and High Flux Membranes. J. Membr. Sci. 2012, 401–402, 222–231.
- Li, J.; Zhang, M.; Feng, W.; Zhu, L.; Zhang, L. PIM-1 pore-filled thin film composite membranes for tunable organic solvent nanofiltration. J. Membr. Sci. 2020, 601, 117951.
- Cook, M.; Gaffney, P.R.J.; Peeva, L.G.; Livingston, A.G. Roll-to-roll dip coating of three different PIMs for Organic Solvent Nanofiltration. J. Membr. Sci. 2018, 558, 52–63.
- Thompson, K.A.; Mathias, R.; Kim, D.; Kim, J.; Rangnekar, N.; Johnson, J.R.; Hoy, S.J.; Bechis, I.; Tarzia, A.; Jelfs, K.E.; et al. N-Aryl–linked spirocyclic polymers for membrane separations of complex hydrocarbon mixtures. Science 2020, 369, 310–315.
- Shi, X.; Xiao, A.; Zhang, C.; Wang, Y. Growing covalent organic frameworks on porous substrates for moleculesieving membranes with pores tunable from ultra- to nanofiltration. J. Membr. Sci. 2019, 576, 116–122.
- Yaun, S.; Li, X.; Zhu, J.; Zhang, G.; Puyvelde, P.V.; Bruggen, B.V.D. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681.
- Li, J.; Zhou, X.; Wang, J.; Li, X. Two-Dimensional Covalent Organic Frameworks (COFs) for Membrane Separation: A Mini Review. Ind. Eng. Chem. Res. 2019, 58, 15394–15406.
- Kandambeth, S.; Biswal, B.P.; Chaudhari, H.D.; Rout, K.C.; Kunjattu H., K.; Mitra, S.; Karak, S.; Das, A.; Mukherjee, R.; Kharul, U.K.; et al. Selective Molecular Sieving in Self-Standing Porous Covalent-Organic-Framework Membranes. Adv. Mater. 2017, 29, 1603945–1603953.
- Gong, J.; Lin, R.B.; Chen, B. Conjugated Microporous Polymers with Rigid Backbones for Organic Solvent Nanofiltration. Chem 2018, 4, 2260–2277.
- Shinde, D.B.; Sheng, G.; Li, X.; Ostwal, M.; Emwas, A.H.; Huang, K.W.; Lai, Z. Crystalline 2D Covalent Organic Framework Membranes for High-Flux Organic Solvent Nanofiltration. J. Am. Chem. Soc. 2018, 140, 14342–14349.
- Uribe, R.F.J.; Doonan, C.J.; Furukawa, H.; Oisaki, K.; Yaghi, O.M. Crystalline Covalent Organic Frameworks with Hydrazone Linkages. J. Am. Chem. Soc. 2011, 133, 11478–11481.
- Liu, G.; Jiang, Z.; Yang, H.; Li, C.; Wang, H.; Wang, M.; Song, Y.; Wu, H.; Pan, F. High-efficiency water-selective membranes from the solution-diffusion synergy of calcium alginate layer and covalent organic framework (COF) layer. J. Membr. Sci. 2019, 572, 557–566.
- Wang, R.; Shi, X.; Xiao, A.; Zhou, W.; Wang, Y. Interfacial polymerization of covalent organic frameworks (COFs) on polymeric substrates for molecular separations. J. Membr. Sci. 2018, 566, 197–204.
- Matsumoto, M.; Valentino, L.; Stiehl, G.M.; Balch, H.B.; Corcos, A.R.; Wang, F.; Ralph, D.C.; Marinas, B.J.; Dichtel, W.R. Lewis-Acid-Catalyzed Interfacial Polymerization of Covalent Organic Framework Films. Chem 2018, 4, 308–317.
- Liang, B.; Wang, H.; Shi, X.; Shen, B.; He, X.; Ghazi, Z.A.; Khan, N.A.; Sin, H.; Khattak, A.M.; Li, L.; et al. Microporous membranes comprising conjugated polymers with rigid backbones enable ultrafast organic-solvent nanofiltration. Nat. Chem. 2018, 10, 961–967.
- Park, S.H.; Alammar, A.; Fulop, Z.; Pulido, B.A.; Nunes, S.P.; Szekely, G. Hydrophobic thin film composite nanofiltration membranes derived solely from sustainable sources. Green Chem. 2021, in press.
- He, M.; Sun, H.; Sun, H.; Yang, X.; Li, P.; Niu, Q.J. Non-organic solvent prepared nanofiltration composite membrane from natural product tannic acid (TA) and cyclohexane-1,4-diamine (CHD). Sep. Purif. Technol. 2019, 223, 250–259.
- Guo, H.; Yao, Z.; Yang, Z.; Ma, X.; Wng, J.; Tang, C.Y. A One-Step Rapid Assembly of Thin Film Coating Using Green Coordination Complexes for Enhanced Removal of Trace Organic Contaminants by Membranes. Environ. Sci. Technol. 2017, 51, 12638–12643.
- Zhang, X.; Lv, Y.; Yang, H.C.; Du, Y.; Xu, Z.K. Polyphenol Coating as an Interlayer for Thin-Film Composite Membranes with Enhanced Nanofiltration Performance. ACS Appl. Mater. Interfaces 2016, 8, 32512–32519.
- Perez, M.L.; Neelakanda, P.; Peinemann, K.V. Tannin-based thin-film composite membranes for solvent nanofiltration. J. Membr. Sci. 2017, 541, 137–142.
- Abdellah, M.H.; Perez, M.L.; Puspasari, T.; Scholes, C.A.; Kentish, S.E.; Peinemann, K.V. Effective Interfacially Polymerized Polyester Solvent Resistant Nanofiltration Membrane from Bioderived Materials. Adv. Sustain. Syst. 2018, 2, 1800043–1800049.
- Perez, M.L.; Neelakanda, P.; Peinemann, K.V. Morin-based nanofiltration membranes for organic solvent separation processes. J. Membr. Sci. 2018, 554, 1–5.
- Liu, J.; Hua, D.; Zhang, Y.; Japip, S.; Chung, T.S. Precise Molecular Sieving Architectures with Janus Pathways for Both Polar and Nonpolar Molecules. Adv. Mater. 2018, 30, 1705933–1705939.
- Villalobos, L.F.; Huang, T.; Peinemann, K.V. Cyclodextrin Films with Fast Solvent Transport and Shape-Selective Permeability. Adv. Mater. 2017, 29, 1606641–1606647.
- Xu, S.J.; Shen, Q.; Xu, Z.L.; Dong, Z.Q. Novel designed TFC membrane based on host-guest interaction for organic solvent nanofiltration (OSN). J. Membr. Sci. 2019, 588, 117227–117237.
- Shakeri, A.; Salehi, H.; Rastgar, M. Chitosan-based thin active layer membrane for forward osmosis desalination. Carbohydr. Polym. 2017, 174, 658–668.
- Zhou, A.; Almijbilee, M.M.A.; Zheng, J.; Wang, L. A thin film composite membrane prepared from monomers of vanillin and trimesoyl chloride for organic solvent nanofiltration. Sep. Purif. Technol. 2021, 263, 118394–118400.
- Phuong, H.A.L.; Blanford, C.F.; Szekely, G. Reporting the unreported: The reliability and comparability of the literature on organic solvent nanofiltration. Green Chem. 2020, 22, 3397–3409.
- Siddique, H.; Peeva, L.G.; Stoikos, K.; Pasparakis, G.; Vamvakaki, M.; Livingston, A.G. Membranes for Organic Solvent Nanofiltration Based on Preassembled Nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 1109–1121.
- Gevers, L.E.M.; Vankelecom, I.F.J.; Jacobs, P.A. Solvent-resistant nanofiltration with filled polydimethylsiloxane (PDMS) membranes. J. Membr. Sci. 2006, 278, 199–204.
- Vanherck, K.; Aerts, A.; Martens, J.; Vankelecom, I. Hollow filler based mixed matrix membranes. Chem. Commun. 2010, 46, 2492–2494.
- Jimenez, S.M.F.; Gorgojo, P.; Munoz, I.M.; Livingston, A.G. Beneath the surface: Influence of supports on thin film composite membranes by interfacial polymerization for organic solvent nanofiltration. J. Membr. Sci. 2013, 448, 102–113.
- Wu, X.; Zhou, G.; Cui, X.; Li, Y.; Wang, J.; Cao, X.; Zhang, P. Nanoparticle-Assembled Thin Film with Amphipathic Nanopores for Organic Solvent Nanofiltration. ACS Appl. Mater. Interfaces 2019, 11, 17804–17813.
- Li, Y.; Li, C.; Li, S.; Su, B.; Han, L.; Mandal, B. Graphene oxide (GO)-interlayered thin-film nanocomposite (TFN) membranes with high solvent resistance for organic solvent nanofiltration (OSN). J. Mater. Chem. A 2019, 7, 13315–13330.
- Hao, L.; Zhang, H.; Wu, X.; Zhang, J.; Wang, J.; Li, Y. Novel thin-film nanocomposite membranes filled with multi-functional Ti3C2Tx nanosheets for task-specific solvent transport. Compos. Part A 2017, 100, 139–149.