
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sophie Alexandra Baron | + 2324 word(s) | 2324 | 2021-03-16 04:40:34 | | | |
| 2 | Peter Tang | Meta information modification | 2324 | 2021-03-20 06:05:17 | | |
Video Upload Options
Colistin is an old polypeptide antibiotic of the group E, discovered in 1947 by Y. Koyama from Paenibacillus polymyxa subspecies colistinus cultures. It is a bactericidal, narrow-spectrum molecule directed against most GNB, but ineffective against Gram-positive bacteria, anaerobic bacteria, and mycoplasmas.
1. Introduction
Over the last 70 years, the polymyxin family of antibiotics, including polymyxin B and colistin (also called polymyxin E), has experienced an uncommon fate. The polymyxins were initially considered “miracle” antibiotics when they were first commercialized in the 1950s, with a bactericidal efficacy against Gram-negative bacteria (GNB) and a low level of resistance [1]. Colistin was subsequently abandoned in the 1980s in favour of other less toxic broad-spectrum antibiotics before regaining the forefront in the 2000s for the treatment of multidrug-resistant GNB infections [2]. As a result, the pharmacokinetic (PK) and pharmacodynamic (PD) properties, as well as the resistance mechanisms developed by the target bacteria, remain poorly understood [3]. Several studies deciphered the mechanism of action of colistin without being able to elucidate it completely in the 1950s. At that time, polymyxin resistance was revealed by the detection of in vitro resistant mutants. Moreover, 30 years of clinical disuse resulted in a lack of knowledge of the minimum inhibitory concentration (MIC) determination in vitro and its optimal use in the clinic [3]. In 2007, the reclassification of polymyxins as a major agent for the treatment of multidrug-resistant GNB infections by the World Health Organization (WHO) revived interest in clinical research on this antibiotic [1]. Consequently, data on PK/PD were collected, and new resistance mechanisms were elucidated. The discovery of the first transferable colistin resistance gene in 2015, the mcr-1 gene (for mobile colistin resistance gene), is the most significant example. This finding highlighted the major role of the animal reservoir in the transmission and diffusion of this antibiotic-resistance gene [4]. In fact, colistin has been used for many years in veterinary medicine as a growth factor and in the prophylaxis and treatment of livestock infections [3]. Investigations into colistin resistance mechanisms revealed the complexity of the pathways by which bacteria defend themselves against colistin activity [2]. It appears that the first targets identified as being responsible for colistin resistance were not sufficient to explain resistance in every isolate, suggesting the existence of other mechanisms involved in polymyxin resistance [1].
New technological tools have become available for researchers over the last few years and have generated a multitude of data to analyse. The remaining challenge is to understand and analyse such information in order to identify new pathways involved in colistin resistance. The aim of this review, through the scientific and clinical history of colistin, is to identify technological methods and interesting targets currently responsible for colistin resistance.
2. Pathways Leading to Colistin Action and Mechanisms of Resistance
2.1. Mode of Action
Colistin is an old polypeptide antibiotic of the group E, discovered in 1947 by Y. Koyama from Paenibacillus polymyxa subspecies colistinus cultures [5]. It is a bactericidal, narrow-spectrum molecule directed against most GNB, but ineffective against Gram-positive bacteria, anaerobic bacteria, and mycoplasmas [3]. The main target of the polymyxins is the lipopolysaccharide (LPS) of GNB membranes [1]. The lipid A of the outer part of the LPS is negatively charged and interacts with divalent cations (mainly Mg2+ and Ca2+), allowing an overall stabilization of the outer membrane [2]. Colistin, a net positively charged molecule, has therefore a strong affinity to bind to the LPS, leading to a displacement of cations by electrostatic interaction. It results in a disorganization of the membrane structure, with release of the LPS [1]. Colistin is then introduced into the outer membrane through its lipophilic acid-fat chain. Colistin alters the permeability of the outer membrane, allowing it to insert itself and reach the inner membrane. A disorganization of this inner membrane then occurs by breaking the integrity of the phospholipid bilayer [6]. Eventually, lysis of this membrane results in the release of intracellular contents and death of the bacteria (Figure 1). This process is the most commonly used mechanism to explain the antibacterial action of colistin, but the ultimate mechanisms leading to cell death are still not well understood [6].
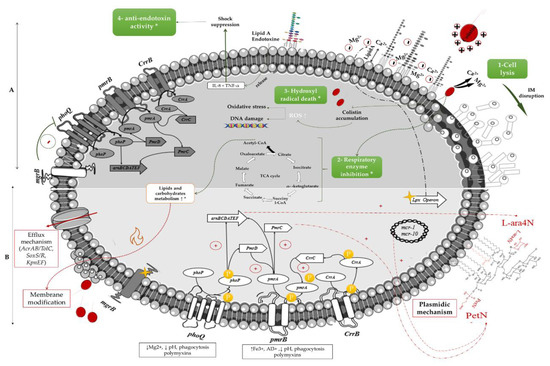
Figure 1. Mechanisms of action and resistance to colistin in Gram-negative organisms. (A): mechanisms of action of colistin, (B): colistin-resistance mechanisms, * on respiratory enzyme inhibition, Hydroxyl radical death, anti-endotoxin activity and the metabolism of lipids and carbohydrates represent the recently described mechanisms of action and resistance, which remain unclear so far; The yellow stars represent mutations resulting in the inactivation of regulatory genes. Green arrows indicate the mechanism of action for a susceptible strain, the red arrows indicate the different pathways involved in colistin resistance, the grey shades demonstrate the differences in the gene expression between the mechanism of action and resistance
Other potential mechanisms of action have been identified, such as vesicle-vesicle contact. After colistin crosses the outer membrane, lipid exchanges between the inner and outer membrane take place, causing structural changes in the membranes with a loss of phospholipids, leading to an osmotic imbalance that lyses the bacteria [7]. The accumulation of free radicals linked to the oxidative stress induced by colistin is also a pathway responsible for DNA, protein, and lipid damage, leading to bacterial death. Inhibition of vital respiratory enzymes and endotoxin activity of lipid A have been described as well. Endotoxin activity involves the inhibition of this activity of lipid A of LPS by colistin, by binding to LPS molecules and neutralizing them, resulting in the release of tumour necrosis factor-alpha (TNF-a) and Interleukin 8 (IL-8) cytokines and thus the suppression of the shock [6]. (Figure 1).
2.2. Mechanisms of Resistance
The most common mechanism of colistin resistance is due to chromosomal mutation in genes associated with the modification of the lipid A of LPS, the primary target of colistin, as an adaptative strategy [2]. Such alterations can be obtained by the addition of phosphoethanolamine (PEtN) and 4-amino-4-deoxy-L-arabinose (L-Ara4N) to the phosphate groups of lipid A [1]. The genes that encode enzymes involved in lipid A synthesis are pmrHFIJFKLM (also known as arnBCADTEF-pmrE). These genes are up-regulated by the two-component systems (TCS) PmrAB and PhoPQ, the latter being negatively regulated by the mgrB gene [8](Figure 1). Other strategies include the use of efflux pumps and capsule formation [1]. Moreover, the horizontal transfer of the plasmid-carried gene mcr-1 encoding for PEtN has become an important cause of the spread of polymyxin resistance among various GNB [4]. The origin of mcr enzymes can be retraced back to the 1980s in China [9].
In the following, we will focus on the methods that enabled discovery of these major mechanisms and on the methods that are currently of the greatest interest in enabling us to understand the unresolved mechanisms.
3. From Electron Microscopy to the Discovery of Regulatory Genes
Electron microscopy was the first tool used to explain colistin’s mechanism of action, demonstrating morphologic changes such as loss of nuclear material and granularity of the cytoplasm [10]. Observations in Escherichia coli and Pseudomonas aeruginosa demonstrated that treatment with polymyxin B or E modifies the cell wall, forming cell wall projections or “blebs” and the release of cytoplasmic contents through splits in the cell envelope [11]. Their presence grew with the polymyxin concentration [12]. Using a fluorescent method, Newton et al. demonstrated in 1954 that Mg2+ and other divalent cations antagonized the activity of polymyxin B at a negatively charged site on P. aeruginosa [13].The researchers compared polymyxins to cationic detergents, in that they are charged similarly at neutral pH, since detergents disorganize the cell membrane and denature certain proteins. They suggested that polymyxin bactericidal activity is due to its ability to combine with bacterial cell structures and disorganize them, leading to loss of the cell’s osmotic balance [14]. To elucidate it, lipid extraction procedures and chromatography showed that polymyxins not only bind to these lipids but also significantly alter their structures [15].Polymyxins rapidly became the treatment of first choice for GNB infections, as it was supposed that one of the characteristics of polymyxins was bacterial difficulty in developing resistance [16]. Thereafter, an enzyme capable of inactivating colistin has been identified, called colistinase, a putative serine protease produced by P. polymyxa. Which seems to be necessary for survival in the presence of polymyxin, its secretion by a gram-positive bacterium remains an intriguing question [17]. Recently, a polymyxin inhibitory enzyme of Bacillus licheniformis strain DC-1 has been identified [18]. It is an alkaline Apr protease, which cleaves colistin at two peptide bonds: one between the tripeptide side chain and the heptapeptide ring, and the other between l-Thr and l-Dab within the heptapeptide ring [18]. Thereafter a chronological characterization of the different regulatory genes (pmrAB, phoPQ, arn operon, mgrB and ccrAB) has occurred. Recently, colistin plasmid mediated resistance genes mcr has been characterized through the advent of the whole genome sequencing, this is demonstrated by the growing number of known mcr resistance genes, which has occurred with the rapid decrease in sequencing costs [19]. Using an amino acid homology sequence, they identified mcr-1 codes for a plasmid phosphoethanolamine transferase that catalyzes the addition of pEtN to lipid A, increasing the cationic charges on LPS, thus decreasing the affinity of colistin for LPS [4]. Through genetic and bioinformatic analyses, the origin of the gene could be linked to the genus Moraxella. Mass spectrometry has helped also in the characterization of colistin resistance by highlighting lipid A modifications. Matrix Assisted Laser Desorption Ionization Time-Of-Flight MS directly assesses the biochemical cause of colistin resistance and the presence, absence and modification of lipid A, the capsule and the LPS structure, which are components of colistin resistance [20]. Optimization of these method has enabled to discriminate chromosomally encoded colistin resistance and mcr-mediated colistin resistance, by comparing the percentage of modification of Lipid A by either L-Ara4N or pEtN [21].
Unlike the PCR-based approach, functional genomics and mutagenesis enabled the identification and characterization of new antibiotic resistance genes and the pathways involved in colistin resistance. These techniques decipher the colistin resistance mechanism in Shewanella algae MARS 14, suggesting the involvement of a phosphoethanolamine transferase EptA coding for pmrC in polymyxin resistance [22], and the implication acrAB-tolC efflux pump of Enterobacter spp, in colistin heteroresistance [23]. In addition, omics technologies have experienced significant growth, sequence-based known resistance markers represent a small proportion, transcriptome analysis could possibly afford a more comprehensive phenotypic profile [24]. The study of RNA provides a viable alternative to the research for resistance genes in genome sequences [25]. Using a combination of genome sequencing and transcriptional profiling by RNA sequencing analysis (RNA-Seq), the identification of crr genes previously described as an uncharacterized histidine kinase as additional regulators of colistin resistance broadens the available genes that regulate the phenotype and illustrates the multiplicity of ways in which bacteria respond to antimicrobial peptides [26]. The increased expression of cation transporters and other efflux pumps are among the transcription modifications commonly found in colistin-resistant strains [26]. Proteomics and metabolomics, other new “omics” approaches, are used to elucidate the development process of specific biologicals and regulatory mechanisms, as well as the expression of the entire protein and changes in metabolites in specific tissues or cells at the system level [27]. The use of these “omics” techniques has enabled the large-scale discovery of new potential targets to investigate colistin resistance [28]. An unlabelled quantitative proteomics study compared the proteomes of mcr-1-mediated colistin-resistant and colistin-sensitive E. coli. The authors identified a large amount of differentially expressed proteins that may contribute to mcr-1-mediated antimicrobial resistance through regulation of glycerophospholipid metabolism, LPS biosynthesis and phosphoethanolamine substrate accumulation [27].
Over the past 10 years, clustered regularly interspaced short palindromic repeats, the CRISPR-Cas Type II system of Streptococcus pyogenes, have become the easier, faster and predominant choice for engineering applications of the genomes [29]. This system requires the co-expression of the Cas9 protein and two RNAs that guide Cas9 to the target site in the intrusive DNA for recognition and subsequent cleavage. Genome editing with Cas9 has become easier since both RNAs have been enhanced to become a chimeric single-guided RNA (sgRNA). The gRNA targeting specific sequences recruits the Cas9 protein to form the compound, and the Cas9 protein acts as a nuclease and generates a blunt end double-strand break [30]. So far, the CRISPR/Cas9 system has been used as a new microbial gene therapy technology to combat colistin resistance, to confirm its use among resistant bacteria [31]. Some recent original work provides a convincing demonstration of its potential to overcome the problem of multi-drug resistance, by demonstrating that it can limit the spread of drug resistance genes by countering multiple horizontal gene transfer pathways [32]. This system has demonstrated high efficiency in destroying the plasmid harbouring mcr-1 in E. coli [31]. CRISPR system allowed to insert single nucleotide point (SNP) mutation in crrA, crrB, ArnT, mgrB, and pmrA genes. crrB gene mutations lead to resistance to polymyxins and mediate the addition of both L-Ara4N and pEtN to lipid A, thus efficiently validating the functions of these genes [33].
4. Conclusions
Due to its disuse in clinical medicine for years, the mechanisms of action and resistance to colistin remained unclear for a long time. This delay is compounded by a complex mechanism involving multiple metabolic pathways. However, the scientific impetus of recent years has enabled us to increase our knowledge in this field, supported by the use and development of innovative tools. For example, whole genome sequencing represents a real added value to more traditional in vitro methods. It also enables faster detection of the presence of mutations in genes known to be involved in colistin resistance, although in vitro confirmation is still required. High throughput transcriptomics tools have considerably increased the data pool on colistin resistance. However, they remain expensive, difficult to interpret and isolate-dependent. Only a few such studies have been conducted, and the accumulation of data, combined with genome sequencing data, may allow us to identify the pathways involved in resistance. Finally, new knock-in/knock-out strategies and mutagenesis methods represent powerful tools for research in this field. Modern biotechnology techniques, high-throughput sequencing, omics techniques, and genome manipulation have facilitated a new era of discovery. Despite spectacular progress, challenges to the detection and understanding of antimicrobial resistance persist, especially for colistin, due to the unexpectedly large number of mechanisms described in various bacterial species.
References
- Baron S., Hadjadj L., Rolain J.M., Olaitan A.O.; Molecular mechanisms of polymyxin resistance: Knowns and unknowns. . Int. J. Antimicrob. Agents. 2016, 48, 583–591, doi: 10.1016/j.ijantimicag.2016.06.023..
- Olaitan A.O., Morand S., Rolain J.M.; Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. . Front. Microbiol. 2014, 5 , 643, doi: 10.3389/fmicb.2014.00643. .
- Poirel L., Jayol A., Nordmann P.; Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes.. Clin. Microbiol. Rev. 2017, 30, 557–596, doi: 10.1128/CMR.00064-16..
- Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., et al.; Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study.. Lancet Infect. Dis. 2016, 16, 161–168, doi: 10.1016/S1473-3099(15)00424-7..
- Stansly P.G., Schlosser M.E.; Studies on Polymyxin: Isolation and Identification of Bacillus polymyxa and Differentiation of Polymyxin from Certain Known Antibiotics.. J. Bacteriol. 1947, 54 , 549–556, doi: 10.1128/JB.54.5.549-556.1947..
- Ahmed E.S.M.A.E.G., Zhong L.L., Shen C., Yang Y., Doi Y., Tian G.B.; Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019) . Emerg. Microbes Infect. 2020, 9, 868–885, doi: 10.1080/22221751.2020.1754133. .
- Kaye K.S., Pogue J.M., Tran T.B., Nation R.L., Li J.; Agents of Last Resort: Polymyxin Resistance.. Infect. Dis. Clin. North Am. 2016, 30, 391–414, doi: 10.1016/j.idc.2016.02.005..
- Sekyere O.J., Govinden U., Bester L.A., Essack S.Y. J.; Colistin and tigecycline resistance in carbapenemase-producing Gram-negative bacteria: Emerging resistance mechanisms and detection methods. . Appl. Microbiol. 2016, 121, 601–617, doi: 10.1111/jam.13169..
- Haenni M., Poirel L., Kieffer N., Châtre P., Saras E., Métayer V., Dumoulin R., Nordmann P., Madec J.Y.; Co-occurrence of extended spectrum lactamase and MCR-1 encoding genes on plasmids. . Lancet Infect. Dis. 2016, 16, 281–282, doi: 10.1016/S1473-3099(16)00007-4..
- Lopes J., Inniss W.E.; Electron microscopy of effect of polymyxin on Escherichia coli lipopolysaccharide.. J. Bacteriol 1969, 100, 1128–1129., doi: 10.1128/JB.100.2.1128-1130.1969..
- Koike M., Iida K., Matsuo T.; Electron microscopic studies on mode of action of polymyxin.. J. Bacteriol. 1969, 97, 448–452., doi: 10.1128/JB.97.1.448-452.1969..
- Storm D.R., Rosenthal K.S., Swanson P.E.; Polymyxin and related peptide antibiotics.. Annu. Rev. Biochem. 1977, 46, 723–763, doi: 10.1146/annurev.bi.46.070177.003451. .
- Newton B.A. J.; Site of action of polymyxin on Pseudomonas aeruginosa: Antagonism by cations. . Gen. Microbiol. 1954, 10, 491–499, doi: 10.1099/00221287-10-3-491..
- Newton B.A. T; The properties and mode of action of the polymyxins. . Microbiol. Mol. Biol. Rev. 1956;, 20, 14, oi: 10.1128/MMBR.20.1.14-27.1956..
- Few A.V.; The interaction of polymyxin E with bacterial and other lipids. . BBA-Biochim. Biophys. Acta. 1955, 16, 137–145. , doi: 10.1016/0006-3002(55)90191-8. .
- Petersdorf R.G., Plorde J.J. JAMA J.; Colistin-A Reappraisal.. Am. Med. Assoc. 1963, 183, 123–125, doi: 10.1001/jama.1963.63700020022014..
- Kagawa I.M., Koyama Y.; Selective cleavage of a peptide antibiotic, colistin by colistinase.. J. Antibiot. 1980, 33, 1551–1555, doi: 10.7164/antibiotics.33.1551..
- Yin J., Wang G., Cheng D., Fu J., Qiu J., Yu Z.; Inactivation of polymyxin by hydrolytic mechanism. . Antimicrob. Agents Chemother. 2019, 63, 2378–2396. , doi: 10.1128/AAC.02378-18..
- Crofts T.S., Gasparrini A.J., Dantas G.; Next-generation approaches to understand and combat the antibiotic resistome. . Nat. Rev. Microbiol. 2017, 15, 422–434, doi: 10.1038/nrmicro.2017.28..
- Sekyere O.J., Govinden U., Bester L.A., Essack S.Y.; Colistin and tigecycline resistance in carbapenemase-producing Gram-negative bacteria: Emerging resistance mechanisms and detection methods.. J. Appl. Microbiol. 2016, 121, 601–617, doi: 10.1111/jam.13169. .
- Dortet L., Broda A., Bernabeu S., Glupczynski Y., Bogaerts P., Bonnin R., Naas T., Filloux A., Maumus L.G.; Optimization of the MALDIxin test for the rapid identification of colistin resistance in Klebsiella pneumoniae using MALDI-TOF MS.. J. Antimicrob. Chemother. 2020, 75, 110–116, doi: 10.1093/jac/dkz405..
- Telke A.A., Rolain J.M.; Functional genomics to discover antibiotic resistance genes: The paradigm of resistance to colistin mediated by ethanolamine phosphotransferase in Shewanella algae MARS 14. . Int. J. Antimicrob. Agents. 2015, 46, 648–652, doi: 10.1016/j.ijantimicag.2015.09.001. .
- Telke A.A., Olaitan A.O., Morand S., Rolain J.M.; SoxRS induces colistin hetero-resistance in Enterobacter asburiae and Enterobacter cloacae by regulating the acrAB-tolC efflux pump.. J. Antimicrob. Chemother. 2017, 72, 2715–2721, doi: 10.1093/jac/dkx215. .
- Barcz A.K., Gomez J.E., Kaufmann B.B., Hinson E.R., Cosimi L., Borowsky M.L., Onderdonk A.B., Stanley S.A., Kaur D., Bryant K.F., et al.; RNA signatures allow rapid identification of pathogens and antibiotic susceptibilities. . Proc. Natl. Acad. Sci. 2012, 109, 6217–6222, doi: 10.1073/pnas.1119540109. .
- Dunne W.M., Jaillard M., Rochas O., Van Belkum A.; Microbial genomics and antimicrobial susceptibility testing. . Expert Rev. Mol. Diagn. 2017, 17, 257–269, doi: 10.1080/14737159.2017.1283220..
- Wright M.S., Suzuki Y., Jones M.B., Marshall S.H., Rudin S.D., Van Duin D., Kaye K., Jacobs M.R., Bonomo R.A., Adamsa M.D.; Genomic and transcriptomic analyses of colistin-resistant clinical isolates of Klebsiella pneumoniae reveal multiple pathways of resistance.. Antimicrob. Agents Chemother. 2015, 59, 536–543, doi: 10.1128/AAC.04037-14..
- Li H., Wang Y., Meng Q., Wang Y., Xia G., Xia X., Shen J.; Comprehensive proteomic and metabolomic profiling of mcr-1-mediated colistin resistance in Escherichia coli. . Int. J. Antimicrob. Agents. 2019, 53, 795–804, doi: 10.1016/j.ijantimicag.2019.02.014..
- Vranakis I., Goniotakis I., Psaroulaki A., Sandalakis V., Tselentis Y., Gevaert K., Tsiotis G. J.; Proteome studies of bacterial antibiotic resistance mechanisms. . Proteomics. 2014, 97, 88–99, doi: 10.1016/j.jprot.2013.10.027..
- Luo M.L., Leenay R.T., Beisel C.L.; Current and future prospects for CRISPR-based tools in bacteria. . Biotechnol. Bioeng. 2016, 113, 930–943, doi: 10.1002/bit.25851..
- Doudna J.A., Charpentier E.; The new frontier of genome engineering with CRISPR-Cas9.. Science. 2014, 346, 8096, doi: 10.1126/science.1258096..
- Dong H., Xiang H., Mu D., Wang D., Wang T.; Exploiting a conjugative CRISPR/Cas9 system to eliminate plasmid harbouring the mcr-1 gene from Escherichia coli. Int. J. Antimicrob. Agents. 2019;53:1–8. doi: 10.1016/j.ijantimicag.2018.09.017.Dong H., Xiang H., Mu D., Wang D., Wang T. Exploiting a conjugative CRISPR/Cas9 system to eliminate plasmid harbouring the mcr-1 gene from Escherichia coli.. Int. J. Antimicrob. Agents. 2019, 53, 1–8, doi: 10.1016/j.ijantimicag.2018.09.017..
- Vercoe R.B., Chang J.T., Dy R.L., Taylor C., Gristwood T., Clulow J.S., Richter C., Przybilski R., Pitman A.R., Fineran P.C.; Cytotoxic Chromosomal Targeting by CRISPR/Cas Systems Can Reshape Bacterial Genomes and Expel or Remodel Pathogenicity Islands.. PLoS Genet. 2013, 9 , 4, doi: 10.1371/journal.pgen.1003454..
- McConville T.H., Annavajhala M.K., Giddins M.J., Macesic N., Herrera C.M., Rozenberg F.D., Bhushan G.L., Ahn D., Mancia F., Trent M.S., et al.; CrrB Positively Regulates High-Level Polymyxin Resistance and Virulence in Klebsiella pneumoniae. . Cell Rep. 2020, 33, 8313, doi: 10.1016/j.celrep.2020.108313..




