
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marie Nováková | + 1380 word(s) | 1380 | 2021-03-10 05:02:26 | | | |
| 2 | Vivi Li | Meta information modification | 1380 | 2021-03-19 02:16:37 | | |
Video Upload Options
According to the World Health Organization, cardiovascular diseases are the main cause of death worldwide. They may be caused by various factors or combinations of factors. Frequently, endothelial dysfunction is involved in either development of the disorder or results from it. On the other hand, the endothelium may be disordered for other reasons, e.g., due to infection, such as COVID-19. The understanding of the role and significance of the endothelium in the body has changed significantly over time—from a simple physical barrier to a complex system encompassing local and systemic regulation of numerous processes in the body. Endothelium disorders may arise from impairment of one or more signaling pathways affecting dilator or constrictor activity, including nitric oxide–cyclic guanosine monophosphate activation, prostacyclin–cyclic adenosine monophosphate activation, phosphodiesterase inhibition, and potassium channel activation or intracellular calcium level inhibition.
1. Introduction
According to the World Health Organization (WHO), almost 18 million people died worldwide in 2017 due to cardiovascular disorders. Numerous experimental and clinical studies are, therefore, focused on the cardiovascular system under both physiological and pathological conditions.
The cardiovascular system consists of the heart and vessels of various types. Three layers form a typical vessel: the tunica intima, tunica media, and tunica adventitia. The thickness ratio of a vessel wall depends on the functional requirements of that particular part of circulation system. Nevertheless, endothelial cells are a standard part of the tunica intima in any vessel.
2. The Endothelium: From a Simple Barrier to a Specialized Organ
2.1. Morphology of the Endothelium
A single layer of flat endothelial cells covers the inner surface of a vessel, which is in direct contact with the blood. Thus, this inner lining provides an anticoagulant barrier between the vessel wall and blood. All endothelial cells form a large organ consisting of approximately 1–6 × 1013 of cells, a mass of almost one kilogram [1].
The endothelium originates from the splanchnopleuric mesoderm [1]. Vascular endothelial growth factor (VEGF) and its high-affinity flk-1 and flt-1 receptor tyrosine kinases represent a paracrine signaling system that is critical for endothelial cell differentiation and vascular system development [2][3]. It has been proven that VEGF is the only specific mitogen for endothelial cells. It stimulates their growth, inhibits apoptosis, increases vascular permeability in various tissues, and promotes vasculogenesis and angiogenesis. Angiogenesis plays a protective role in coronary artery disease and myocardial infarction [4].
Endothelial cells consist of four basic compartments: the glycocalyx, cell cortex, cytoplasm, and nucleus (Figure 1). The structure and mechanical properties of these compartments directly affect physiological processes [1]. The endothelial glycocalyx is a thick, carbohydrate-rich layer that surrounds the endothelial lumen surface; it is composed of proteoglycans and glycoproteins. On the inner side of a cell membrane, the cell cortex is found, containing actin organized in a dynamic net. Actin fibers represent a support network for the plasma membrane and membrane proteins. The cell is also penetrated by actin microtubules and intermediate filaments. All components of the cell cytoskeleton are associated with the nucleus. Mechanical stimuli perceived by actin fibers, microtubules, or intermediate filaments are integrated in the nucleus [5]. Endothelial cells contain so-called Weibel–Palade bodies, measuring 0.1 µm wide and 0.3 µm long. These membrane-bound structures are a kind of storage organelle for von Willebrand’s factor (vWf) (Figure 1) [1].
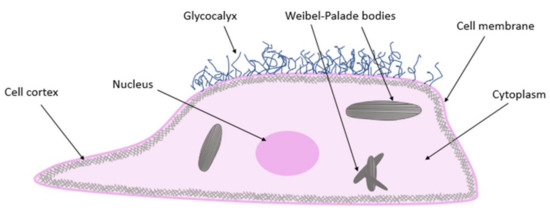
Figure 1. Endothelial cell structure.
2.2. Physiological Roles of Endothelium
For a long time, the role of the simple barrier was attributed to the endothelium. Since then, its concept has changed significantly and new functions of endothelial cells have been reported. It is now considered a specialized organ with numerous physiological functions [1].
First of all, the barrier function of the endothelium is viewed in a less static way than in the original concept, where the endothelium was believed to simply separate blood from the surrounding tissues. Nowadays, it is considered a dynamic barrier, the integrity of which is essential for maintaining physiological blood flow. On the other hand, endothelial cells communicate among themselves on one side and with circulating blood elements on the other side; the latter involves thrombocytes and leukocytes. Communication with other cells, even distant ones, via various paracrine and endocrine substances has also been described. All of these cells, cooperatively with the blood flow, affect the behavior of the endothelium [6].
Based on the above, it can be presumed that both endothelial cell injury and its dysfunction may lead to a number of pathological situations. Endothelial dysfunction results in various seemingly unrelated pathological processes, such as loss of semipermeable membrane function, hyperlipoproteinemia (often accompanied by atherogenesis), diabetes mellitus, vascular spasms, and arterial hypertension. Together with certain risk factors (e.g., smoking), these processes progress to uniform vascular changes. Subsequent organ hypoperfusion leads to failure in the target structure, for example heart failure [1].
The basic humoral and metabolic functions of the endothelium are summarized in Figure 2. Various types of autocrine, paracrine, and endocrine communication systems are presented.
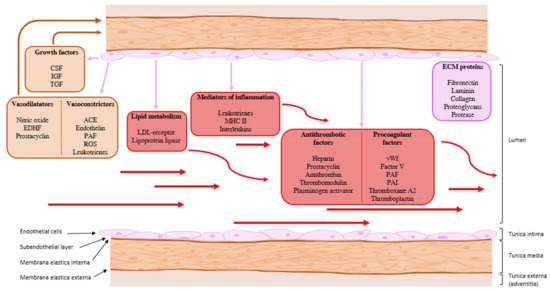
Figure 2. The basic humoral and metabolic functions of the endothelium. ACE: angiotensin converting enzyme; CSF: colony-stimulating factor; ECM: extracellular matrix; EDH: endothelium-derived hyperpolarization; IGF: insulin-like growth factor; LDL receptor: low-density lipoprotein receptor; MHC II: major histocompatibility complex type 2; PAF: platelet-activating factor; PAI: plasminogen activator inhibitor; ROS: reactive oxygen species; TGF: transforming growth factor; vWf: von Willebrand’s factor. Purple arrow: paracrine communication, red arrow: endocrine communication.
3. Substances Affecting Vascular Tone
3.1. Substances with Vasoconstriction Activity
Most research is focused on substances with vasodilatory potential, since these are of high clinical relevance. Although there are also some substances with vasoconstriction activity, research studies focus on them quite rarely. In folk medicine, some plants are used for their vasoconstriction activity, e.g., Cissus sicyoides L. (Vitaceae Juss.) [7], Nicotiana tabacum L. (Solanaceae Juss.) [8][9], Potentilla erecta (L.) Räusch. (Rosaceae L.) [10], Paspalidium flavidum (Retz.) A. Camus (Poaceae Barnhart) [11], and Haloxylon recurvum Bunge ex Boiss. (Amaranthaceae Juss.) [12][13].
3.1.1. Thromboxane A2
Thromboxane A2 (as well as PGI2) is a metabolite of ARA. For a long time, TXA2 was known to be released from platelets. Nowadays, it is known to be released by a variety of cells, including the endothelial ones. It stimulates platelet activation, aggregation, and proliferation, as well as vasoconstriction [14][15]. It counterbalances the effects of PGI2, especially in pathological situations, such as tissue injury and inflammation [15]. ARA is metabolised by COX to form unstable PGH2. PGH2 is further converted into TXA2 by thromboxane synthase (TXAS) [14]. TXA2 binds to TXA2–prostanoid receptor (TPR), resulting in an influx of Ca2+ ions and VSMC contraction [14][15]. Production of TXA2 can be evoked by acetylcholine, among others. TXA2 level reduction and TPR antagonism may be promising therapeutic targets to prevent cardiovascular disease [14][16].
As mentioned above, the production of synergic TXA2 and PGI2 is catalyzed by COX enzymes. The two COX isoforms, cyclooxygenase 1 (COX-1) and cyclooxygenase 2 (COX-2), metabolise ARA to PGH2, the common substrate for TXA2 and PGI2 synthesis. TXA2 is the predominant COX-1-derived product, in contrast to PGI2, which is synthetized as a result of COX-2 activation [17][18].
3.1.2. Endothelin
The common name endothelin (ET) is used for three peptides, namely endothelin-1, -2, and -3 (ET-1, ET-2, and ET-3). ET-1 is the most examined endothelin and is considered the most potent vasoconstrictive substance to date. Its expression is stimulated by shear stress, thrombin, insulin, adrenaline, AT II, cortisol, and also by hypoxia; it is inhibited by NO and natriuretic peptides. ET-1 is produced by endothelial cells, smooth muscle cells, macrophages, fibroblasts, cardiomyocytes, neurons, and endocrine pancreas cells. ET-2 is formed in the ovaries and intestinal epithelial cells. ET-3 is expressed in endothelial cells, placenta, brain neurons, melanocytes, and renal tubular epithelial cells [19][20][21][22][23].
Formation of the final, biologically active ET-1 is catalyzed by endothelin-converting enzymes 1–3 (ECE 1–3), each occurring in several isoforms. ECE-1 is the major enzyme, which catalyzes all endothelin isoform formation.
Endothelin receptors ETA, ETB1, ETB2, and ETC are G-protein-coupled receptors, differing in their affinity for individual ETs. ET-1 via ETA mediates vasoconstriction (ETA is expressed mainly in smooth muscle cells). Moreover, bronchoconstriction and secretion of aldosterone are mediated via ETA. ETB1 and ETB2 occur in both endothelial and smooth muscle cells. ETB1 agonist causes vasodilation by stimulating NO, PGI2, and EDH. On the contrary, ETB2 mediates vasoconstriction [19][20][21][22][23].
3.1.3. Platelet-Activating Factor
Platelet-activating factor (PAF) is a phospholipid mediator, synthesis and degradation of which are catalyzed enzymatically. PAF plays a role in numerous pathophysiological reactions—it potentiates aggregation and chemotaxis, as well as formation of neutrophils, eosinophils, and monocytes. In other words, by increasing vascular permeability, it induces local inflammatory processes and edema [24].
References
- Sumpio, B.E.; Riley, J.T.; Dardik, A. Cells in focus: Endothelial cell. Int. J. Biochem. Cell Biol. 2002, 34, 1508–1512.
- Roberts, D.M.; Kearney, J.B.; Johnson, J.H.; Rosenberg, M.P.; Kumar, R.; Bautch, V.L. The vascular endothelial growth factor (VEGF) receptor Flt-1 (VEGFR-1) modulates Flk-1 (VEGFR-2) signaling during blood vessel formation. Am. J. Pathol. 2004, 164, 1531–1535.
- Patan, S. Vasculogenesis and angiogenesis. Cancer Treat. Res. 2004, 117, 3–32.
- Kajdaniuk, D.; Marek, B.; Borgiel-Marek, H.; Kos-Kudła, B. Vascular endothelial growth factor (VEGF)—Part 1: In physiology and pathophysiology. Endokrynol. Pol. 2011, 62, 444–455.
- Fels, J.; Jeggle, P.; Liashkovich, I.; Peters, W.; Oberleithner, H. Nanomechanics of vascular endothelium. Cell Tissue Res. 2014, 355, 727–737.
- Galley, H.F.; Webster, N.R. Physiology of the endothelium. Br. J. Anaesth. 2004, 93, 105–113.
- García, X.; Cartas-Heredia, L.; Lorenzana-Jímenez, M.; Gijón, E. Vasoconstrictor effect of Cissus sicyoides on guinea-pig aortic rings. Gen. Pharmacol. 1997, 29, 457–462.
- Bull, H.A.; Pittilo, R.M.; Blow, D.J.; Blow, C.M.; Rowles, P.M.; Woolf, N.; Machin, S.J. The effects of nicotine on PGI2 production by rat aortic endothelium. Thromb. Haemost. 1985, 54, 472–474.
- Oakes, J.M.; Xu, J.; Morris, T.M.; Fried, N.D.; Pearson, C.S.; Lobell, T.D.; Gilpin, N.W.; Lazartigues, E.; Gardner, J.D.; Yue, X. Effects of Chronic Nicotine Inhalation on Systemic and Pulmonary Blood Pressure and Right Ventricular Remodeling in Mice. Hypertension 2020, 75, 1305–1314.
- Wölfle, U.; Hoffmann, J.; Haarhaus, B.; Rao Mittapalli, V.; Schempp, C.M. Anti-inflammatory and vasoconstrictive properties of Potentilla erecta—A traditional medicinal plant from the northern hemisphere. J. Ethnopharmacol. 2017, 204, 86–94.
- Hayat-Malik, M.N.; Bashir, S.; Khan, I.U.; Karim, S.; Mushtaq, M.N.; Khan, H.U.; Rashid, M.; Naz, H.; Samreen, S. Cardiotonic and vasoconstriction effects of aqueous methanolic extract of Paspalidium flavidum L. Pak. J. Pharm. Sci. 2015, 28, 437–441.
- Gilani, A.U.H.; Shaheen, F. Vasoconstrictor and cardiotonic actions of Haloxylon-recurvum extract. Phytother. Res. 1994, 8, 115–117.
- Wahab, A.; Ahmed, E.; Nawaz, S.A.; Sharif, A.; Ul Haq, R.; Malik, A.; Choudhary, M.I.; Raza, M. A pharmacological and toxicological evaluation of Haloxylon recurvum. Nat. Prod. Res. 2008, 22, 1317–1326.
- Chen, H. Role of thromboxane A. Prostaglandins Other Lipid Med. 2018, 134, 32–37.
- Rucker, D.; Dhamoon, A.S. Physiology, Thromboxane A2. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020.
- Grann, M.; Comerma-Steffensen, S.; Arcanjo, D.D.; Simonsen, U. Mechanisms Involved in Thromboxane A. Basic Clin. Pharmacol. Toxicol. 2016, 119 (Suppl. 3), 86–95.
- Sandoo, A.; van Zanten, J.J.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302–312.
- Caughey, G.E.; Cleland, L.G.; Penglis, P.S.; Gamble, J.R.; James, M.J. Roles of cyclooxygenase (COX)-1 and COX-2 in prostanoid production by human endothelial cells: Selective up-regulation of prostacyclin synthesis by COX-2. J. Immunol. 2001, 167, 2831–2838.
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharmacol. Rev. 2016, 68, 357–418.
- Drawnel, F.M.; Archer, C.R.; Roderick, H.L. The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth. Br. J. Pharmacol. 2013, 168, 296–317.
- Barton, M.; Yanagisawa, M. Endothelin: 30 Years From Discovery to Therapy. Hypertension 2019, 74, 1232–1265.
- Stow, L.R.; Jacobs, M.E.; Wingo, C.S.; Cain, B.D. Endothelin-1 gene regulation. FASEB J. 2011, 25, 16–28.
- Unic, A.; Derek, L.; Hodak, N.; Marijancevic, D.; Ceprnja, M.; Serdar, T.; Krhac, M.; Romic, Z. Endothelins—Clinical perspectives. Biochem. Med. 2011, 21, 231–242.
- Camussi, G.; Tetta, C.; Baglioni, C. The role of platelet-activating factor in inflammation. Clin. Immunol. Immunopathol. 1990, 57, 331–338.




