
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alan Graham Pockley | + 3960 word(s) | 3960 | 2021-03-17 04:56:29 | | | |
| 2 | Bruce Ren | Meta information modification | 3960 | 2021-03-19 02:03:07 | | | | |
| 3 | Bruce Ren | Meta information modification | 3960 | 2021-03-19 02:03:45 | | | | |
| 4 | Bruce Ren | Meta information modification | 3960 | 2021-03-23 09:50:14 | | |
Video Upload Options
Prostate cancer (PCa) is the second-most common cancer in men worldwide. Treatment options for patients with advanced or aggressive prostate cancer or recurrent disease continue to be of limited success and are rarely curative. Despite the efficacy of immune checkpoint blockade (ICB) in some melanoma, lung, kidney and breast cancers, this approach has been remarkably unsuccessful in PCa. One potentially explanation for this lack of efficacy is the generation of a distinctly immunosuppressive prostate tumor microenvironment (TME) by regulatory T (Treg) cells, myeloid-derived suppressor cells (MDSCs), and type 2 macrophages, all of which have been implicated in a variety of pathological conditions including solid cancers. In PCa, Treg cells and MDSCs are recruited into TME by low-grade chronic inflammatory signals, whereas tissue-resident type 2 macrophages are induced by cytokines such as IL-4, IL-10, IL-13, transforming growth factor beta (TGFβ) or prostaglandin E2 (PGE2) produced by Th2 cells. These then drive tumor progression, therapy resistance and the generation of castration (hormone) resistant disease, ultimately conferring a poor prognosis. The biology of MDSCs and Treg cells is highly complex and the development, proliferation, maturation or function can each be pharmacologically mediated to counteract the immunosuppressive effects of these cells.
1. Prostate Cancer Background
Prostate cancer (PCa) is the second-most common cancer in men worldwide, accounting for 13.5% of all new cancer cases in men in 2018 [1]. Incidence rates vary considerably worldwide, with the highest frequencies in Westernized countries such as Oceania (79.1 per 100,000 people), America (73.7) and Europe (62.1), whereas less-developed areas such as Africa and Asia experience a significantly lower incidence, with rates of 26.6 and 11.5, respectively [2]. Interestingly, mortality rates do not align with incidence rates, with elevated mortality rates in Sub-Saharan Africa and the Caribbean. There may be multiple explanations for this global disparity such as (i) genetic background, (ii) lifestyle and environmental factors, (iii) proportion of aging populations, (iv) over diagnosis in well-developed countries, and (v) other factors of which we are not yet aware. Despite declining, global mortality rates and the age-adjusted incidence is significantly increasing, although this is at least in part due to increased PCa testing. Following the commercial availability of prostate-specific antigen (PSA) testing in the mid-to-late 1980s, the incidence has dramatically increased in proportion to amount of testing carried out by individual countries, thereby leading to over diagnosis [3]. Epidemiological over diagnosis is defined by detection which would otherwise not have been diagnosed within the patient’s lifetime and is a key influencing factor in the disparity between the incidence rates in well-developed and developing countries. Over diagnosis rates vary widely and range from 1.7% to as high as 67% [4]. Conversely, because disease progression is most often indolent and asymptomatic, latent undiagnosed tumors are found in 36% of autopsied men aged 70–79 [5], with numbers increasing exponentially in later years of life.
For a disease as common as PCa, very little is known about its etiology and only a few risk factors have been identified. Incidence rates and mortality for PCa also vary greatly between ethnic groups, suggesting ethnic and genetic predisposition. Mortality rates are highest among males of African descent in Sub-Saharan Africa, the Caribbean and the United States [1]. An analysis of biopsy detected PCa in six Sub-Saharan countries found substantially higher Gleason scores than in both African Americans and European Americans, with a score of +8 predominating in Sudan and Uganda [6]. Another study found that Black South African men are at a 2.1-fold and 4.9-fold higher risk of presenting with a Gleason score ≥8 and PSA ≥ 20 ng/mL at diagnosis, respectively, than their African American counterparts [7]. However, there are significant restrictions to producing direct comparisons between Sub-Saharan Africans and African Americans due to differences in the methods of testing, availability of high-quality cancer population data, as well as under-diagnosis and late detection in Africa. Interestingly, a recent study linked chromosomal loci from the KhoeSan ancestry to an increased presentation of high-risk prostate cancer, which may partially explain the increased incidence in Black South Africans compared with African Americans [8].
Ancestral differences in the incidence of PCa, in addition to family history, demonstrate a genetic contribution to PCa risk and etiology. Twin studies have indicated that PCa is among the most heritable of all common cancers [9]. Thus far, Genome-Wide Association Studies have identified 147 loci that account for 28.4% of familial risk, most of which occur commonly, but are of low penetrance [10]. Some loci are clinically relevant to the etiology of prostate cancer due to their positioning to nearby oncogenes, DNA damage repair genes, tumor suppressor genes, etc.
Despite the relatively high heritability of PCa, there is overwhelming evidence that lifestyle and environmental factors play a role, though much of the evidence is correlative. Given that the incidence is uniformly significantly higher in developed countries, it has long been suspected that diet and other factors of a “Westernized” lifestyle contribute to increased risk. This is supported by multiple migrant studies where PCa incidence rates increased in Japanese [11], Korean [12] and Chinese [13], Vietnamese [14] migrants after moving to North America. Additionally, there is strong evidence that obesity, which is highly prevalent in Westernized countries, increases the risk of aggressive prostate cancer [15][16]. However, a recent systematic review and meta-analysis suggests that this correlation may be due to the inverse relationship that exists between body mass index (BMI) and PSA levels, resulting in late diagnosis in obese men [17]. Although little evidence supports the notion that a high-fat diet increases prostate cancer risk [18], a high-fat diet may influence disease progression and mortality through interleukin-6 (IL-6)-mediated intratumoral infiltration of MDSCs [19]. Interestingly, adult height has been found to have a direct impact on PCa risk, which may be due to polygenic interactions between genes involved in growth, such as IGF-1 [20]. The link between PCa and IGF-1 has also been made in the dietary setting, where a systematic review of 172 studies found milk consumption associate with a greater risk of developing PCa, probably through IGF-1 signaling pathways [21]. This leads to the hypothesis of an intimate interaction between host IGF-1 genes, dietary IGF-1 and growth signaling pathways that ultimately influences adult height and risk of PCa. Finally, although it has not been found to be associated with PCa incidence, there is a small but modest association between smoking and mortality from PCa [22].
2. Diagnosis
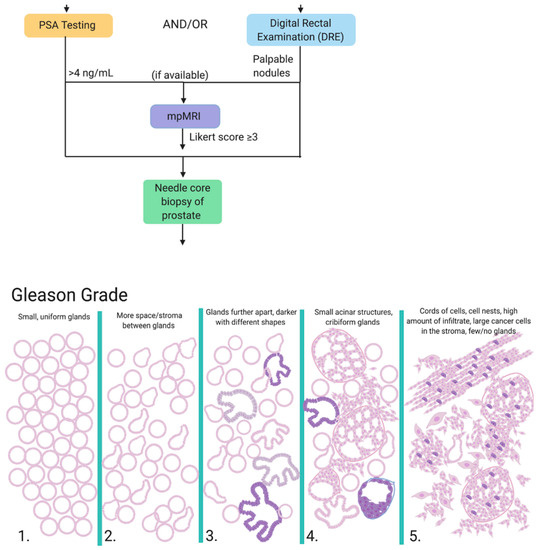
Men who present with symptoms such as urination difficulty and/or a family history of PCa are referred to PSA testing and/or digital rectal examination (DRE). PSA is a glycoprotein secreted exclusively by the epithelial cells lining the acini and ducts of the prostate gland that functions to promote sperm motility and dissolve cervical mucus [23]. PSA is physiologically present in serum at low concentrations (0–4 ng/mL), but can become elevated through prostatic irritation, prostate infection, benign prostatic hyperplasia or the development of PCa, as a consequence of which PSA levels are used as a 'biomarker' for PCa risk and disease progression. Although a useful diagnostic and prognostic aid, PSA levels cannot be considered without supporting clinical evidence; for example, 2% of patients with PCa harbor an aggressive form of prostate cancer (Gleason score ≥ 7) despite PSA levels < 4 ng/mL [24], and false positives are also common. The DRE is a simple, yet efficient, screening measure whereby the physician feels for palpable nodules, asymmetry or diffuse firmness on the prostate gland through the rectal passageway. However, this technique similarly lacks accuracy, with a reported positive predictive value to be between 5 to 30% [25]. When available, the first line of investigation for clinically suspected localized PCa is multiparametric MRI (mpMRI), which is a non-invasive imaging technique that has been successful in more accurately characterizing lesions and ruling out non-clinically relevant PCa [26]. The mpMRI is assigned a Likert score from 1 to 5; those with a Likert score of 3 or greater are referred to prostate biopsy. Prostate biopsy is a requirement for diagnosis and is referred to patients with PSA levels repeatedly exceeding 4 ng/mL and/or palpable nodules upon DRE and/or high Likert score on mpMRI and/or clinical suspicion of PCa [27].
Transrectal ultrasound (TRUS)-guided systematic prostate biopsy is the current standard of care approach for diagnosing PCa and is carried out via needle biopsy through the rectal passageway to extract 10–12 samples in a grid-like pattern over the apical and far lateral regions [28]. The TRUS biopsy is the least invasive technique and is widely accessible. However, this approach suffers several drawbacks including higher infection rates, higher false negative rates and underestimation of Gleason grade. Contemporary MRI-guided biopsies offer enhanced detection of clinically important lesions and include MRI-guided (in-bore) biopsy, fusion biopsy and MRI–TRUS fusion biopsy [26]. Although currently less common, transperineal biopsies are considered preferable to TRUS biopsies by many urologists due to more comprehensive sampling, decreased risk of post-operational infection and comparable cancer detection rates [29]. Although not yet recommended by the UK’s National Institute for Health and Care Excellence (NICE) guidelines (unless as part of a clinical trial) [27], transperineal biopsies may be useful in cases that have previously returned a negative TRUS biopsy, but for which there is clinical suspicion of PCa based on other parameters such as PSA, DRE and high Likert score. Indeed, freehand transperineal biopsies have completely replaced TRUS biopsies at one of the UK’s largest hospitals [30] and is likewise being adopted elsewhere around the world. Finally, transurethral biopsy represents the third prostate biopsy type. However, this method has largely been surpassed by other techniques due to poor diagnostic accuracy.
Regardless of the biopsy type, samples are given a primary and secondary Gleason Grade from 1 to 5 based on the architecture and state of differentiation of the predominant and second-most prevalent pattern in the sample. These are added together to get the resulting Gleason score, which is assigned a Gleason Grade Group (Figure 1). An important distinction is a 3 + 4 score versus a 4 + 3 score, as patients with a 4 + 3 score present with higher levels of PSA at diagnosis and are at a 3-fold increased risk of metastasis [31]. Additionally, it is notoriously difficult to adequately sample or even correctly capture prostate tumors; 21–28% of tumors that are located on the anterior side of the prostate are missed and 14–17% are under-graded through current techniques [32].
3. Treatment
Given the generally indolent progression of the disease and the impact of treatment-related side-effects such as incontinence, erectile dysfunction and impotence on quality of life, treatment is considered in the context of age and risk group. Approximately 45% of tumors will not progress in the patient’s lifetime and are deferred to ‘Active Surveillance’ or ‘Watchful Waiting’, both of which aim to minimize treatment-related toxicity [33]. Multiple randomized clinical trials have established that radical prostatectomy does not significantly reduce mortality from PCa in those with localized PCa [34][35], and the risk of death from alternative causes supersedes the risk of death from PCa itself, particularly in men over 60 years old. There has been an increasing role for patient preferences in treatment decision making, as similar health outcomes are achieved through sharing such decisions [36]. Some of the main concerns leading to patients preferring deferred management over treatment options such as prostatectomy and radiation therapy relate to the poyential side effects of these treatments, with incontinence and erectile dysfunction being reported to occur in approximately 20% and 70% of patients, respectively [37].
Beyond expectant management, patients with localized PCa have two primary options: radical prostatectomy (RP) or radiation therapy. RP may be conducted through laparoscopic or robot-assisted (RARP) techniques and may be performed within as little as 2 weeks from the time of biopsy [38]. A recent systematic review and meta-analysis of two randomized controlled trials and 9 prospective studies indicated no significant difference between the open, laparoscopic or RARP techniques in terms of complications, biochemical reccurrence, urinary continence and erectile function, although RARP resulted in significantly lower blood loss [39]. The type of external beam radiation used for the treatment of PCa is intensity-modulated radiotherapy (IMRT), which may also be image-guided (IGRT) [27]. Less commonly, low-dose (permanent implantation) and high-dose (temporary implantation) brachytherapy may be used in conjunction with IMRT/IGRT on patients with intermediate- and high-risk localized PCa who satisfy certain conditions [27]. Other, currently experimental, treatment modalities include cryosurgery [40], high-intensity focal ultrasound [41], irreversible electroporation [42] and photodynamic therapy (PDT) [43]. However, these are not recommended unless part of a clinical trial. Once diagnosed and a baseline level is established, PSA testing represents an essential monitoring measure for detection of local reccurrence and metastatic disease, although it is not possible to differentiate between these two scenarios from a PSA test alone. A definitive diagnosis of biochemical reccurrence is determined through rising PSA levels, radiology and clinical signs of deterioration.
Androgen Deprivation Therapy (ADT) remains the mainstay treatment for high-risk and recurrent patients and involves various approaches for inhibiting interactions between androgen hormones and their receptors. ADT was pioneered in 1941 by Huggins and Hodges, who observed that ablation of androgen hormones, either through chemical or physical castration, inhibited tumor growth and relieved cancer-related symptoms [44]. In the decades that followed, various agents to interfere with the hypothalamus–pituitary–gonadal axis and functioning of the androgen receptor (AR) signaling pathway were developed, including anti-androgens, luteinizing hormone-releasing hormone (LHRH) agonists and LHRH antagonists [45] (Figure 2). Anti-androgens work by inhibiting the interaction between dihydrotestosterone (DHT; a derivative of testosterone that is formed in the prostate gland) and the AR; one such anti-androgen is enzalutamide. As a monotherapy, anti-androgens are less effective than bilateral orchiectomy or other chemical forms of ADT in patients due to the fact that they do not reduce serum testosterone levels and are typically used in conjunction with LHRH agonists/antagonists to achieve “complete androgen blockade” (CAB). LHRH agonists stimulate the LHRH receptor, resulting in a downregulation of the receptor following 2–3 weeks of treatment, whereas LHRH antagonists competitively inhibit the receptor from binding to LHRH. The main difference between the LHRH drugs is that through activating the LHRH receptor and subsequent signaling pathway, LHRH agonists result in a transient surge in luteinizing hormone and testosterone levels, while LHRH antagonists achieve testosterone suppression without this initial surge. Additionally, testosterone production can be successfully inhibited from all sources (testes, adrenal glands, PCa cells) via oral administration of abiraterone acetate, which is an inhibitor of the androgen biosynthesis enzyme CYP17. CYP17 functions by hydroxylating 17-hydroxypregnenolone to produce dehydroepiandrosterone (DHEA), which is subsequently converted to testosterone. By targeting biosynthesis in completely different pathway, abiraterone acetate compliments other forms of ADT to successfully achieve very low testosterone levels with improved outcome. Although physical castration via bilateral orchiectomy remains an effective and cost-efficient form of ADT, it is less common due to the invasiveness of surgery and permanent consequences. Regardless of the method used, a testosterone level of <20 ng/dL is desirable to maximize therapeutic outcomes.
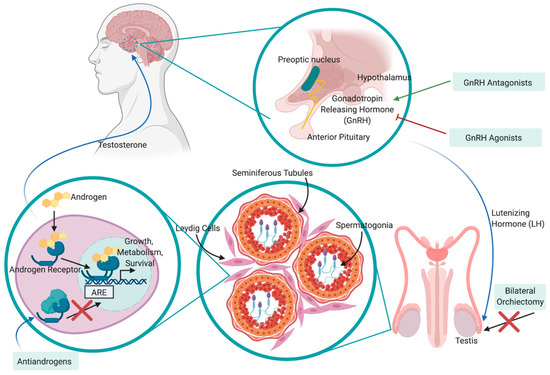
Figure 2. Mechanism of action and feedback loop of ADT on the hypothalamic–pituitary–gonadal (HPG) axis in the treatment of PCa. Luteinizing hormone-releasing hormone (LHRH) is released from the preoptic nucleus of the hypothalamus to induce the secretion of luteinizing hormone (LH) from the anterior pituitary gland. LH moves through peripheral circulation to act on the Leydig cells of the testis, inducing release of testosterone. Testosterone promotes the progression of PCa by binding to androgen receptors (ARs), translocating to the nucleus, binding to androgen-responsive elements (AREs) and promoting the expression of proteins involved in growth, metabolism and survival. This pathway may be disrupted by physical castration (bilateral orchiectomy) or chemical castration via LHRH agonists/antagonists, anti-androgens and CYP17 inhibitors.
Although PCa is initially highly responsive to ADT, it almost invariably develops resistance within 2–3 years to become castration-resistant PCa (CRPC, or mCRPC if the cancer has metastasized), in which tumors continue to grow despite an absence of testosterone [46]. CRPC is determined by a continuous increase in PSA levels, the progression of a pre-existing disease or the development of new metastases, despite castration-level concentrations of testosterone. The development of CRPC is due to a variety of molecular mechanisms involving AR reactivation, including production of androgens via the adrenal glands and PCa cells, androgen-independent activation of ARs, gene amplifications and mutations, activation of aberrant co-regulators and ligand-independent splice variants. The castration-resistant tumor contains a heterogeneous population of cells consisting of fully androgen-insensitive cells, partially androgen-insensitive cells and androgen-sensitive cells, thus warranting ongoing, effective ADT therapy. However, the proliferation of the castration-insensitive population cannot be prevented. Patients with mCRPC have a poor prognosis - a median survival of 1.14 years [47] and 17% survival rate over 3.5 years [48]. Treatment of mCRPC involves combining ADT with the chemotherapeutic agent docetaxel. Failing this, the patient may be referred to the taxane cabazitaxel [49] or radiation therapy with radium 223 to target bone metastases [50]. Despite the availability of these new agents and the improved overall survival with sequential use, there is no consensus on proper sequencing and the median overall survival remains poor, ranging from 21 to 29 months [51]. This poor survival is due, at lest so some extent, to the development of resistance and cross-resistance mechanisms. For example, it has been demonstrated that acquired resistance to docetaxel induces cross-resistance to cabazitaxel, and that resistance to enzalutamide induces cross resistance to abiraterone [52].
4. Immunotherapy
Given the current lack of curative treatment options for patients with mCRPC, various immunotherapies have been clinically investigated with the goal of initiating a robust antitumor response in vivo. Despite the efficacy of immune checkpoint inhibitors such as ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1) in a variety of cancers including melanoma [53], lung [54], kidney [55] and breast [56], clinical trials of these drugs in PCa have found them to be relatively inert or even toxic. In two large, randomized phase III clinical trials, ipilimumab did not increase Overall Survival (OS) when given either before [57] or after [58] treatment with docetaxel. A phase I clinical trial assessing the safety and antitumor activity of nivolumab on patients with mCRPC also showed negligible response [59]. For the 2–3% of patients with DNA mismatch repair genes, the increased mutational burden in which renders either of these drugs effective; pembrolizumab is already FDA approved and investigations for ipilimumab plus nivolumab [60] and durvalumab (anti-PD-L1) plus Olaparib [61] are currently underway. Most recently, a phase II clinical study on pre-treated patients with mCRPC found positive antitumor activity after treatment with pembrolizumab and a 29% decrease in tumor size [62]. However, no control arm was assessed for statistical analysis of this result.
Although clinical trials for monoclonal antibodies are still ongoing, various antitumor vaccines are currently in the pipeline in the form of antigen-loaded antigen-presenting cells (APCs) [63], peptides [64][65] or loaded DNA vectors [66]. Intratumoral CD8+ T cells have a canonical exhausted phenotype, with a diminished ability to proliferate and produce effector cytokines such as IL-2, IFN-γ and TNF-α in addition to enhanced expression of immune checkpoint receptors such as PD-1, TIM-3 and LAG-3 [67]. Thus, vaccine approaches aim to present the host immune system with tumor-specific antigens and elicit robust CD8+ T cell activation. Currently, the only FDA-approved immunotherapy for mCRPC is sipuleucel-T (PROVENGE®), which is an ex vivo autologous peripheral blood mononuclear cell (PBMC) vaccine [68]. To generate the vaccine, the patient’s PBMCs are isolated through leukapheresis and cultured with the entire prostatic acid phosphatase (PAP) protein conjugated to granulocyte-macrophage colony-stimulating factor (GM-CSF) by a single Gly-Ser linker (PA2024). This is taken up, processed and presented on APCs as PAP epitopes. The GM-CSF conjugate induces maturation of many of these APCs into dendritic cells (DCs) and induces in vitro antigen processing. The entire PMBC cell culture is subsequently re-infused back into the patient, in whom DCs are thought to activate CD4+ and vaccine-specific CD8+ T cells (Figure 3). PAP is an ideal prostate tumor antigen due to its prostate restricted expression and overexpression that correlates with disease progression [69]. Sipuleucel-T treatment prior to RP has been associated with intratumoral infiltration of CD3+, CD4+, FOXP3- and CD8+ T cells, IFNɣ-detectable responses and Th1-biased activation, which is important for host tumor immunity [70][71]. Additionally, T-cell receptor (TCR) diversity decreases in peripheral blood, but increases intratumorally, thereby indicating recruitment of T-specific clones from the peripheral blood to the prostate TME [72]. Although Sipuleucel-T has also been explored in neoadjuvant [70][71] and biochemical recurrent settings [73], it is not FDA approved for these uses.
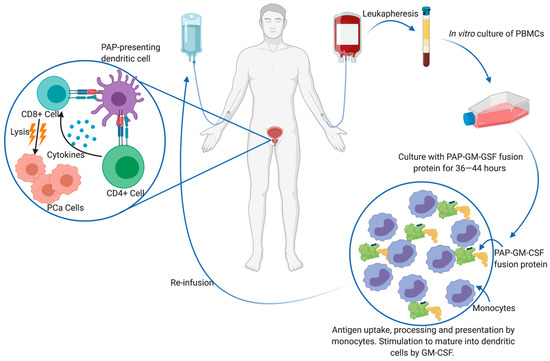
Interestingly, no study to date has demonstrated solid evidence of PAP only specific CD8+ T cell following Sipuleucel-T treatment. In the landmark study that granted the drug FDA-approval, increased OS was achieved (reduced risk of death of 22%, p = 0.03), and immune responses against PA2024 were observed via antibody titers measured using ELISA, and T-cell proliferation assays [68]. Further phase III trials found cellular and humoral responses against PA2024 and/or PAP [74]. However, these response assays were produced against the entire PA2024 antigen without a GM-CSF control, which begs the question whether the in vivo effects of Sipuleucel-T can be attributed to an adaptive immune response against the fusion protein construct containing GM-CSF, rather than the perceived tumor antigen PAP. Indeed, GM-CSF is secreted by activated DCs and exogenous GM-CSF has been suggested to have antitumor activity in patients with advanced PCa [75]. Clinical and pre-clinical research leading up to this study reported T-cell proliferation in response to PA2024 and antibodies against in PA2024 in 100% of patients, whereas much weaker and infrequent immune responses were generated against PAP or GM-CSF alone [76][77]. The only report of PA2024-specific CD4+ and CD8+ T cell proliferation and activation is that of Antonarakis et al. [78]. However, these findings remain to be externally validated. Although OS is significantly improved by the administration of Sipuleucel-T, this improvement is limited to a median increase of 4.1 months, with no difference in progression-free survival (PFS) or time-to-clinical progression [68]. From a cost perspective, Sipuleucel-T requires three infusions at two-week intervals for one month and costs a total upwards of £47,000 [27]. The preparation of each dose of Sipuleucel-T is also demanding for patients that are already immunocompromised. A total of 8–14 L of blood (i.e., approximately 1–2 times total blood volume) must be collected. This generates approximately 600 mL of PBMCs and reduces the patient’s peripheral WBC blood count by 15–20%. With such a burdensome procedure, low benefit-cost ratio, and unclear capability of CD8+ T cell activation, efforts have been redirected at either improving Sipuleucel-T efficacy or producing an alternative form of immunotherapy.
One reason for the lack of clinical efficacy of immunotherapy in PCa is the low mutational burden of the disease, which results in low mutation-associated neoantigens and low immunogenicity. To improve upon the design of sipuleucel-T and determine the exact immunogenic region of the PAP protein, the John van Geest Cancer Research group identified a 15 mer PAP epitope of amino acids 114–128 that are capable of initiating CD4+ and CD8+ T-cell responses in mice [79]. The identified peptide was selected based on predictive binding to the human leukocyte antigen HLA*A02:01 and 100% homology between the human and murine PAP protein. However, the haplotype HLA*A02:01 represents only ~40% of US Caucasians and ~15% of US African Americans [80], warranting the need for a broader-spectrum peptide. Based on the observation that vaccination with long peptides (20 aa or more) induces more robust immune responses in a wider range of phenotypically diverse individuals [81], the identified peptide was elongated to 42 amino acids and mutated with a Leucine to Alanine substitution at position 14 for additional immunogenicity [82]. As an intermediate-sized peptide, this vaccine meets the requirements of balancing immunogenicity and broad haplotype specificity, with the enhanced ease of purification and lower cost compared to producing a whole protein. In mouse models this immune-vaccine induced robust anti-PAP immunity via MHC class I and delayed tumor growth.
The lack of efficacy of immunotherapy techniques such as immune checkpoint blockade and Sipuleucel-T in PCa suggests other factors which result in inhibition of antitumor immunity to be at play. One hypothesis for this lack of efficacy is the generation of a potently immunosuppressive TME by Treg cells, type 2 macrophages (TAMs) and MDSCs, all of which have been implicated in a variety of solid cancers. Herein, we critically assess the role of MDSCs in the progression of PCa resistance to immunotherapy, assess methods of targeting them, and propose combinatorial treatment modalities with the ultimate goal of improving the efficacy of immunotherapeutics.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 86, 394–424.
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63–98.
- Ilic, D.; Djulbegovic, M.; Jung, J.H.; Hwang, E.C.; Zhou, Q.; Cleves, A.; Agoritsas, T.; Dahm, P. Prostate cancer screening with prostate-specific antigen (PSA) test: A systematic review and meta-analysis. Br. Med. J. 2018, 362.
- Loeb, S.; Bjurlin, M.; Nicholson, J.; Tammella, T.L.; Penson, D.; Carter, H.B.; Carrol, P.; Etzioni, R. Overdiagosis and overtreatment of prostate cancer. Eur. Urol. 2014, 65, 1046–1055.
- Lahn, J.L.; Giovannucci, E.L.; Stampfer, M.J. The high prevalence of undiagnosed prostate cancer at autopsy: Implications for epidemiology and treatment of prostate cancer in the Prostate-specific Antigen-era. Int. J. Cancer 2015, 137, 2795–2802.
- Jalloh, M.; Friebel, T.M.; Thiam, F.S.; Niang, L.; Sy, C.; Siby, T.; Fernandez, P.; Mapulanga, V.; Maina, S.; Doodu Mante, S.; et al. Evaluation of 4,672 routine prostate biopsies performed in six African countries. J. Afr. Cancer 2013, 5, 144–154.
- Tindall, E.A.; Monare, L.R.; Petersen, D.C.; Zyl, S.V.; Hardie, R.A.; Segone, A.M.; Venter, P.A.; Bornman, M.S.R.; Hayes, V.M. Clinical presentation of prostate cancer in Black South Africans. Prostate 2014, 74, 880–891.
- Petersen, D.C.; Jaratlerdsiri, W.; Van Wyk, A.; Chan, E.K.F.; Fernandez, P.; Lyons, R.J.; Mutambirw, S.B.A.; Van der Merwe, A.; Venter, P.A.; Bates, W.; et al. African KhoeSan ancestry linked to high-risk prostate cancer. BMC Med. Genom. 2019, 12, 82.
- Mucci, L.A.; Hjelmborg, J.B.; Harris, J.R.; Czene, K.; Havelick, D.J.; Scheike, T.; Graff, R.E.; Holst, K.; Möller, S.; Unger, R.H.; et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA 2016, 315, 68–76.
- Schumacher, F.R.; Al Olama, A.A.; Berndt, S.I.; Benlloch, S.; Ahmed, M.; Saunders, E.J.; Dadaev, T.; Leongamornlert, D.; Anokian, E.; Cieza-Borrella, C.; et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat. Genet. 2018, 50, 928–936.
- Maskarinec, G.; Noh, J.J. The effect of migration on cancer incidence among Japanese in Hawaii. Ethn. Dis. 2004, 14, 431–439.
- Lee, J.; Demissie, K.; Lu, S.; Rhoads, G.G. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control 2007, 14, 78–85.
- Hanley, A.J.; Choi, B.C.; Holowaty, E.J. Cancer mortality among Chinese migrants: A review. Int. J. Epidemiol. 1995, 24, 255–265.
- Le, G.M.; Gomez, S.L.; Clarke, C.A.; Glaser, S.L.; West, D.W. Cancer incidence patterns among Vietnamese in the United States and Ha Noi, Vietnam. Int. J. Cancer 2002, 102, 412–417.
- Dickerman, B.A.; Torfadottir, J.E.; Valdimarsdottir, U.A.; Giovannucci, E.; Wilson, K.M.; Aspelund, T.; Tryggvadottir, L.; Sigurdardottir, L.G.; Harris, T.B.; Launer, L.J.; et al. Body fat distribution on computed tomography imagine and prostate cancer risk and mortality in the AGES-Reykjavik study. Cancer 2019, 125, 2730–2731.
- Dickerman, B.A.; Ahearn, T.U.; Giovannucci, E.; Stampfer, M.J.; Nguyen, P.L.; Mucci, L.A.; Wilson, K.M. Weight change, obesity and risk of prostate cancer aggression among men with clinically localized prostate cancer. Int. J. Cancer 2017, 141, 933–944.
- Harrison, S.; Tilling, K.; Turner, E.L.; Martin, R.M.; Lennon, R.; Lane, J.A.; Donovan, J.L.; Hamdy, F.C.; Neal, D.E.; Ruud Bosch, J.H.L.; et al. Systematic review and meta-analysis of the associations between body mass index, prostate cancer, advanced prostate cancer, and prostate-specific antigen. Cancer Causes Contol 2020, 31, 431–449.
- Xu, C.; Han, F.F.; Zeng, X.T.; Liu, T.Z.; Li, S.; Gao, Z.Y. Fat intake is not limited to prostate cancer: A systematic review and dose-response meta-analysis. PLoS ONE 2015, 10, e0131747.
- Hayashi, T.; Fujita, K.; Nojima, S.; Hayashi, Y.; Nakano, K.; Ishizuya, Y.; Wang, C.; Yamamoto, Y.; Kinouchi, T.; Matsuzaki, K.; et al. High-fat diet-induced inflammation accelerates prostate cancer growth via IL6 signaling. Clin. Cancer Res. 2018, 24, 4309–4318.
- Lophatananon, A.; Stewart-Brown, S.; Kote-Jarai, Z.; Al Olama, A.A.; Garcia, S.B.; Neal, D.E.; Hamdy, F.C.; Donovan, J.L.; Giles, G.G.; Fitzgerald, L.M.; et al. Height, selected genetic markers and prostate cancer risk: Results from the PRACTICAL consortium. Br. J. Cancer 2018, 118.
- Harrison, S.; Lennon, R.; Holly, J.; Higgins, J.P.T.; Gardner, M.; Perks, C.; Gaunt, T.; Tan, V.; Borwick, C.; Emmet, P.; et al. Does milk intake promote prostate cancer initiation or progression via effects of insulin-like growth factors (IGFs)? A systematic review and meta-analysis. Cancer Causes Control 2017, 28, 297–528.
- Islami, F.; Moreira, D.M.; Boffetta, P.; Freedland, S.J. A systemaic review and meta-analysis of tobacco use and prostate cancer mortality and incidence in prospective cohort studies. Eur. Urol. 2014, 66, 1054–1064.
- Arneth, B.M. Clinical significance of measuring prostate-specific antigen. Lab.Med. 2009, 40, 487–491.
- Thompson, I.M.; Pauler, D.K.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Parnes, H.L.; Minasian, L.M.; Ford, L.G.; Lippman, S.M.; Crawford, E.D. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0ng per milliliter. N. Engl. J. Med. 2004, 350, 2239–2246.
- Naji, L.; Randhawa, H.; Sohani, Z.; Dennis, B.; Lautenbach, D.; Kavanagh, O.; Bawor, M.; Banfield, L.; Profetto, J. Digital rectal examination for prostate cancer screening in primary care: A systematic review and meta-analysis. Ann. Fam. Med. 2018, 16, 149–154.
- Streicher, J.; Lee Myerson, B.; Karivedu, V.; Sidana, A. A review of optimal prostate biopsy: Indications and techniques. Ther. Adv. Urol. 2019, 11, 1–8.
- National Institute for Health and Care Excellence. Prostate Cancer: Diagnosis and Management. Available online: (accessed on 26 February 2021).
- Litwin, M.S.; Tan, H.J. The diagnosis and treatment of prostate cancer: A review. JAMA 2017, 317, 2532–2542.
- Omer, A.; Lamb, A.D. Optimizing prostate biopsy techniques. Curr. Opin. Urol. 2019, 29, 578–586.
- Kum, F.; Elhage, O.; Maliyil, J.; Wong, K.; Faure Walker, N.; Kulkarni, M.; Namdarian, B.; Callacombe, B.; Cathcart, P.; Popert, R. Initial outcomes of local anaesthetic freehand transperineal prostate biopsies in the outpatient setting. BJU Int. 2020, 125, 244–252.
- Kamel, M.H.; Khalil, M.I.; Alobuia, W.M.; Su, J.; Davis, R. Incidence of metastasis and prostate-specific antigen levels at diagnosis in Gleason 3+4 versus 4+3 prostate cancer. Urol. Ann. 2018, 10, 203–208.
- Bjurlin, M.A.; Carter, H.B.; Schellhammer, P.; Cookson, M.S.; Gomella, L.G.; Troyer, D.; Wheeler, T.M.; Schlossberg, S.; Penson, D.F.; Taneja, S.S. Optimization of initial prostate biopsy in clinical practice: Sampling, labelling and specimen processing. J. Urol. 2013, 189, 2039–2046.
- Mottet, N.; Van den Bergh, R.C.N.; Briers, E.; Cornford, P.; De Santis, M.; Fanti, S.; Gillessen, J.; Grummet, A.M.; Henry, T.B.; Lam, M.D.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Available online: (accessed on 26 February 2021).
- Wilt, T.J.; Brawer, M.K.; Jones, K.M.; Barry, M.J.; Aronson, W.J.; Fox, S.; Gingrich, J.R.; Wei, J.T.; Gilhooly, P.; Grob, B.M. Radical prostatectomy versus observation for localized prostate cancer. N. Engl. J. Med. 2012, 367, 203–213.
- Bill-Axelson, A.; Holmberg, L.; Garmo, H.; Rider, J.R.; Taari, K.; Busch, C.; Nordling, S.; Haggman, M.; Andersson, S.O.; Spangberg, A. Radical prostatectomy or watchful waiting in early prostate cancer. N. Engl. J. Med. 2014, 370, 932–942.
- Violette, P.D.; Agoritsas, T.; Alexander, P.; Piikonen, J.; Santti, H.; Agarwal, A.; Bhatnagar, N.; Dahm, P.; Montori, V.; Guyatt, G.H.; et al. Decision aids for localized prostate cancer treatment choice: Systematic review and meta-analysis. CA Cancer J. Clin. 2015, 65, 239–251.
- Haglind, E.; Carlsson, S.; Strane, J.; Wallerstedt, A.; Wilderäng, U.; Thorsteindottie, T.; Lagerkvist, M.; Damber, J.E.; Bjartell, A.; Hugosson, J.; et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: A prospective, controlled, nonrandomised trial. Eur. Urol. 2015, 68, 216–225.
- Minke, H.; Yaohui, L.; Zhuoyi, X.; Li-an, S.; Yanjun, Z.; Xiaoyi, H.; Jianming, G.; Hang, W. Short internval of biopsy to robotic-assisted laparoscopic radical prostatectomy does not render any adverse effects on the perioperative outcomes. Medicine 2018, 97, e11686.
- Cao, L.; Yang, Z.; Qi, L.; Chen, M. Robot-assisted and laparoscopic vs. open radical prostatectomy in clinically localized prostate cancer perioperative, function and oncological outcomes: A systematic review and meta-analysis. Medicine 2019, 98, e15770.
- Gao, L.; Yang, L.; Qian, S.; Tang, Z.; Qin, F.; Wei, Q.; Han, P.; Yuan, J. Cryosurgery would be an effect option for clinically localized prostate cancer: A meta-analysis and systematic review. Sci. Rep. 2016, 7, 27490.
- Bonekamp, D.; Wolf, M.B.; Roethke, M.C.; Pahernik, S.; Hadaschik, B.A.; Hatiboglu, G.; Kuru, T.H.; Popeneciu, I.V.; Chin, J.L.; Billia, M.; et al. Tweleve-month prostate volume reduction after MRI-guided transurethral ultrasound ablation of the prostate. Eur. Radiol. 2019, 29, 299–308.
- Van den Bos, W.; Scheltema, M.J.; Siriwardana, A.R.; Kalsbeek, A.M.F.; Thompson, J.E.; Ting, F.; Böhm, M.; Haynes, A.; Shnier, R.; Delprado, W.; et al. Focal irreversible electroporation as primary treatment for localized prostate cancer. BJU Int. 2018, 121, 716–724.
- Gill, I.S.; Azzouzi, A.R.; Emberton, M.; Coleman, J.A.; Coeytaux, E.; Scherz, A.; Scardino, P.T.; PCM301 Study, Group. Randomized trial of partial gland ablation with vascular targeted phototherapy versus active surveillance for low risk prostate cancer: Extended followup and analyses of effectiveness. J. Urol. 2018, 200, 786–793.
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer: I. the effect of castration, of estrogen, and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941, 1, 293–297.
- Crawford, E.D.; Heidenreich, A.; Lawrentschuk, N.; Tombal, B.; Pompeo, A.C.L.; Mendoza-Valdes, A.; Miller, K.; Debruyne, F.M.J.; Klotz, L. Androgen-targeted therapy in men with prostate cancer: Evolving practice and future considerations. Prosate Cancer Prostatic Dis. 2019, 22, 24–38.
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate-resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511.
- Mehtälä, J.; Zong, J.; Vassilev, Z.; Brobert, G.; Gabarró, M.S.; Stattin, P.; Khanfir, H. Overall survival and second primary malignancies in men with metastatic prostate cancer. PLoS ONE 2020, 15, e0227552.
- Moreira, D.M.; Howard, L.E.; Sourbeer, K.N.; Amarasekara, H.S.; Chow, L.C.; Cockrell, D.C.; Pratson, C.L.; Hanyok, B.T.; Aronson, W.J.; Kane, C.J.; et al. Predicting time from metastasis to overall survival in castration-resistant prostate cancer: Results from SEARCH. Clin. Gent. Cancer 2017, 15, 60–66.
- De Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Roessner, M.; et al. Prenisone plus cabazitaxel or mitoxanone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomized open-label trial. Lancet 2010, 376, 1147–1154.
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223.
- Caffo, O.; Wissing, M.; Bianchini, D.; Bergman, A.; Thomsen, F.B.; Schmid, S.; Yu, E.Y.; Bournakis, E.; Sella, A.; Zagonel, V.; et al. Survival outcomes from a cumulative analysis of worldwide observational studies on sequential use of new agents in metastatic castration-resistant prostate cancer. Clin. Gent. Cancer 2019, 18, 69–76.
- Lombard, A.P.; Liu, L.; Cucchiara, V.; Liu, C.; Armstrong, C.M.; Zhao, R.; Yang, J.C.; Evans, C.P.; Gao, A.C. Intra vs. inter cross resistance determines treatment sequence between taxane and AR-targeting therapies in advanced prostate cancer. Mol. Cancer Ther. 2018, 17, 2197–2205.
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.; Cowey, L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017, 377, 1345–1356.
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, K.; et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028.
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Frontera, O.A.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290.
- Swoboda, A.; Nanda, R. Immune checkpoint blockade for breast cancer. Cancer Treat. Res. 2018, 173, 155–165.
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, double-blind, Phase II trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naïve castration-resistant prostate cancer. J. Clin. Oncol. 2017, 35, 40–47.
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; Van den Eertwegh, A.J.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, Phase 3 trial. Lancet Oncol. 2014, 15, 700–712.
- Topalian, S.L.; Hodi, S.H.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Cavajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454.
- Boudadi, K.; Suzman, D.L.; Luber, B.; Wang, H.; Silberstein, J.; Sullivan, R.; Dowling, D.; Harb, R.; Nirschl, T.; Dittamore, R.V.; et al. Phase 2 biomarker-driven study of ipilimumab plus nivolumab (Ipi/Nivo) for ARV7-positive metastatic castrate-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2017, 35, 5035.
- Karzai, F.; VanderWeele, D.; Madan, R.A.; Owens, H.; Cordes, L.M.; Hankin, A.; Couvillon, A.; Nichols, E.; Bilusic, M.; Beshiri, M.L. Activity of durvalumab plus Olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J. Immunother. Cancer 2018, 6.
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: Multicohort, open label Phase II KEYNOTE-199 study. J. Clin. Oncol. 2020, 38, 395–405.
- Sonpavde, G.; McMannis, J.D.; Bai, Y.; Seethammangari, M.R.; Bull, J.M.V.; Hawkins, V.; Dancsak, T.K.; Lapteva, N.; Levitt, J.M.; Moseley, A.; et al. Phase I trial of antigen-targeted autologous dendritic cell-based vaccine with in vivo activation of inducible CD40 for advanced prostate cancer. Cancer Immunol. Immunother. 2017, 66, 1345–1357.
- Lilleby, W.; Gaudernack, G.; Brunsvig, P.F.; Vlatkovic, L.; Schulz, M.; Mills, K.; Hole, K.H.; Inderberg, E.M. Phase I/IIa clinical trial of novel hTERT peptide vaccine in men with metastatic hormone-naïve prostate cancer. Cancer Immunol. Immunother. 2017, 66, 891–901.
- Noguchi, M.; Arai, G.; Egawa, S.; Ohyama, C.; Naito, S.; Matsumoto, K.; Uemura, H.; Nakagawa, M.; Nasu, Y.; Eto, M.; et al. Mixed 20-peptide cancer vaccine in combination with docetaxel and dexamethasone for castration-resistant prostate cancer: A randomized phase II trial. Cancer Immunol. Immunother. 2020, 69, 847–857.
- McNeel, D.G.; Eickhoff, J.C.; Johnson, L.E.; Roth, A.R.; Perk, T.G.; Fong, L.; Antonarakis, E.S.; Wargowski, E.; Jarej, R.; Liu, G. Phase II trial of a DNA vaccine encoding prostatic acid phosphatase (pTVG-HP [MVI-816]) in patients with progressive, nonmetastatic, castration-sensitive prostate cancer. J. Clin. Oncol. 2019, 37, 3507–3517.
- Zhang, Z.; Liu, S.; Zhang, B.; Qiao, L.; Zhang, Y.; Zhang, Y. T cell dysfunction and exhaustion in cancer. Front. Cell Dev. Biol. 2020, 8.
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy in castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422.
- Xu, H.; Wang, F.; Li, H.; Ji, J.; Cao, Z.; Lyu, J.; Shi, X.; Zhu, Y.; Zhang, C.; Guo, F.; et al. Prostatic acid phosphatase (PAP) predicts prostate cancer progress in a population-based study: The renewal of PAP? Dis. Markers 2019, 2019, 7090545.
- Fong, L.; Carroll, P.; Weinberg, V.; Chan, S.; Lewis, J.; Corman, J.; Amling, C.L.; Stephenson, R.A.; Simko, J.; Sheikh, N.A.; et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J. Natl. Cancer Inst. 2014, 10611.
- Hagihara, K.; Chan, S.; Zhang, L.; Oh, D.Y.; Wei, X.X.; Simko, J.; Fong, L. Neoadjuvant sipuleucel-T induces both Th1 activation and immune regulation in localized prostate cancer. Oncoimmunology 2019, 8, e1486953.
- Sheikh, N.; Cham, J.; Zhang, L.; DeVries, T.; Letarte, S.; Pufnock, J.; Hamm, D.; Trager, J.; Fong, L. Clonotypic diversification of intratumoral T cells following sipuleucel-T treatment in prostate cancer subjects. Cancer Res. 2016, 76, 3711–3718.
- Antonarakis, E.S.; Kibel, A.S.; Yu, E.Y.; Karsh, L.I.; Elfiky, A.; Shore, N.D.; Vozelgang, N.J.; Corman, J.M.; Millard, F.E.; Maher, J.C.; et al. Sequencing of sipuleucel-T and androgen deprivation therapy in men with hormone-sensitive biochemically recurrent prostate cancer: A phase II randomized trial. Clin. Cancer Res. 2017, 23, 2451–2459.
- Sheikh, N.A.; Patrylak, D.; Kantoff, P.W.; Dela Rosa, C.; Stewart, F.P.; Kuan, L.; Whitmore, J.B.; Trager, J.B.; Poehlein, C.H.; Frohlich, M.W.; et al. Sipuleucel-T immune parameters correlate with survival: An analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol. Immunother. 2013, 62, 137–147.
- Small, E.J.; Reese, D.M.; Whisenant, D.; Dixon, S.C.; Figg, W.D. Therapy of advanced prostate cancer with granulocyte macrophage colony-stimulating factor. Clin. Cancer. Res. 1999, 5, 1738–1744.
- Small, E.J.; Fratesi, P.; Reese, D.M. Immunotherapy for hormone-refractory prostate cancer with antigen-loaded dendritic cells. J. Clin. Oncol. 2000, 18, 3894–3903.
- Burch, P.A.; Breen, J.K.; Buckner, J.C.; Gastineau, D.A.; Kaur, J.A.; Laus, R.L.; Padley, D.J.; Peshwa, M.V.; Pitot, H.C.; Richardson, R.L.; et al. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin. Cancer Res. 2000, 6, 2175–2182.
- Antonarakis, E.S.; Small, E.J.; Petrylak, D.P.; Quinn, D.I.; Kibel, A.S.; Chang, N.N.; Dearstyne, E.; Harmon, M.; Campogan, D.; Haynes, H.; et al. Antigen-specific CD8 lytic phenotype induced by sipuleucel-T in hormone-sensitive or castration-resistant prostate cancer and association with overall survival. Clin. Cancer Res. 2018, 24, 4662–4671.
- Saif, J.M.; Vadakekolathu, J.; Rane, S.S.; McDonald, D.; Ahmad, M.; Mathieu, M.; Pockley, A.G.; Durrent, L.; Metheringham, R.; Rees, R.C.; et al. Novel prostate acid phosphatase-based peptide vaccination strategy induces antigen-specific T-cell responses and limits tumour growth in mice. Eur. J. Immunol. 2014, 44, 994–1004.
- Gonzalez-Garzala, F.F.; McCabe, A.; Santos, E.J.; Jones, J.; Takeshita, L.Y.; Ortega-Rivera, N.D.; Del Cid-Pavon, G.M.; Ramsbottom, K.; Ghattaoraya, G.S.; Alfirevic, A.; et al. Allele frequency net database (AFND) 2020 update: Gold-standard data classification, open access genotype data and new query tools. Nucleic Acid Res. 2020, 48, D783–D788.
- Speetjens, F.M.; Kuppen, P.J.; Welters, M.J.; Essahsah, F.; Voet van den Brink, A.M.; Lantrua, M.G.; Valentijn, A.R.; Oostendorp, J.; Fathers, L.M.; Nijman, H.W.; et al. Induction of p53-specific immunity by a p53 synthetic long peptide vaccine in patients treated for metastatic colorectal cancer. Clin. Cancer Res. 2009, 15, 1086–1095.
- Le Vu, P.; Vadakekolathu, J.; Nicholls, H.; Christensen, D.; Durrant, L.; Pockley, A.; McArdle, S.E. Novel PAP-derived vaccine for the treatment of advanced prostate cancer. Eur. J. Cancer 2018, 92, S18.




