
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Darren Candow | + 3421 word(s) | 3421 | 2021-03-12 06:57:21 | | | |
| 2 | Vivi Li | Meta information modification | 3421 | 2021-03-18 09:32:56 | | |
Video Upload Options
Sarcopenia, defined as age-related reduction in muscle mass, strength, and physical performance, is associated with other age-related health conditions such as osteoporosis, osteosarcopenia, sarcopenic obesity, physical frailty, and cachexia. From a healthy aging perspective, lifestyle interventions that may help overcome characteristics and associated comorbidities of sarcopenia are clinically important. One possible intervention is creatine supplementation (CR). Accumulating research over the past few decades shows that CR, primarily when combined with resistance training (RT), has favourable effects on aging muscle, bone and fat mass, muscle and bone strength, and tasks of physical performance in healthy older adults. However, research is very limited regarding the efficacy of CR in older adults with sarcopenia or osteoporosis and no research exists in older adults with osteosarcopenia, sarcopenic obesity, physical frailty, or cachexia.
1. Introduction
Sarcopenia refers to age-related reductions in muscle strength (dynapenia), muscle mass (quantity), relative strength (strength per unit of muscle mass), muscle quality (architecture and composition), and/or physical performance (i.e., tasks of functionality) [1]. Sarcopenia typically occurs in 8–13% of adults ≥60 years of age [2] and is associated with other age-related health conditions such as osteoporosis, osteosarcopenia, sarcopenic obesity, physical frailty, and cachexia. Annually, muscle mass decreases by 0.45% in men and by 0.37% in women, but these decrements climb to 0.9% for men and to 0.7% for women starting in their seventh decade [3]. The age-related decrease in muscle strength, which is a strong predictor of poor health outcomes (mobility disability, falls, fractures, and mortality) in older adults [1], occurs more rapidly (2–5 fold faster) than the reduction in lean (muscle) mass [4].
From a global health perspective, the World Health Organization established a code (ICD-10-CM; M62.84) for sarcopenia as a means for better diagnosis, assessment, and treatment of the condition. While several definitions and subcategories of sarcopenia exist, the European Working Group on Sarcopenia in Older People (EWGSOP) defines individuals with low muscle strength (as assessed by grip-strength or chair-stand test) as having probable sarcopenia; those with low muscle strength and low muscle quantity (as assessed by dual energy X-ray absorptiometry, magnetic resonance imaging and spectroscopy, computed tomography, bioelectrical impedance, creatine dilution, and/or muscle biopsy) as having confirmed sarcopenia; and those with low muscle strength, low muscle quantity, and poor physical performance (as assessed by gait speed, short physical performance battery test, timed-up-and-go test, or 400 m walk test) as having severe sarcopenia [1]. Sarcopenia is classified as primary when its etiology is age dependent whereas secondary sarcopenia is influenced by age and/or other factors such as physical inactivity and undernutrition [1]. Contributing factors to the pathophysiology of sarcopenia include changes in neuromuscular function, skeletal muscle morphology and architecture, protein kinetics, hormonal regulation, growth factors and satellite cells, vascularization, inflammation, mitochondrial function, nutrition, and physical activity [1][3][4]. From a healthy aging perspective, interventions that may help overcome characteristics and associated comorbidities of sarcopenia (i.e., osteoporosis, osteosarcopenia, sarcopenic obesity, physical frailty, and cachexia) are clinically important.
Accumulating research over the past few decades shows that creatine supplementation (CR), primarily when combined with resistance training (RT), has some favourable effects on muscle accretion and bone mineral density, bone and muscle strength, and tasks of functionality in older adults (for reviews, see Candow et al. [5], Chilibeck et al. [6], Forbes et al. [7], Gualano et al. [8], and Kreider et al. [9]). However, research is very limited regarding the efficacy of CR in older adults with sarcopenia or osteoporosis and no research exists in older adults with osteosarcopenia, sarcopenic obesity, physical frailty, or cachexia. Therefore, the purpose of this narrative review is (1) to evaluate and summarize current research involving CR, with and without RT, on properties of muscle and bone in older adults and (2) to provide a rationale and justification for future research involving CR in older adults with osteosarcopenia, sarcopenic obesity, physical frailty, or cachexia.
2. Potential of Creatine Supplementation for Sarcopenia
The majority of aging research involving CR has focused on measures of muscle accretion and strength in response to RT. Studies published to date involving >600 older adults (>48 years of age) show divergent results, possibly because of methodological differences across studies (Table 1). We have previously reviewed the majority of these studies in detail elsewhere [5][8][10][11][12][13]. Most studies (n = 16) involved healthy older adults, whereas 4 studies involved older adults with knee osteoarthritis, osteopenia or osteoporosis, type II diabetes, or chronic obstructive pulmonary disease (COPD). The results are equivocal regarding the efficacy of CR on measures of muscle accretion and strength, with half of the studies showing greater gains from CR vs. placebo (PLA) and the other half showing similar effects between the two interventions during an RT program. Individual studies typically lack adequate statistical power to detect small changes in muscle accretion and strength from CR over time, and the responsiveness to CR in older adults may be influenced by initial resting PCr levels in different muscle regions, changes in type II muscle fibre size and quantity, and habitual dietary intake of creatine [12]. To overcome the limitations of low statistical power and high variability amongst older adult populations, three meta-analyses have been performed to determine the efficacy of CR (≥3 g/day) vs. PLA during an RT program (≥7 weeks) on measures of muscle accretion and strength [6][14][15]. Collectively, these meta-analyses showed that the combination of CR and RT augmented muscle accretion (≈1.2 kg), and upper- and lower-body strength more than PLA and RT in older adults. Mechanistically, the greater increase in muscle accretion and strength from CR may be related to its ability to influence phosphate metabolism, calcium and glycogen regulation, cellular swelling, muscle protein signaling and breakdown, myogenic transcription factors and satellite cells, growth factors (i.e., IGF-1 and myostatin), inflammation, and oxidative stress (for reviews, see Candow et al. [5], Chilibeck et al. [6], Gualano et al. [8], and Kreider et al. [9]). Upon CR cessation, the gains in muscle accretion and strength seem to persist for up to 12 weeks when RT is maintained in older adults [16].
Table 1. Summary of studies examining creatine and resistance training on muscle outcomes in older adults.
| First Author, Year | Population | Supplement Dose | Resistance Training | Duration | Outcomes |
|---|---|---|---|---|---|
| Aguiar et al. 2013 [17] | N = 18; healthy women; Mean age = 65 y | CR (5 g/day), PLA | RT = 3 x/wk | 12 wks | CR ↑ gains in fat-free mass (+3.2%), muscle mass (+2.8%), 1 RM bench press, knee extension, and biceps curl compared to PLA |
| Alves et al. 2013 [18] | N = 47; healthy women, Mean age = 66.8 y (range: 60–80 y) | CR (20 g/day for 5 days, followed by 5 g/day thereafter), PLA with and without RT | RT = 2 x/wk | 24 wks | ↔1 RM strength compared to RT + PLA |
| Bemben et al. 2010 and Eliot et al. 2008 [19][20] | N = 42; healthy men; age = 48–72 y | CR (5 g/day), PRO (35 g/day), CR + PRO, PLA | RT = 3 x/wk | 14 wks | ↔lean tissue mass, 1 RM strength |
| Bermon et al. 1998 [21] | N = 32 (16 men, 16 women); healthy; age = 67–80 y | CR (20 g/day for 5 days followed by 3 g/day), PLA | RT = 3 x/wk | 7.4 wks (52 days) | ↔lower limb muscular volume, 1- and 12-repetitions maxima, and isometric intermittent endurance |
| Bernat et al. 2019 [22] | N = 24 healthy men; age = 59 ± 6 y | CR (0.1 g/kg/day), PLA | High-velocity RT = 2 x/wk | 8 wks | ↔muscle thickness, physical performance, upper-body muscle strength; CR ↑ leg press strength, total lower body strength |
| Brose et al. 2003 [23] | N = 28 (15 men, 13 women); healthy; age: men = 68.7, women = 70.8 y | CR (5 g/day), PLA | RT = 3 x/wk | 14 wks | CR ↑ gains in lean tissue mass and isometric knee extension strength; ↔ type 1, 2 a, 2 x muscle fibre area |
| Candow et al. 2008 [24] | N = 35; healthy men; age = 59–77 y | CR (0.1 g/kg/day), CR + PRO (PRO: 0.3 g/kg/day), PLA | RT = 3 x/wk | 10 wks | CR ↑ muscle thickness compared to PLA. CR ↑1 RM bench press ↔ 1 RM leg press |
| Candow et al. 2015 [25] | N = 39 (17 men, 22 women); healthy; age = 50–71 y | CR (0.1 g/kg) before RT, CR (0.1 g/kg) after RT, PLA | RT = 3 x/wk | 32 wks | CR after RT ↑ lean tissue mass, 1 RM leg press, 1 RM chest press compared to PLA |
| Candow et al. 2020 [26] | N = 38; healthy men; age = 49–67 y | CR (On training days: 0.05 g/kg before and 0.05 g/kg after exercise) + 0.1 g/kg/day on non-train-ing days (2 equal doses) or PLA | RT = 3 x/wk | 12 months | ↔lean tissue mass, muscle thickness, or muscle strength |
| Chilibeck et al. 2015 [27] | N = 33; healthy women; Mean age = 57 y | CR (0.1 g/kg/day), PLA | RT = 3 x/wk | 52 wks | ↔lean tissue mass and muscle thickness gains between groups; ↑ relative bench press strength compared to PLA. |
| Chrusch et al. 2001 [28] | N = 30; healthy men; age = 60–84 y | CR (0.3 g/kg/d for 5 days followed by 0.07 g/kg/day), PLA | RT = 3 x/wk | 12 wks | CR ↑ gains in lean tissue mass; CR ↑1 RM leg press, 1 RM knee extension, leg press endurance, and knee extension endurance; ↔ 1 RM bench press or bench press endurance. |
| Cooke et al. 2014 [29] | N = 20; healthy men; age = 55–70 y | CR (20 g/day for 7 days followed by 0.1 g/kg/day on training days) | RT = 3 x/wk | 12 wks | ↔lean tissue mass, 1 RM bench press, 1 RM leg press |
| Deacon et al. 2008 [30] | N = 80 (50 men, 30 women); COPD; age = 68.2 y | CR (22 g/day for 5 day followed by 3.76 g/day), PLA | RT = 3 x/wk | 7 wks | ↔lean tissue mass or muscle strength |
| Eijnde et al. 2003 [31] | N = 46; healthy men; age = 55–75 y | CR (5 g/day), PLA | Cardiorespiratory + RT = 2–3 x/wk | 26 wks | ↔lean tissue mass or isometric maximal strength |
| Gualano et al. 2011 [32] | N = 25 (9 men, 16 women); type 2 diabetes; age = 57 y | CR (5 g/day), PLA | RT = 3 x/wk | 12 wks | ↔lean tissue mass |
| Gualano et al. 2014 [33] | N = 30; "vulnera-ble" women; Mean age = 65.4 y | CR (20 g/day for 5 days; 5 g/day thereafter), PLA with and without RT | RT = 2 x/wk | 24 wks | CR + RT ↑ gains in 1RM bench press and appendicular lean mass compared to PLA + RT |
| Johannsmeyer et al. 2016 [34] | N = 31 (17 men, 14 women); healthy; age = 58 y | CR (0.1 g/kg/day), PLA | RT = 3 x/wk | 12 wks | CR ↑ gains in lean tissue mass; ↔ 1RM strength and endurance; CR attenuated magnitude increase in time to complete balance test compared to PLA |
| Neves et al. 2011 [35] | N = 24 (postmen-opausal women with Knee osteo-arthritis); Age = 55–65 y | CR (20 g/day for 1 week, followed by 5 g/day), PLA | RT = 3 x/wk | 12 wks | CR ↑ gains in limb lean mass. ↔ 1RM leg press |
| Pinto et al. 2016 [36] | N = 27 (men and women); healthy; age = 60–80 y | CR (5 g/day), PLA | RT = 3 x/wk | 12 wks | CR ↑ gains in lean tissue mass; ↔ 10 RM bench press or leg press strength |
| Smolarek et al. 2020 [37] | N = 26 (5 men, 21 women); long-term care residence; age = 68.9 ± 6.8 y | CR (5 g/day), PLA | RT = 2 x/wk | 16 wks | CR ↑ dominant and non-dominant handgrip strength |
CR = creatine; PRO = protein; RM = repetition maximum; ↑ = significant greater; ↔ no difference between conditions; wk = weeks; y = years; g = grams; kg = kilograms.
Only three studies have determined the effects of CR and RT in older adults with different classifications of sarcopenia. Pinto et al. [36] showed that, in older adults with either probable sarcopenia (n = 3; skeletal muscle mass index (SMI): appendicular skeletal muscle mass/height2 <7.26 kg/m2 for men and <5.45 kg/m2 for women), sarcopenia (n = 1; SMI + handgrip strength <30 kg and <20 kg for women or gait speed <0.8 m/s), or severe sarcopenia (n = 1; SMI + handgrip strength <30 kg and <20 kg for women and gait speed <0.8 m/s), 12 weeks of CR (5 g/day) and supervised RT eliminated the probable and severe sarcopenia designations in 3 participants. However, creatine had no effect on the individual with sarcopenia. Furthermore, it is unknown whether creatine and RT reduced the level of severe sarcopenia to sarcopenia or probable sarcopenia. In seven older adults considered to be pre-sarcopenic (defined as relative skeletal muscle index >7.26 kg/m2 for men and >5.5 kg/m2 for women [38]), 8 months of CR (0.1 g/kg/day or ≈8 g/day) and supervised whole-body RT eliminated the pre-sarcopenic designation in 5 of the participants [25].
Finally, in four postmenopausal women (>60 years) who were sarcopenic (defined by appendicular lean mass, adjusted for height and weight [39]), CR (20 g/day for 5 days + 5 g/day for 23 weeks) during supervised whole-body RT (3 sets of 8–12 repetitions, 2 days per week) eliminated the sarcopenia classification in two of the women [33]. While limited by very low sample sizes, these preliminary results across studies suggest that CR (≥5 g/day) and supervised RT (>12 weeks) has some potential to mitigate sarcopenia in older adults.
Regarding physical performance (functionality), two meta-analyses of older adults demonstrated that CR in conjunction with RT resulted in greater improvements in sit-to-stand performance when compared to RT (plus PLA) alone [5][15]. These findings are of clinical relevance given that improving sit-to-stand performance may reduce the risk of falls in older adults [40].
Independent of RT, research is mixed regarding the effectiveness of CR on aging muscle, with 5 studies showing greater effects from CR vs. PLA and 5 studies showing similar effects between the two interventions (for review, see Forbes et al. [41]). While it is difficult to compare results across studies, these inconsistent findings may be related to the CR protocol and/or dosage used. The majority of studies that found beneficial effects from CR incorporated a CR loading phase (20 g/day) or used a high relative daily dosage of creatine (0.3 g/kg/day), whereas several of the studies that failed to observe beneficial effects did not use these strategies.
In summary, CR (≥3 g/day) and RT (≥7 weeks; primarily whole-body routines) can improve some measures of muscle accretion, strength, and physical performance in older adults. Independent of RT, a CR loading phase and/or high relative daily dosage of creatine (≥0.3 g/kg/day) may be required to produce some muscle benefits in older adults. It is unknown whether the combination of CR and RT provides greater fitness benefits compared to CR alone. Furthermore, the effects of CR in sarcopenic older adults is relatively unknown. No research exists regarding the efficacy of CR in older adults with inborn creatine synthesis deficiencies involving arginine–glycine amidinotransferase (AGAT), guanidinoacetate methyl transferase (GAMT), solute carrier 6 (SLC6AB), or CT1 (creatine transporter). Future research should investigate the effects of CR, with and without RT, in older clinical populations with possible musculoskeletal disorders and creatine synthesis/transporter deficiencies.
3. Potential of Creatine Supplementation for Osteoporosis
Osteoporosis refers to age-related loss of bone mineral density (BMD) and architecture [42] that increases bone fragility and the risks of falls and fractures [43]. There are 8 published studies that have examined the combined effects of CR and RT on properties of bone in older adults, with only 3 of these studies showing greater effects from creatine compared to PLA (Table 2). In healthy older men, 12 weeks of CR (loading phase: 0.3 g/kg/day for 5 days; maintenance phase: 0.07 g/kg/day for an additional 79 days) and supervised whole-body RT increased upper-limb bone mineral content (assessed by dual energy X-ray absorptiometry [DXA]) compared to PLA [44]. Additional work in healthy older men showed that 10 weeks of CR (0.1 g/kg/day) and supervised whole-body RT decreased the urinary excretion of cross-linked N-telopeptides of type I collagen (indicator of bone resorption) compared to PLA [24]). Most recently, Chilibeck et al. [27] showed that CR (0.1 g/kg/day) and supervised whole-body RT for 52 weeks attenuated the rate of bone mineral loss in the femoral neck (assessed by DXA) (Figure 1) and increased femoral shaft subperiosteal width (indicator of bone bending strength) in postmenopausal women compared to PLA.
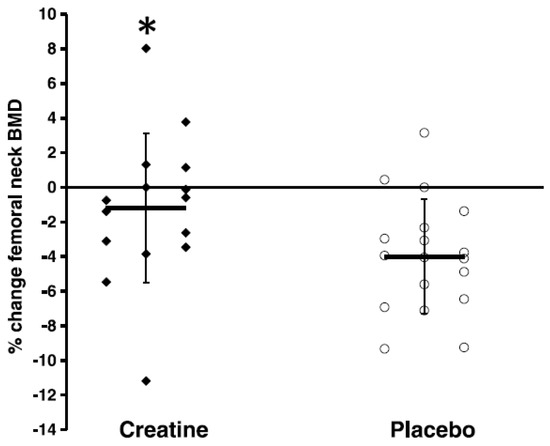
Figure 1. Relative changes in femoral neck bone mineral density (BMD). “Closed diamonds” represent changes for individual creatine group participants, and “open circles” represent placebo group participants. The “horizontal bars” represent the group means, and the “vertical bars” represent the SD. * Creatine participants lost significantly less BMD at the femoral neck compared with placebo participants (p < 0.05). (Reproduced with permission from Chilibeck et al. 2015 [27]).
Table 2. Study characteristics and outcomes of research examining the influence of creatine with a resistance training program on bone.
| First Author, Year | Study Population | Intervention | Duration | Outcomes |
|---|---|---|---|---|
| Brose et al. 2003 [23] | N = 28; healthy (15 men, 13 women); age ≥ 65 y (men = 68.7 y, women = 70.8 y) | RCT; CR + RT, PLA + RT. CR = 5 g/day; RT = 3 x/wk | 14 wks | ↔on osteocalcin |
| Candow et al. 2008 [24] | N = 35; older men (age: 59–77 y) | RCT; CR + PRO + RT; CR + RT, PLA + RT; CR = 0.1 g/kg/day; RT = 3 x/wk | 10 wks | CR ↓ NTx |
| Candow et al. 2019 [5] | N = 39; healthy (17 men; 22 women); age ≥ 50 y (mean ~55 y) | RCT; CR-Before + RT, CR-After + RT, PLA + RT; CR = 0.1 g/kg/day; RT = 3 x/wk | 8 mths | ↔BMD and BMC of the whole-body, limbs, femoral neck, lumbar spine, and total hip |
| Candow et al. 2020 [26] | N = 38; healthy men; age = 49–67 y | RCT; CR + RT, PLA + RT; CR = 0.1 g/kg/day; RT = 3 x/wk | 12 mths | ↔ BMD and geometry, bone speed of sound; CR ↑ (p = 0.06) section modulus of the narrow part of the femoral neck |
| Chilibeck et al. 2005 [44] | N = 29; older men (71 y). | RCT; CR + RT, PLA + RT; CR = 0.3 g/kg/day for 5 days followed by 0.07 g/kg/day for the remaining; RT = 3 x/wk | 12 wks | ↑ arm BMC greater in the CR group com-pared to PLA; ↔ between groups for whole-body and leg BMD |
| Chilibeck et al. 2015 [27] | N = 33; postmenopausal women; age: 57 ± 6 y | RCT; PLA + RT, CR + RT; CR = 0.1 g/kg/day (0.05 g/kg provided immediately before and 0.05 g/kg after training on training days and with two meals on non-training days); RT = 3 x/wk | 12 mths | CR attenuated rate of femoral neck BMD loss compared to PLA and CR ↑ femoral shaft subperiosteal width; ↔ between groups on all other outcome measures |
| Gualano et al. 2014 [33] | N = 60; older vulnerable women (age: 66 y) | RCT; PLA, CR, PLA + RT, CR + RT; CR = 20 g/day for 5 days followed by 5 g/day for the remaining; RT = 2 x/wk | 24 wks | ↔bone mineral and serum bone markers between groups |
| Pinto et al. 2016 [36] | N = 32; healthy, non-athletic men and women between 60–80 y | RCT; PLA + RT, CR + RT; CR = 5 g/day; RT = 3 x/wk. Muscle groups (i.e., upper and lower body) alternated between training days, 1.5 x/wk per muscle group | 12 wks | ↔BMD and BMC of all assessed sites between groups |
RCT = randomized controlled trial; PLA = placebo; RT = resistance training; CR = creatine; PRO = protein; RM = repetition maximum; NTx = cross-linked N-telopeptides of type I collagen; BMD = bone mineral density; BMC = bone mineral content; ↑ = significant greater; ↔ no difference be-tween conditions; wk = weeks; mth = months; y = years; g = grams; kg = kilograms.
In contrast to these studies, Brose et al. [23] was unable to find a beneficial effect from 14 weeks of CR (5 g/day) and whole-body RT on serum osteocalcin (indicator of bone formation) compared to PLA in healthy older adults. Furthermore, Gualano et al. [33] found no effect from CR (loading phase: 20 g/day for 5 days; maintenance phase: 5 g/day for an additional 24 weeks) and supervised whole-body RT on changes in bone mineral (density and content; assessed by DXA) or serum concentrations of procollagen type 1 N-propeptide (P1NP; indicator of bone formation) and type 1 collagen C-telopeptide (CTX; indicator of bone resorption) compared to PLA in older women. In addition, 12 weeks of CR (5 g/day) and supervised whole-body RT had no greater effect on measures of BMD or content (assessed by DXA) compared to PLA in healthy older adults [36]. Similarly, Candow et al. [45] was unable to find greater effects from CR (0.1 g/kg/day) and 32 weeks of supervised whole-body RT on measures of bone mineral (density and content; assessed by DXA) compared to PLA in healthy older adults. Most recently, Candow et al. [26] failed to show a beneficial effect from 52 weeks of CR (0.1 g/kg/day) and supervised whole-body RT on measures of BMD or bone geometric properties (assessed by DXA and ultrasound) in older men compared to PLA.
There are only three studies that have investigated the effects of CR alone (no exercise training stimulus) on properties of aging bone. In postmenopausal women with osteopenia or osteoporosis, 24 weeks of CR (loading phase: 20 g/day for 5 days; maintenance phase: 5 g/day for an additional 23 weeks) had no effect on measures of BMD (whole-body, lumbar, total femur, and femoral neck; assessed by DXA) or serum markers of bone turnover (CTX, P1NP) compared to PLA [33]. In two additional studies involving postmenopausal women, CR (1 g/day for 52 weeks) had no effect on measures of BMD (assessed by DXA), bone microarchitecture (assessed by high-resolution peripheral quantitative computed tomography (HR-pQCT)), CTX, or P1NP compared to PLA [46]. Increasing the dosage of creatine to 3 g/day for an additional 52 weeks (104 weeks in total) also had no effect on the same bone measures in postmenopausal women. Furthermore, creatine had no effect on the number of falls or fractures experienced [47].
Collectively, the vast majority of studies show no greater effect from CR, with and without RT, on properties of bone in older adults. In the few studies that did show beneficial effects, CR was combined with supervised whole-body RT. Importantly, no study showed any detrimental effect from CR on bone mineral or geometry. The combined effects of CR and RT on reducing the risk and incidence of falls and fractures in older adults is largely unknown. Bone tissue typically takes a long time (i.e., several months) to turnover [48], especially in older adults [49]. Future research should investigate the longer-term effects (i.e., ≥2 years) of CR, with and without RT, on properties of bone mineral and geometry and risk of falls and fractures in older adults.
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2 Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31.
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 1–10.
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Joint Bone Spine 2019, 86, 309–314.
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260.
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R.B. Effectiveness of Creatine Supplementation on Aging Muscle and Bone: Focus on Falls Prevention and Inflammation. J. Clin. Med. 2019, 8, 488.
- Chilibeck, P.D.; Kaviani, M.; Candow, D.G.; Zello, G.A. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: A meta-analysis. Open Access J. Sports Med. 2017, 8, 213–226.
- Forbes, S.C.; Chilibeck, P.D.; Candow, D.G. Creatine Supplementation During Resistance Training Does Not Lead to Greater Bone Mineral Density in Older Humans: A Brief Meta-Analysis. Front. Nutr. 2018, 5, 27.
- Gualano, B.; Rawson, E.S.; Candow, D.G.; Chilibeck, P.D. Creatine supplementation in the aging population: Effects on skeletal muscle, bone and brain. Amino Acids 2016, 48, 1793–1805.
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 1–18.
- Candow, D.G. Sarcopenia: Current theories and the potential beneficial effect of creatine application strategies. Biogerontology 2011, 12, 273–281.
- Candow, D.G.; Forbes, S.C.; Little, J.P.; Cornish, S.M.; Pinkoski, C.; Chilibeck, P.D. Effect of nutritional interventions and resistance exercise on aging muscle mass and strength. Biogerontology 2012, 13, 345–358.
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R.B. Variables Influencing the Effectiveness of Creatine Supplementation as a Therapeutic Intervention for Sarcopenia. Front. Nutr. 2019, 6, 124.
- Forbes, S.C.; Little, J.P.; Candow, D.G. Exercise and nutritional interventions for improving aging muscle health. Endocrine 2012, 42, 29–38.
- Candow, D.G.; Chilibeck, P.D.; Forbes, S.C. Creatine supplementation and aging musculoskeletal health. Endocrine 2014, 45, 354–361.
- Devries, M.C.; Phillips, S.M. Creatine supplementation during resistance training in older adults-a meta-analysis. Med. Sci. Sports Exerc. 2014, 46, 1194–1203.
- Candow, D.G.; Chilibeck, P.D.; Chad, K.E.; Chrusch, M.J.; Davison, K.S.; Burke, D.G. Effect of ceasing creatine supplementation while maintaining resistance training in older men. J. Aging Phys. Act. 2004, 12, 219–231.
- Aguiar, A.F.; Januario, R.S.; Junior, R.P.; Gerage, A.M.; Pina, F.L.; do Nascimento, M.A.; Padovani, C.R.; Cyrino, E.S. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur. J. Appl. Physiol. 2013, 113, 987–996.
- Alves, C.R.; Merege Filho, C.A.; Benatti, F.B.; Brucki, S.; Pereira, R.M.; de Sa Pinto, A.L.; Lima, F.R.; Roschel, H.; Gualano, B. Creatine supplementation associated or not with strength training upon emotional and cognitive measures in older women: A randomized double-blind study. PLoS ONE 2013, 8, e76301.
- Bemben, M.G.; Witten, M.S.; Carter, J.M.; Eliot, K.A.; Knehans, A.W.; Bemben, D.A. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J. Nutr. Health Aging 2010, 14, 155–159.
- Eliot, K.A.; Knehans, A.W.; Bemben, D.A.; Witten, M.S.; Carter, J.; Bemben, M.G. The effects of creatine and whey protein supplementation on body composition in men aged 48 to 72 years during resistance training. J. Nutr. Health Aging 2008, 12, 208–212.
- Bermon, S.; Venembre, P.; Sachet, C.; Valour, S.; Dolisi, C. Effects of creatine monohydrate ingestion in sedentary and weight-trained older adults. Acta Physiol. Scand. 1998, 164, 147–155.
- Bernat, P.; Candow, D.G.; Gryzb, K.; Butchart, S.; Schoenfeld, B.J.; Bruno, P. Effects of high-velocity resistance training and creatine supplementation in untrained healthy aging males. Appl. Physiol. Nutr. Metab. 2019, 44, 1246–1253.
- Brose, A.; Parise, G.; Tarnopolsky, M.A. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 11–19.
- Candow, D.G.; Little, J.P.; Chilibeck, P.D.; Abeysekara, S.; Zello, G.A.; Kazachkov, M.; Cornish, S.M.; Yu, P.H. Low-dose creatine combined with protein during resistance training in older men. Med. Sci. Sports Exerc. 2008, 40, 1645–1652.
- Candow, D.G.; Vogt, E.; Johannsmeyer, S.; Forbes, S.C.; Farthing, J.P. Strategic creatine supplementation and resistance training in healthy older adults. Appl. Physiol. Nutr. Metab. 2015, 40, 689–694.
- Candow, D.G.; Chilibeck, P.D.; Gordon, J.; Vogt, E.; Landeryou, T.; Kaviani, M.; Paus-Jensen, L. Effect of 12 months of creatine supplementation and whole-body resistance training on measures of bone, muscle and strength in older males. Nutr. Health 2020, 260106020975247.
- Chilibeck, P.D.; Candow, D.G.; Landeryou, T.; Kaviani, M.; Paus-Jenssen, L. Effects of Creatine and Resistance Training on Bone Health in Postmenopausal Women. Med. Sci. Sports Exerc. 2015, 47, 1587–1595.
- Chrusch, M.J.; Chilibeck, P.D.; Chad, K.E.; Davison, K.S.; Burke, D.G. Creatine supplementation combined with resistance training in older men. Med. Sci. Sports Exerc. 2001, 33, 2111–2117.
- Cooke, M.B.; Brabham, B.; Buford, T.W.; Shelmadine, B.D.; McPheeters, M.; Hudson, G.M.; Stathis, C.; Greenwood, M.; Kreider, R.; Willoughby, D.S. Creatine supplementation post-exercise does not enhance training-induced adaptations in middle to older aged males. Eur. J. Appl. Physiol. 2014, 114, 1321–1332.
- Deacon, S.J.; Vincent, E.E.; Greenhaff, P.L.; Fox, J.; Steiner, M.C.; Singh, S.J.; Morgan, M.D. Randomized controlled trial of dietary creatine as an adjunct therapy to physical training in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 178, 233–239.
- Eijnde, B.O.; Van Leemputte, M.; Goris, M.; Labarque, V.; Taes, Y.; Verbessem, P.; Vanhees, L.; Ramaekers, M.; Vanden Eynde, B.; Van Schuylenbergh, R.; et al. Effects of creatine supplementation and exercise training on fitness in men 55-75 yr old. J. Appl. Physiol. (1985) 2003, 95, 818–828.
- Gualano, B.; de Salles Painelli, V.; Roschel, H.; Lugaresi, R.; Dorea, E.; Artioli, G.G.; Lima, F.R.; da Silva, M.E.; Cunha, M.R.; Seguro, A.C.; et al. Creatine supplementation does not impair kidney function in type 2 diabetic patients: A randomized, double-blind, placebo-controlled, clinical trial. Eur. J. Appl. Physiol. 2011, 111, 749–756.
- Gualano, B.; Macedo, A.R.; Alves, C.R.; Roschel, H.; Benatti, F.B.; Takayama, L.; de Sa Pinto, A.L.; Lima, F.R.; Pereira, R.M. Creatine supplementation and resistance training in vulnerable older women: A randomized double- blind placebo-controlled clinical trial. Exp. Gerontol. 2014, 53, 7–15.
- Johannsmeyer, S.; Candow, D.G.; Brahms, C.M.; Michel, D.; Zello, G.A. Effect of creatine supplementation and drop-set resistance training in untrained aging adults. Exp. Gerontol. 2016, 83, 112–119.
- Neves, M.; Gualano, B.; Roschel, H.; Fuller, R.; Benatti, F.B.; Pinto, A.L.; Lima, F.R.; Pereira, R.M.; Lancha, A.H.; Bonfa, E. Beneficial effect of creatine supplementation in knee osteoarthritis. Med. Sci. Sports Exerc. 2011, 43, 1538–1543.
- Pinto, C.L.; Botelho, P.B.; Carneiro, J.A.; Mota, J.F. Impact of creatine supplementation in combination with resistance training on lean mass in the elderly. J. Cachexia Sarcopenia Muscle 2016, 7, 413–421.
- Smolarek, A.C.; McAnulty, S.R.; Ferreira, L.H.; Cordeiro, G.R.; Alessi, A.; Rebesco, D.B.; Honorato, I.C.; Laat, E.F.; Mascarenhas, L.P.; Souza-Junior, T.P. Effect of 16 weeks of strength training and creatine supplementation on strength and cognition in older adults: A pilot study. J. Exerc. Physiol. Online 2020, 23, 88–94.
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763.
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.; Goodpaster, B.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Health ABC Study Investigators Sarcopenia: Alternative definitions and associations with lower extremity function. J. Am. Geriatr. Soc. 2003, 51, 1602–1609.
- Macrae, P.G.; Lacourse, M.; Moldavon, R. Physical performance measures that predict faller status in community- dwelling older adults. J. Orthop. Sports Phys. Ther. 1992, 16, 123–128.
- Forbes, S.C.; Candow, D.G.; Ferreira, L.H.B.; Souza-Junior, T.P. Effects of Creatine Supplementation on Properties of Muscle, Bone, and Brain Function in Older Adults: A Narrative Review. J. Diet. Suppl. 2021, 1–18.
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117.
- Reginster, J.Y.; Beaudart, C.; Buckinx, F.; Bruyere, O. Osteoporosis and sarcopenia: Two diseases or one? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 31–36.
- Chilibeck, P.D.; Chrusch, M.J.; Chad, K.E.; Shawn Davison, K.; Burke, D.G. Creatine monohydrate and resistance training increase bone mineral content and density in older men. J. Nutr. Health Aging 2005, 9, 352–353.
- Candow, D.G.; Forbes, S.C.; Vogt, E. Effect of pre-exercise and post-exercise creatine supplementation on bone mineral content and density in healthy aging adults. Exp. Gerontol. 2019, 119, 89–92.
- Lobo, D.M.; Tritto, A.C.; da Silva, L.R.; de Oliveira, P.B.; Benatti, F.B.; Roschel, H.; Niess, B.; Gualano, B.; Pereira, M. Effects of long-term low-dose dietary creatine supplementation in older women. Exp. Gerontol. 2015, 70, 97–104.
- Sales, L.P.; Pinto, A.J.; Rodrigues, S.F.; Alvarenga, J.C.; Goncalves, N.; Sampaio-Barros, M.M.; Benatti, F.B.; Gualano, B.; Rodrigues Pereira, R.M. Creatine Supplementation (3 g/d) and Bone Health in Older Women: A 2- Year, Randomized, Placebo-Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 931–938.
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227.
- Boskey, A.L.; Coleman, R. Aging and bone. J. Dent. Res. 2010, 89, 1333–1348.




