
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eugenia Gallardo | + 2007 word(s) | 2007 | 2021-03-08 03:12:27 | | | |
| 2 | Peter Tang | Meta information modification | 2007 | 2021-03-18 12:25:39 | | | | |
| 3 | Peter Tang | Meta information modification | 2007 | 2021-03-21 07:16:00 | | |
Video Upload Options
Both active and second-hand exposure can be measured and controlled using specific biomarkers of tobacco and its derivatives, allowing the development of more efficient public health policies. Exposure to these compounds can be measured using different methods (involving for instance liquid- or gas-chromatographic procedures) in a wide range of biological specimens to estimate the type and degree of tobacco exposure.
1. Introduction
Tobacco is the only hazardous product legally available that is harmful to everyone exposed to its action [1]. Despite the awareness to the risks entailed to tobacco use, there are more than 1.3 billion users worldwide and over 8 million deaths per year due to tobacco smoke (TS) [2]. However, the health issues related to TS are not limited to active smokers, but also to those exposed to passive or second-hand smoke [2]. The term “passive smoking” is associated with the involuntary inhalation of TS within the immediate surroundings, generally formed as a result of the burning of a cigarette (side-stream smoke 57–85%) or the inhalation of the smoke from a smoker (mainstream smoke 15–43%) [1]. On the other hand, the exposure to environmental tobacco smoke (ETS), also called second-hand smoke, has been associated with an increased risk of developing cardiovascular diseases [3], lung cancer, and other respiratory diseases [4]. Nowadays, more than 5000 constituents have been identified in TS, including a wide variety of inorganic substances, such as ethers, hydrocarbons, phenols, alcohols, ketones, aldehydes, carboxylic acids, and amines, including at least 60 different carcinogenic products [5]. Depending on the amount of smoke present, the type of ventilation, along with other environmental conditions and the level of exposure, many countries have developed restricted smoking policies and active strategies to decrease exposure to ETS in workplaces and homes.
2. Biomarkers
Typically, biomarkers can give a useful insight into environmental exposures, and in this particular case, into tobacco-related exposure [6]. The levels of these substances can be determined by measuring either the parent molecule or its metabolites in biological matrices [6]. To be considered an ideal ETS biomarker the substance should be highly sensitive, specific, have a usable biological half-life and should allow distinguishing between active and non-tobacco users [7]. The following sections summarize the most commonly-used biomarkers related to tobacco smoking and ETS from several chemical classes.
2.1. Nicotine and Tobacco Alkaloids
Nicotine is the most abundant alkaloid found in the tobacco leaf and the primary reason for tobacco dependence, due to its addictiveness [8]. Despite the existing evidence of the presence of nicotine in certain fruits and vegetables, for example, tomatoes and potatoes, the difference in the magnitude of concentrations by comparison with those in cigarette smoke or in nicotine replacement therapy (NRT) (e.g., chewing gum or nicotine patches) are much lower [9][10]. One cigarette contains in average from 7 to 24 mg of nicotine and the average nicotine air concentration ranges from 1 to 10 μg/m3 in indoor smoking environments, with approximately 0.3 to 3.0 mg being absorbed into the body per cigarette consumed via inhalation or absorbed through the skin [9]. Thus, nicotine was one of the first tobacco biomarkers used to access ETS, due to its high concentrations in the organism and its specificity [6]. However, its half-life is short (~2 h) and the metabolism rate is variable, limiting the time-window for its monitoring [7]. Considering all these factors, multiple nicotine metabolites started to be studied as biomarkers of ETS exposure [11]. It was reported that approximately 70/80% of the nicotine absorbed by the body was rapidly metabolized into cotinine [6] by cytochrome P450 2A6 (CYP2A6), whose values described in the literature are above 70% [12][13]. Additionally, cotinine can be further metabolized by the UGT enzymes into cotinine-N-glucuronide; nicotine is also converted by these enzymes into nicotine-N-glucuronide. Both nicotine and cotinine, as well as their glucuronides can be detected and measured in biological fluids [7]. Other compounds are formed in the metabolization process, such as trans-3′-hydroxycotinine, norcotinine, nornicotine, and the glucuronized compounds, among others [12][13][14][15]. Figure 1 resumes the metabolic profile of nicotine.
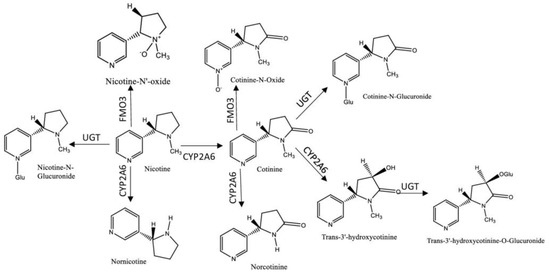
Figure 1. Metabolic profile of nicotine. Legend: (UGT-UPD-glucuronosyltransferases; FMO3-Flavin-containing monooxigenase 3; CYP2A6- cytochrome P450 2A6).
Cotinine has a half-life of about 18 h in the organism and persists in biological matrices due to its poor lipid solubility, facilitating its identification in several tissues and biological fluids, including hair, blood, oral fluid, urine, and breast milk, making it an efficient and widely used biomarker [16][17][18][19][20]. Despite cotinine levels are affected by several factors, such as gender, genetic variability, pregnancy, as well as certain diseases, generally active smokers display two to three times higher concentrations of cotinine than non-smokers, allowing a simple differentiation between active- and non-smokers [21]. Although cotinine is considered an efficient biomarker to measure nicotine consumption, it does not provide information regarding the remaining metabolites. Thus, researchers usually measure the total nicotine equivalents (TNE), which is the sum of urinary nicotine, cotinine, as well as several other metabolites in the nicotine metabolic profile, to fully evaluate nicotine intake; as such, compounds as hydroxy-PAHs and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) may be measured [22][23]. Trans-3′-hydroxycotinine is, in most individuals, the major metabolite of cotinine, making it an important biomarker to measure the ETS exposure [24]. In urine, the levels of trans-3′-hydroxycotinine surpass those of cotinine by about three to four fold, but in plasma and oral fluid cotinine levels are higher than the former’s, allowing the differentiation between smokers and non-smokers. [25]. Other substances, like anabansine and anatabine, minor tobacco alkaloids, are also established tobacco intake biomarkers. Additionally these compounds are not present in NRT, and therefore may be used to indicate tobacco use by individuals undergoing NRT [26].
2.2. Carbon Monoxide (CO)
CO is a product generated by incomplete combustion of organic materials during combustions, both from tobacco and non-tobacco sources, for example motor vehicles, forest fires, etc. [27]. Exposure to CO can be measured by measuring the concentration of carboxyhemoglobin (COHb), a complex formed from carbon monoxide and hemoglobin in red blood cells (percent of hemoglobin saturation) and the concentration of CO in exhaled breath (COex) [27]. Both are valid markers to identify individuals that recently used a combustible tobacco product [8]. Despite the short half-life of COHb (1 to 4 h) and COex (5 to 55 min), these biomarkers allow distinguishing between tobacco users and non-users and are considered to be the most useful biomarkers for verifying smoking cessation in clinical trials [28]. For instance, COex concentration in non-smokers ranges from 4 to 7 ppm, while levels from 20 to 30 ppm are seen in smokers (over 50 ppm in heavy smokers). Concerning COHb, non-smokers present values from 1 to 2%, smokers from 4 to 7% and heavy smokers higher 12% [27].
2.3. Tobacco-Specific N-Nitrosamines (TSNA)
TSNAs are formed from tobacco alkaloids during the curing process and are present in tobacco and TS, being mostly nicotine and nornicotine derivatives [29]. The most studied TSNAs are nicotine-derived nitrosamine ketone (NNK), a potent lung carcinogenic, and N’-nitrosonornicotine (NNN), an oral cavity and esophageal carcinogenic [30]. This class of compounds and their metabolites (Figure 2), which include NNK, NNN, NNN-glucuronide, NNAL-N-glucuronide, NNAL-O-glucuronid; the glucuronide metabolites, also referred to as NNAL-Gluc, and N′-nitrosoanabasine (NAB), NAB-glucuronide, N′-nitrosoanatabine (NAT), and NAT-glucuronide, are considered the most relevant biomarkers for ETS monitoring [29][31][32]. From these, NNAL and NNAL-Gluc, the main metabolites of NNK, are the most widely studied biomarkers of this class, displaying a long half-life in biological fluids (~10 to 45 days), they are completely tobacco specific and may be detected in urine [33][34]. In several studies ranges of 1–2 pmol NNAL/mL of urine in smokers have been reported, while in non-smokers exposed to ETS the concentrations were 1–5% of the amount found in smokers [35][36]. In addition, this biomarker is never found in non-tobacco users that are not exposed to ETS, and correlates well with other tobacco-specific markers such as cotinine and TNE [8]. Monitoring NNAL has already been used in investigations regarding exposure to NNK in non-smokers, for example in newborns, children and women living with smoking partners [37][38]. For instance, NNN and its glucuronides, can also be measured in urine and in toenails. However, due to differences in their biological pathways, these compounds exist in lower concentrations than NNAL in the organism, which can difficult their monitoring [29]. In urine, it is possible to distinguish smokers from non-smokers based on the levels of NNN, and it was also demonstrated that it correlates with increasing cigarettes per day and the total levels of cotinine found in urine [39].
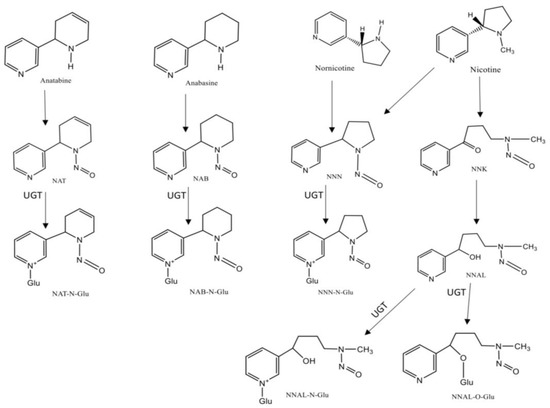
Figure 2. Chemical structures and metabolism of Tobacco-Specific N-Nitrosamines (TSNA). Legend: (UGT-UPD-glucuronosyltransferases).
2.4. Polycyclic Aromatic Hydrocarbons (PAHs)
PAHs are chemicals formed by the incomplete combustion and pyrolysis of tobacco and other organic matter, such as pyrene, fluorene, phenanthrene, and naphthalene [40]. Therefore, both non- and carcinogenic PAHs exhibit high correlation with TS [8]. PAHs biomarkers include 1-hydroxypyrene, less used due to its low specificity to ETS, tetrols of benzo[a]pyrene and phenanthrene, considered effective biomarkers of PAH uptake and metabolic activation [41]. In several studies it was demonstrated that the levels of PAHs biomarkers are higher in smokers when compared to non-smokers [42]. Specifically, the levels of benzo[a]pyrene and phenanthrene-tetrols are two to three times higher in smokers than in non-smokers [43].
2.5. Volatile Organic Compounds (VOCs)
VOCs are substances that are generally formed by the incomplete combustion of organic materials, such as some components of paint, cleaning supplies, pesticides, and are also present in TS [44]. This class includes a wide variety of compounds that can be measured in biological fluids [44]. In blood, 2,5-dimethylfuran, which is comparable to serum cotinine in sensitivity and specificity, benzene, toluene, ethylbenzene, xylene, and styrene are directly correlated with active smoking and have dose-response relationships with cigarettes-per-day [45]. The main VOCs found in urine are mercapturic acids of ethylene oxide, acrolein, crotonaldehyde, butadiene, benzene, acrylonitrile, and acrylamide; these compounds are present in higher amounts in smokers that non-smokers [46]. Particularly, the concentration of 3-hydroxypropylmercapturic acid, a mercapturic acid metabolite of acrolein, is present in smokers at concentrations four times higher than those of non-smokers [47]. Most mercapturic acid metabolites of VOCs present in urine only remain for one day following smoking cessation, making them good biomarkers to distinguish between active and non-smokers [8].
2.6. Aromatic Amines and Heterocyclic Amines
Both aromatic and heterocyclic amines are combustion products that are present in the particulate phase of TS with great potential for evaluation of the exposure to ETS [48][49]. Most of the amines identified as ETS biomarkers are found in small amounts in biological fluids, often in the low part per billion (ppb) range [49], and therefore hardly differentiate between smokers and non-smokers [8]. Indeed, urinary levels of 1-naphthylamine, 2-naphthylamine, ortho-toluidine, 3-aminobiphenyl, 4-aminobiphenyl and the majority of heterocyclic amines are generally low, with the exception of 2-amino-1,7-dimethylimidazo[4,5-b]pyridine (DMIP) and 2-amino-9H-pyrido[2,3-b]indole (AαC), that can be used to distinguish smokers from non-smokers [49].
2.7. Metals
Metals, such as lead and cadmium, may be also considered ETS biomarkers, despite being widespread in the environment, since the tobacco plant can absorb these metals from the soil [8]. Previously, arsenic was also considered a tobacco biomarker because of the use of arsenic pesticides in tobacco cultivation, but this action was discontinued recently. Cadmium exhibits elevated levels in smokers compared to non-smokers in biological fluids like blood and urine, presenting very long half-lives (urine—11 to 30 years/blood—7 to 16 years) [50][51]. Hence, urine cadmium is a biomarker of cumulative and long-term ETS. On the other hand, lead is mostly detected in blood and urine, and many studies associate exposure to this metal to increased risks to develop cardiovascular diseases [52].
2.8. Thiocyanates
One of the first biomarkers of exposure to ETS discovered in biological fluids was thiocyanate ion (SCN−); indeed, it was demonstrated that this ion was found at higher concentrations in smokers than in non-smokers [27]. This ion is a metabolite of cyanides (HCN), which are found in foods like almonds, nuts, leguminous plants, cow’s milk, etc., however in small quantities [27]. However, larger concentrations are produced by the metabolism of some constituents of TS [53]. SCN− exhibits a longer half-life than most biomarkers (~6 days) and the levels of this compound in urine and serum are two to three times higher in smokers than in non-smokers [27]. The average concentration of SCN− in serum and urine samples is 150 µmol/dm3 in smokers, compared to 50 µmol/dm3 in non-smokers, while in oral fluid the levels are in the order of 3000 µmol/dm3 in smokers and 1200 µmol/dm3 in non-smokers [27].
References
- Narkowicz, S.; Polkowska, Ż.; Namieśnik, J. Analysis of Markers of Exposure to Constituents of Environmental Tobacco Smoke (ETS). Crit. Rev. Anal. Chem. 2012, 42, 16–37.
- Yousuf, H.; Hofstra, M.; Tijssen, J.; Leenen, B.; Lindemans, J.W.; van Rossum, A.; Narula, J.; Hofstra, L. Estimated Worldwide Mortality Attributed to Secondhand Tobacco Smoke Exposure, 1990–2016. JAMA Netw. Open 2020, 3, e201177.
- Kawachi, I.; Colditz, G.A. Workplace exposure to passive smoking and risk of cardiovascular disease: Summary of epidemiologic studies. Environ. Health Perspect. 1999, 107 (Suppl. 6), 847–851.
- Hackshaw, A.K.; Law, M.R.; Wald, N.J. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ 1997, 315, 980–988.
- Narkowicz, S.; Polkowska, Ż.; Kiełbratowska, B.; Namieśnik, J. Environmental Tobacco Smoke: Exposure, Health Effects, and Analysis. Crit. Rev. Environ. Sci. Technol. 2013, 43, 121–161.
- Ramdzan, A.N.; Almeida, M.I.G.S.; McCullough, M.J.; Segundo, M.A.; Kolev, S.D. Determination of salivary cotinine as tobacco smoking biomarker. TrAC Trends Anal. Chem. 2018, 105, 89–97.
- Peck, M.J.; Sanders, E.B.; Scherer, G.; Lüdicke, F.; Weitkunat, R. Review of biomarkers to assess the effects of switching from cigarettes to modified risk tobacco products. Biomarkers 2018, 23, 213–244.
- Chang, C.M.; Edwards, S.H.; Arab, A.; Del Valle-Pinero, A.Y.; Yang, L.; Hatsukami, D.K. Biomarkers of Tobacco Exposure: Summary of an FDA-Sponsored Public Workshop. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 291–302.
- Yildiz, D. Nicotine, its metabolism and an overview of its biological effects. Toxicon 2004, 43, 619–632.
- Husgafvel-Pursiainen, K. Biomarkers in the assessment of exposure and the biological effects of environmental tobacco smoke. Scand. J. Work. Environ. Health 2002, 28 (Suppl. 2), 21–29.
- Benowitz, N.L.; Jacob, P. Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin. Pharmacol. Ther. 1994, 56, 483–493.
- Miller, E.I.; Norris, H.-R.K.; Rollins, D.E.; Tiffany, S.T.; Wilkins, D.G. A novel validated procedure for the determination of nicotine, eight nicotine metabolites and two minor tobacco alkaloids in human plasma or urine by solid-phase extraction coupled with liquid chromatography–electrospray ionization–tandem mass spectrometr. J. Chromatogr. B 2010, 878, 725–737.
- Schick, S.F.; Blount, B.C.; Jacob, P.; Saliba, N.A.; Bernert, J.T.; El Hellani, A.; Jatlow, P.; Pappas, R.S.; Wang, L.; Foulds, J.; et al. Biomarkers of exposure to new and emerging tobacco delivery products. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L425–L452.
- Scheidweiler, K.B.; Marrone, G.F.; Shakleya, D.M.; Singleton, E.G.; Heishman, S.J.; Huestis, M.A. Oral fluid nicotine markers to assess smoking status and recency of use. Ther. Drug Monit. 2011, 33, 609–618.
- Swortwood, M.J.; Bartock, S.H.; Scheidweiler, K.B.; Shaw, S.; Filis, P.; Douglas, A.; O’Shaughnessy, P.J.; Soffientini, U.; Lucendo-Villarin, B.; Iredale, J.P.; et al. Quantification of ethyl glucuronide, ethyl sulfate, nicotine, and its metabolites in human fetal liver and placenta. Forensic Toxicol. 2018, 36, 102–112.
- Byrd, G.D.; Davis, R.A.; Ogden, M.W. A Rapid LC-MS-MS Method for the Determination of Nicotine and Cotinine in Serum and Saliva Samples from Smokers: Validation and Comparison with a Radioimmunoassay Method. J. Chromatogr. Sci. 2005, 43, 133–140.
- Lewis, S.J.; Cherry, N.M.; Mcl. Niven, R.; Barber, P.V.; Wilde, K.; Povey, A.C. Cotinine levels and self-reported smoking status in patients attending a bronchoscopy clinic. Biomarkers 2003, 8, 218–228.
- Shin, H.-S.; Kim, J.-G.; Shin, Y.-J.; Jee, S.H. Sensitive and simple method for the determination of nicotine and cotinine in human urine, plasma and saliva by gas chromatography–mass spectrometry. J. Chromatogr. B 2002, 769, 177–183.
- Vartiainen, E. Validation of self reported smoking by serum cotinine measurement in a community-based study. J. Epidemiol. Community Heal. 2002, 56, 167–170.
- Dhar, P. Measuring tobacco smoke exposure: Quantifying nicotine/cotinine concentration in biological samples by colorimetry, chromatography and immunoassay methods. J. Pharm. Biomed. Anal. 2004, 35, 155–168.
- Benowitz, N.L.; Bernert, J.T.; Caraballo, R.S.; Holiday, D.B.; Wang, J. Optimal Serum Cotinine Levels for Distinguishing Cigarette Smokers and Nonsmokers Within Different Racial/Ethnic Groups in the United States Between 1999 and 2004. Am. J. Epidemiol. 2009, 169, 236–248.
- Benowitz, N.L.; Dains, K.M.; Dempsey, D.; Wilson, M.; Jacob, P. Racial Differences in the Relationship Between Number of Cigarettes Smoked and Nicotine and Carcinogen Exposure. Nicotine Tob. Res. 2011, 13, 772–783.
- St.Helen, G.; Goniewicz, M.L.; Dempsey, D.; Wilson, M.; Jacob, P.; Benowitz, N.L. Exposure and Kinetics of Polycyclic Aromatic Hydrocarbons (PAHs) in Cigarette Smokers. Chem. Res. Toxicol. 2012, 25, 952–964.
- Jacob, P., III; Yu, L.; Duan, M.; Ramos, L.; Yturralde, O.; Benowitz, N.L. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography–tandem mass spectrometry: Biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2. J. Chromatogr. B 2011, 879, 267–276.
- Hukkanen, J.; Jacob, P.; Benowitz, N.L. Metabolism and Disposition Kinetics of Nicotine. Pharmacol. Rev. 2005, 57, 79–115.
- Jacob, P.; Hatsukami, D.; Severson, H.; Hall, S.; Yu, L.; Benowitz, N.L. Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 1668–1673.
- Scherer, G. Carboxyhemoglobin and thiocyanate as biomarkers of exposure to carbon monoxide and hydrogen cyanide in tobacco smoke. Exp. Toxicol. Pathol. 2006, 58, 101–124.
- Sandberg, A.; Sköld, C.M.; Grunewald, J.; Eklund, A.; Wheelock, Å.M. Assessing Recent Smoking Status by Measuring Exhaled Carbon Monoxide Levels. PLoS ONE 2011, 6, e28864.
- Kavvadias, D.; Scherer, G.; Cheung, F.; Errington, G.; Shepperd, J.; McEwan, M. Determination of tobacco-specific N -nitrosamines in urine of smokers and non-smokers. Biomarkers 2009, 14, 547–553.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 1–538.
- Shah, K.A.; Karnes, H.T. A review of the analysis of tobacco-specific nitrosamines in biological matrices. Crit. Rev.Toxicol. 2010, 40, 305–327.
- Stepanov, I. Tobacco-Specific Nitrosamines and Their Pyridine-N-glucuronides in the Urine of Smokers and Smokeless Tobacco Users. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 885–891.
- Goniewicz, M.L.; Havel, C.M.; Peng, M.W.; Jacob, P.; Dempsey, D.; Yu, L.; Zielinska-Danch, W.; Koszowski, B.; Czogala, J.; Sobczak, A.; et al. Elimination Kinetics of the Tobacco-Specific Biomarker and Lung Carcinogen 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 3421–3425.
- Hecht, S.S.; Carmella, S.G.; Chen, M.; Dor Koch, J.F.; Miller, A.T.; Murphy, S.E.; Jensen, J.A.; Zimmerman, C.L.; Hatsukami, D.K. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999, 59, 590–596.
- Carmella, S.G.; Ming, X.; Olvera, N.; Brookmeyer, C.; Yoder, A.; Hecht, S.S. High throughput liquid and gas chromatography-tandem mass spectrometry assays for tobacco-specific nitrosamine and polycyclic aromatic hydrocarbon metabolites associated with lung cancer in smokers. Chem. Res. Toxicol. 2013, 26, 1209–1217.
- Vogel, R.I.; Carmella, S.G.; Stepanov, I.; Hatsukami, D.K.; Hecht, S.S. The ratio of a urinary tobacco-specific lung carcinogen metabolite to cotinine is significantly higher in passive than in active smokers. Biomarkers 2011, 16, 491–497.
- Hecht, S.S. 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol and its Glucuronides in the Urine of Infants Exposed to Environmental Tobacco Smoke. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 988–992.
- Jensen, J.A.; Schillo, B.A.; Moilanen, M.M.; Lindgren, B.R.; Murphy, S.; Carmella, S.; Hecht, S.S.; Hatsukami, D.K. Tobacco Smoke Exposure in Nonsmoking Hospitality Workers before and after a State Smoking Ban. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 1016–1021.
- Yuan, J.-M.; Knezevich, A.D.; Wang, R.; Gao, Y.-T.; Hecht, S.S.; Stepanov, I. Urinary levels of the tobacco-specific carcinogen N’-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis 2011, 32, 1366–1371.
- Li, Z.; Sandau, C.D.; Romanoff, L.C.; Caudill, S.P.; Sjodin, A.; Needham, L.L.; Patterson, D.G. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ. Res. 2008, 107, 320–331.
- Hecht, S.S.; Chen, M.; Yagi, H.; Jerina, D.M.; Carmella, S.G. r-1,t-2,3,c-4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene in human urine: A potential biomarker for assessing polycyclic aromatic hydrocarbon metabolic activation. Cancer Epidemiol. Biomarkers Prev. 2003, 12, 1501–1508.
- Suwan-ampai, P.; Navas-Acien, A.; Strickland, P.T.; Agnew, J. Involuntary Tobacco Smoke Exposure and Urinary Levels of Polycyclic Aromatic Hydrocarbons in the United States, 1999 to 2002. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 884–893.
- Zhong, Y.; Carmella, S.G.; Upadhyaya, P.; Hochalter, J.B.; Rauch, D.; Oliver, A.; Jensen, J.; Hatsukami, D.; Wang, J.; Zimmerman, C.; et al. Immediate Consequences of Cigarette Smoking: Rapid Formation of Polycyclic Aromatic Hydrocarbon Diol Epoxides. Chem. Res. Toxicol. 2011, 24, 246–252.
- Dong, J.-Z.; Moldoveanu, S.C. Gas chromatography–mass spectrometry of carbonyl compounds in cigarette mainstream smoke after derivatization with 2,4-dinitrophenylhydrazine. J. Chromatogr. A 2004, 1027, 25–35.
- Ashley, D.L.; Bonin, M.A.; Cardinali, F.L.; McCraw, J.M.; Wooten, J.V. Measurement of volatile organic compounds in human blood. Environ. Health Perspect. 1996, 104, 871–877.
- Yuan, J.-M.; Gao, Y.-T.; Wang, R.; Chen, M.; Carmella, S.G.; Hecht, S.S. Urinary levels of volatile organic carcinogen and toxicant biomarkers in relation to lung cancer development in smokers. Carcinogenesis 2012, 33, 804–809.
- Alwis, K.U.; Blount, B.C.; Britt, A.S.; Patel, D.; Ashley, D.L. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal. Chim. Acta 2012, 750, 152–160.
- Riedel, K.; Scherer, G.; Engl, J.; Hagedorn, H.-W.; Tricker, A.R. Determination of Three Carcinogenic Aromatic Amines in Urine of Smokers and Nonsmokers. J. Anal. Toxicol. 2006, 30, 187–195.
- Turesky, R.J.; Le Marchand, L. Metabolism and Biomarkers of Heterocyclic Aromatic Amines in Molecular Epidemiology Studies: Lessons Learned from Aromatic Amines. Chem. Res. Toxicol. 2011, 24, 1169–1214.
- Marano, K.M.; Naufal, Z.S.; Kathman, S.J.; Bodnar, J.A.; Borgerding, M.F.; Garner, C.D.; Wilson, C.L. Cadmium exposure and tobacco consumption: Biomarkers and risk assessment. Regul. Toxicol. Pharmacol. 2012, 64, 243–252.
- Amzal, B.; Julin, B.; Vahter, M.; Wolk, A.; Johanson, G.; Åkesson, A. Population Toxicokinetic Modeling of Cadmium for Health Risk Assessment. Environ. Health Perspect. 2009, 117, 1293–1301.
- Jain, N.B.; Potula, V.; Schwartz, J.; Vokonas, P.S.; Sparrow, D.; Wright, R.O.; Nie, H.; Hu, H. Lead Levels and Ischemic Heart Disease in a Prospective Study of Middle-Aged and Elderly Men: The VA Normative Aging Study. Environ. Health Perspect. 2007, 115, 871–875.
- Glatz, Z.; Nováková, S.; Štěrbová, H. Analysis of thiocyanate in biological fluids by capillary zone electrophoresis. J. Chromatogr. A 2001, 916, 273–277.





