
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pierre-Yves Lozach | + 2380 word(s) | 2380 | 2021-03-11 04:28:04 | | | |
| 2 | Camila Xu | Meta information modification | 2380 | 2021-04-16 12:06:44 | | | | |
| 3 | Camila Xu | Meta information modification | 2380 | 2021-04-16 12:07:09 | | |
Video Upload Options
Phenuiviridae in the Bunyavirales order is a large family of arthropod-borne RNA viruses that comprises 19 genera. Phenuiviruses are unique in the sense that they infect a large spectrum of hosts, including humans and other vertebrates as well as invertebrates and plants. They usually spread to vertebrate hosts by blood-feeding arthropod vectors, such as sandflies and ticks and more rarely mosquitoes.
1. Introduction
With over 100 identified members and a wide geographical distribution, phenuiviruses represent a global threat to human public health and livestock and agricultural productivity [1]. Many members cause serious diseases in humans and domestic animals. For instance, in severe cases, patients infected with Dabie virus (DABV) develop thrombocytopenia and hemorrhagic fever, resulting in a case-fatality rate of 10–30% [2]. Toscana virus (TOSV) can cause meningoencephalitis in humans, and Rift Valley fever virus (RVFV) acute hepatitis and fetal malformations in several mammalian hosts including cattle [3][4][5]. No vaccines or treatments against phenuiviruses are currently approved for human use.
Human activity, international trade, deforestation, and global warming are many of the factors favoring the spread of arthropod vectors to new regions as well as the viruses they carry. Many examples show that phenuiviral infections are no longer limited to tropical or developing countries. DABV, previously known as severe fever with thrombocytopenia syndrome virus (SFTSV), and Heartland virus (HRTV), are two closely related members in the Bandavirus genus transmitted by the Haemaphysalis longicornis and Amblyomma americium ticks, respectively [2][6][7]. DABV emerged in Henan and Hubei provinces, China, and HRTV in Missouri, USA, one decade ago [2][8][9][10][11]. The emergence of these two human pathogens led to a renewed interest in the study of Uukuniemi virus (UUKV), an uukuvirus originally isolated from the Ixodes ricinus tick in the village Uukuniemi, Finland, in the early 1960s [12]. UUKV is not associated with any disease in humans. It is a validated biosafety level (BSL)-2 surrogate that enabled major advances in many aspects of the cell life cycle of phenuiviruses with a higher biosafety classification, such as receptors, cell entry, and assembly [13][14][15].
The Phlebovirus genus includes members that also illustrate the need to seriously take phenuiviruses as potential agents of emerging and reemerging diseases. The phlebovirus Toscana was first isolated in 1971 from Phlebotomus perniciosus and Phlebotomus perfiliewi sandflies in the Tuscany region, Italy [16]. The virus is reemerging in the Mediterranean basin, as shown by the increasing number of outbreaks and reported cases in Spain, south of France, Italy, and Greece [3][17][18]. TOSV is currently the primary cause of arboviral diseases in humans in southern Europe during the summer [3][19]. Another example is RVFV, a phlebovirus transmitted essentially by Aedes and Culex mosquitoes [20]. The virus was discovered in the Great Rift Valley, Kenya, in 1930 [21] and has since spread across Africa and beyond in the 1970s to reach Madagascar and, more recently, Saudi Arabia and Turkey [4][5][22]. RVFV now presents the risk of introduction into southern Europe and Asia. It is listed as high-priority pathogens by the World Health Organization, for which there is an urgent need to develop diagnostics, therapies, and research [23]. The virus is in addition considered as a potential biological weapon by the US army. Overall, it is apparent that phleboviruses and other phenuiviruses are potential agents of emerging and reemerging diseases.
In 2016, we contributed to the first special issue on bunyaviruses from Viruses with a review on early bunyavirus-host cell interactions [24]. Since then, important discoveries have been made in this field. Taxonomic classification has evolved considerably to better reflect the variety of bunyavirus members, vectors, hosts, and diseases [25][26][27]. In this review, we therefore focus on the novel family Phenuiviridae. We address the most current knowledge and advances regarding the entry process of phenuiviruses, from virus binding to penetration into the cytosol. Most of the available information on how phenuiviruses enter cells comes from studies on only a few species, mainly banda-, phlebo-, and uukuviruses infecting animals (Table 1). Nothing is known about the penetration mechanisms in plant cells, and not much regarding penetration in arthropod cells. Hence, the discussion is limited to animal phenuiviruses and mammalian host cells. For information on phenuiviruses and their arthropod vectors, we recommend the following reviews [1][20][28][29][30].
Table 1. Classification within the family of Phenuiviridae [25][26].
| Genus | Species | Representative Species |
|---|---|---|
| Bandavirus | 7 | Dabie bandavirus [previously named severe fever with thrombocytopenia syndrome virus (SFTSV)], Heartland bandavirus (HRTV) |
| Beidivirus | 1 | Dipteran beidivirus |
| Cugovirus | 2 | Citrus coguvirus |
| Entovirus | 1 | Entoleuca entovirus |
| Goukovirus | 3 | Gouleako goukovirus |
| Horwuvirus | 1 | Horsefly horwuvirus |
| Hudivirus | 1 | Dipteran hudivirus |
| Hudovirus | 1 | Lepidopteran hudovirus |
| Ixovirus | 3 | Blackleg ixovirus |
| Laulavirus | 1 | Laurel Lake laulavirus |
| Lentinuvirus | 1 | Lentinula lentinuvirus |
| Mobuvirus | 1 | Mothra mobuvirus |
| Phasivirus | 5 | Badu phasivirus |
| Phlebovirus | 60 | Rift Valley fever phlebovirus (RVFV), Punta Toro phlebovirus (PTV), Sandfly fever Sicilian phlebovirus (SFSV), Sandfly fever Naples phlebovirus (SFNV), Toscana phlebovirus (TOSV) |
| Pidchovirus | 1 | Pidgey pidchovirus |
| Rubodvirus | 2 | Apple rubodvirus 1 |
| Tenuivirus | 8 | Rice stripe tenuivirus |
| Uukuvirus | 17 | Uukuniemi uukuvirus (UUKV) |
| Wenrivirus | 1 | Shrimp wenrivirus |
The genera and species highlighted in this review appear in bold and underlined, respectively.
2. Genomic and Structural Organization of Phenuiviral Particles
Phenuiviruses are enveloped by a lipid bilayer with a trisegmented single-stranded RNA genome, mainly of negative-sense polarity (Figure 1a) [1]. The viral RNA replicates exclusively in the cytosol and encodes at least four structural proteins [1]. The longest segment of genomic RNA, the L segment, encodes an RNA-dependent RNA polymerase, which is necessary to initiate virus replication after release of the viral genome into the cytosol. The medium segment, M, encodes a precursor polypeptide for the two envelope glycoproteins GN and GC (Figure 1b) [31][32]. Phenuiviruses also encode one or two nonstructural proteins, i.e., NSs and NSm. With the vector of transmission, the presence of an NSm protein appears as one of the main distinctions between tick- and dipteran-borne phenuiviruses [1]. Although phenuiviruses are quite distinct from each other, analysis of amino-acid sequences revealed that the structural proteins of phenuiviruses show a rather high similarity in general, up to 30–40%, compared to the nonstructural proteins, about 10–15% [1][2][33]. So far, none of the nonstructural proteins has been shown to be involved in virion cell entry and will therefore not be discussed here.
Proteolytic cleavage of the M precursor takes place in the endoplasmic reticulum or Golgi apparatus, where the virions assemble and acquire their lipid envelope. The exact location and mechanisms of GN and GC glycoprotein maturation and viral particle budding may differ between phenuiviral species and cell types. They often remain to be elucidated. The smallest segment S encodes the nucleoprotein N, which, together with polymerase and viral RNA, constitute the ribonucleoproteins found inside virions (Figure 1a) [1]. Phenuiviruses do not have a classical matrix or a rigid internal structure. The N protein therefore plays an important role in protecting the genetic information of these viruses. In recent years, the crystal structure of the N protein has been resolved for several phenuiviruses, providing new information on the mechanisms of ribonucleoprotein assembly [34].
On the surface of viral particles, the two envelope glycoproteins GN and GC are responsible for virion binding to the target cells and then acid-activated penetration into the cytosol [35]. Electron micrographs show that phenuiviral particles are globally spherical and heterogeneous in size, with a diameter ranging from 80 to 160 nm [1]. Electron cryo-tomography analyses of RVFV and UUKV revealed that the most regular particles have protrusions on their surface forming an icosahedral lattice with an atypical T = 12 triangulation (Figure 1c) [36][37][38].
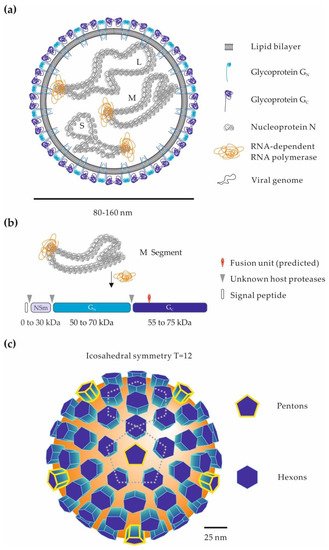
Figure 1. Phenuiviral particles and the glycoproteins GN and GC. (a) Schematic representation of a phenuiviral particle. The three viral genomic RNA segments are named according to their size: S (small), M (medium), and L (large). (b) Proteolytic processing of the phenuivirus M polypeptide precursor. The M precursor and the glycoproteins GN and GC can vary greatly among phenuiviral species. In addition to GN and GC, some phenuiviruses encode an additional nonstructural protein, NSm. Arrow heads indicate the cleavage sites by host cell proteases within the precursor. The position of the fusion peptide is given based on the crystal structure of the Rift Valley fever virus (RVFV) glycoprotein GC [39]. (c) Arrangement of GN and GC glycoproteins on the surface of phenuiviral particles. Electron cryo-tomography analysis of RVFV [36][37] and Uukuniemi virus (UUKV) [38] viral particles shows an icosahedral lattice with an atypical T = 12 triangulation.
3. Cellular Receptors for Phenuiviruses in Mammalian Hosts
To initiate infection, viruses must first attach to target cells and then obtain access to the intracellular environment to replicate. The first step is highly dependent on the presence of surface receptors, such as proteins, lipids, or carbohydrates, to which viral particles bind [40]. Some of these receptors by themselves are capable of triggering the entry of viral particles into the cell. Others limit the free diffusion of virions and/or promote interactions with secondary surface molecules that are responsible for the entry of viral particles [40]. When viruses depend on numerous cellular surface factors for binding and entry, the primary receptor is often referred to as an attachment factor, and the secondary receptors are referred to as coreceptors. Only a few attachment factors and receptors are known for phenuiviruses, and very often, their role in cell entry remains to be discovered (Table 2).
Table 2. Receptors for phenuiviruses in mammalian hosts.
DABV, Dabie virus; DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin; L-SIGN, liver-specific intercellular adhesion molecule-3-grabbing non-integrin; LSECtin, liver and lymph node sinusoidal endothelial cell C-type lectin; NMMHC-IIA, nonmuscle myosin heavy chain IIA; ppDABV, rhabdovirus pseudotyped with the glycoproteins GN and GC of Dabie virus; PTV, Punta Toro virus; RVFV, Rift Valley fever virus; TOSV, Toscana virus; UUKV, Uukuniemi virus.
Interactions between viruses and receptors are often specific and multivalent. Binding to several molecules of the same receptor, concentrating within microdomains, may increase the avidity of low-affinity interactions [40]. For example, glycoproteins and glycolipids present in the extracellular matrix of most mammalian cells are highly polar structures. Despite low affinity interactions, due to their electrostatic nature, these structures serve as attachment factors for many viruses, including phenuiviruses. Glycosaminoglycans (GAGs), such as heparan sulfates, have been shown to facilitate RVFV and TOSV infections (Table 2) [48,49,50]. Infection with these two viruses is greatly reduced in the presence of heparin, a competitor of GAGs on the cell surface. In addition, enzymatic digestion of heparan sulfate on the cell surface prior to exposure to these viruses produces similar results. Finally, cells deficient in heparan sulfate synthesis show a reduced sensitivity to RVFV [48,49]. Interestingly, the glycoproteins of an RVFV strain amplified in cell culture and used in one of these studies do not differ in basic amino acid composition from those on viruses isolated from infected animals [48]. This result suggests that the dependence of RVFV on heparan sulfates does not result from the adaptation of the virus to cell culture. However, the fact that some cell types remain permissive to infection even without GAGs indicates that phenuiviruses may use alternative receptors to enter cells.
A number of studies have indicated that many phenuiviruses can use the human C-type lectin DC-SIGN (also known as CD209) to target and infect dendritic cells (DCs) in the dermis (Table 2) [41][42][43][44][45]. In the presence of neutralizing antibodies, dermal DCs become resistant to infection with RVFV and UUKV [41]. The expression of DC-SIGN at the plasma membrane renders cells that are originally not permissive highly sensitive to several phleboviruses, including RVFV, UUKV, TOSV and Punta Toro virus [41]. The list of phleboviruses described to interact with DC-SIGN has since been extended to include bandaviruses. Recent studies demonstrate, among others, that lectin facilitates infection by DABV or rhabdoviral particles pseudotyped with DABV glycoproteins [42][43][44]. DC-SIGN represents an interesting molecular candidate for linking arthropod-derived viruses to the initial infection in the skin of the human host. This immune receptor is (i) mainly expressed on the surface of immature dermal DCs present in the anatomical site of transmission of these viruses and (ii) specialized in the capture of foreign antigens rich in mannose residues, such as the glycoproteins found in viruses produced from insects [35][41]. For these reasons, interactions between DC-SIGN and arthropod-borne pathogens are considered the most relevant, although several studies have suggested a role of lectin in infection by various microbes not propagated by arthropods [52].
Phenuiviruses generally possess numerous N-glycosylations on the particle surface, distributed between the envelope glycoproteins GN and GC [32][53]. For instance, RVFV has one glycosylation site in GN and four in GC. RVFV appears to rely on the N-glycan sites N438 and N1077 in GN and GC, respectively, for DC-SIGN-mediated infection [45]. It is tempting to draw a parallel with dengue virus in the Flaviviridae family and its envelope glycoprotein E. The engagement of multiple E molecules by homotetramers of DC-SIGN explains the high avidity of the interaction between the lectin and the viral particles [54]. The same is probably true for the interactions between DC-SIGN and phenuiviruses. In addition, the human C-type lectins L-SIGN and LSECtin, both closely related to DC-SIGN but expressed on the surface of the liver endothelium [55], are also used as receptors by several phenuiviruses, including RVFV, TOSV, UUKV, and DABV (Table 2) [42][43][46][47]. It is possible that L-SIGN and LSECtin, by acting as receptors on the surface of the liver endothelium, contribute to the hepatic tropism of some phenuiviruses.
Nonmuscle myosin heavy chain type IIA (NMMHC-IIA) has been proposed to act as an attachment factor for DABV (Table 2) [51]. This factor has been identified using a strategy combining coimmunoprecipitation and mass spectrometry analysis, using a fragment of the GN ectodomain as bait. NMMHC-IIA usually has an intracellular localization but, in some cases, seems to be able to reach the outer surface of the plasma membrane, notably in human umbilical vein endothelial cells and Vero cells [51]. Silencing of the gene coding for NMMHC-IIA by small interfering RNAs (siRNAs) led to a significant decrease in infection by DABV. It is likely that NMMHC-IIA is not the only cellular receptor used by DABV. HeLa cells do not express this gene and are sensitive to infection [51]. However, ectopic expression of NMMHC-IIA resulted in an increased sensitivity of HeLa cells to DABV. It is still not known whether NMMHC-IIA serves as an entry receptor or simply as an attachment factor.
More recently, DABV has been observed in secreted vesicles exhibiting CD63, a marker of extracellular vesicles (Table 2) [56]. It appeared that virions within these vesicles are efficiently delivered to neighboring uninfected cells. This work was the first demonstration of the hijacking of the exocytosis machinery by a phenuivirus to ensure its transmission to surrounding cells in a receptor-independent fashion.
References
- Elliott, R.M.; Brennan, B. Emerging phleboviruses. Curr. Opin. Virol. 2014, 5, 50–57.
- Yu, X.-J.; Liang, M.-F.; Zhang, S.-Y.; Liu, Y.; Li, J.-D.; Sun, Y.-L.; Zhang, L.; Zhang, Q.-F.; Popov, V.L.; Li, C.; et al. Fever with Thrombocytopenia Associated with a Novel Bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532.
- Moriconi, M.; Rugna, G.; Calzolari, M.; Bellini, R.; Albieri, A.; Angelini, P.; Cagarelli, R.; Landini, M.P.; Charrel, R.N.; Varani, S. Phlebotomine sand fly–borne pathogens in the Mediterranean Basin: Human leishmaniasis and phlebovirus infections. PLoS Negl. Trop. Dis. 2017, 11, e0005660.
- Wright, D.; Kortekaas, J.; Bowden, T.A.; Warimwe, G.M. Rift Valley fever: Biology and epidemiology. J. Gen. Virol. 2019, 100, 1187–1199.
- Léger, P.; Nachman, E.; Richter, K.; Tamietti, C.; Koch, J.; Burk, R.; Kummer, S.; Xin, Q.; Stanifer, M.; Bouloy, M.; et al. NSs amyloid formation is associated with the virulence of Rift Valley fever virus in mice. Nat. Commun. 2020, 11, 1–19.
- Zhang, Y.-Z.; Zhou, D.-J.; Qin, X.-C.; Tian, J.-H.; Xiong, Y.; Wang, J.-B.; Chen, X.-P.; Gao, D.-Y.; He, Y.-W.; Jin, D.; et al. The Ecology, Genetic Diversity, and Phylogeny of Huaiyangshan Virus in China. J. Virol. 2011, 86, 2864–2868.
- Savage, H.M.; Godsey, J.M.S.; Lambert, A.; Panella, N.A.; Burkhalter, K.L.; Harmon, J.R.; Lash, R.R.; Ashley, D.C.; Nicholson, W.L. First Detection of Heartland Virus (Bunyaviridae: Phlebovirus) from Field Collected Arthropods. Am. J. Trop. Med. Hyg. 2013, 89, 445–452.
- McMullan, L.K.; Folk, S.M.; Kelly, A.J.; MacNeil, A.; Goldsmith, C.S.; Metcalfe, M.G.; Batten, B.C.; Albariño, C.G.; Zaki, S.R.; Rollin, P.E.; et al. A New Phlebovirus Associated with Severe Febrile Illness in Missouri. N. Engl. J. Med. 2012, 367, 834–841.
- Liu, Q.; He, B.; Huang, S.-Y.; Wei, F.; Zhu, X.-Q. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect. Dis. 2014, 14, 763–772.
- Muehlenbachs, A.; Fata, C.R.; Lambert, A.J.; Paddock, C.D.; Velez, J.O.; Blau, D.M.; Staples, J.E.; Karlekar, M.B.; Bhatnagar, J.; Nasci, R.S.; et al. Heartland Virus-Associated Death in Tennessee. Clin. Infect. Dis. 2014, 59, 845–850.
- Wang, J.; Selleck, P.; Yu, M.; Ha, W.; Rootes, C.; Gales, R.; Wise, T.; Crameri, S.; Chen, H.; Broz, I.; et al. Novel Phlebovirus with Zoonotic Potential Isolated from Ticks, Australia. Emerg. Infect. Dis. 2014, 20, 1040–1043.
- Oker-Blom, N.; Salminen, A.; Brummer-Korvenkontio, M.; Kaeaeriaeinen, L.; Weckstroem, P. Isolation of Some Viruses Other than Typical Tick-Borne Encephalitis Viruses from Ixodes Ricinus Ticks in Finland. Ann. Med. Exp. Biol. Pennine 1964, 42, 109–112.
- Mazelier, M.; Rouxel, R.N.; Zumstein, M.; Mancini, R.; Bell-Sakyi, L.; Lozach, P.-Y. Uukuniemi Virus as a Tick-Borne Virus Model. J. Virol. 2016, 90, 6784–6798.
- Uckeley, Z.M.; Moeller, R.; Kühn, L.I.; Nilsson, E.; Robens, C.; Lasswitz, L.; Lindqvist, R.; Lenman, A.; Passos, V.; Voss, Y.; et al. Quantitative Proteomics of Uukuniemi Virus-host Cell Interactions Reveals GBF1 as Proviral Host Factor for Phleboviruses. Mol. Cell Proteom. 2019, 18, 2401–2417.
- Rezelj, V.V.; Överby, A.K.; Elliott, R.M. Generation of Mutant Uukuniemi Viruses Lacking the Nonstructural Protein NSs by Reverse Genetics Indicates that NSs is a Weak Interferon Antagonist. J. Virol. 2015, 89, 4849–4856.
- Verani, P.; Ciufolini, M.G.; Nicoletti, L.; Balducci, M.; Sabatinelli, G.; Coluzzi, M.; Paci, P.; Amaducci, L. Ecological and epidemiological studies of Toscana virus, an arbovirus isolated from Phlebotomus. Ann. Ist. Super. Sanità 1982, 18, 397–399.
- Charrel, R.; Berenger, J.-M.; Laroche, M.; Ayhan, N.; Bitam, I.; Delaunay, P.; Parola, P. Neglected vector-borne bacterial diseases and arboviruses in the Mediterranean area. New Microbes New Infect. 2018, 26, S31–S36.
- Ayhan, N.; Charrel, R. An update on Toscana virus distribution, genetics, medical and diagnostic aspects. Clin. Microbiol. Infect. 2020, 26, 1017–1023.
- Woelfl, F.; Léger, P.; Oreshkova, N.; Pahmeier, F.; Windhaber, S.; Koch, J.; Stanifer, M.; Sosa, G.R.; Uckeley, Z.M.; Rey, F.A.; et al. Novel Toscana Virus Reverse Genetics System Establishes NSs as an Antagonist of Type I Interferon Responses. Viruses 2020, 12, 400.
- Lumley, S.; Horton, D.L.; Hernandez-Triana, L.L.M.; Johnson, N.; Fooks, A.R.; Hewson, R. Rift Valley fever virus: Strategies for maintenance, survival and vertical transmission in mosquitoes. J. Gen. Virol. 2017, 98, 875–887.
- Daubney, R.; Hudson, J.R. Enzootic Hepatitis or Rift Valley Fever. An Undescribed Virus Disease of Sheep, Cattle and Man from East Africa. J. Pathol. Bacteriol. 1931, 34, 545–579.
- Gür, S.; Kale, M.; Erol, N.; Yapici, O.; Mamak, N.; Yavru, S. The first serological evidence for Rift Valley fever infection in the camel, goitered gazelle and Anatolian water buffaloes in Turkey. Trop. Anim. Health Prod. 2017, 49, 1531–1535.
- World Health Organization. Blueprint for R&D Preparedness and Response to Public Health Emergencies Due to Highly Infectious Pathogens; World Health Organization: Geneva, Switzerland, 2015.
- Albornoz, A.; Hoffmann, A.B.; Lozach, P.-Y.; Tischler, N.D. Early Bunyavirus-Host Cell Interactions. Viruses 2016, 8, 143.
- ICTV. 10th Report of the International Committee for Taxonomy of Viruses. 2017. Available online: (accessed on 15 January 2021).
- Marklewitz, M.; Palacios, G.; Ebihara, H.; Kuhn, J.H.; Junglen, S. Create Four New Genera, Create Seventy-Nine New Species, Rename/Move Seven Species, Rename/Move Three Genera and Abolish One Genus in the Family Phenuiviridae. In Order Bunyavirales; ICTV: Berlin, Germany, 2019.
- Maes, P.; Adkins, S.; Alkhovsky, S.V.; Avšič-Županc, T.; Ballinger, M.J.; Bente, D.A.; Beer, M.; Bergeron, É.; Blair, C.D.; Briese, T.; et al. Taxonomy of the order Bunyavirales: Second update 2018. Arch. Virol. 2019, 164, 927–941.
- Brault, A.C.; Savage, H.M.; Duggal, N.K.; Eisen, R.J.; Staples, J.E. Heartland Virus Epidemiology, Vector Association, and Disease Potential. Viruses 2018, 10, 498.
- Horne, K.M.; VanLandingham, D.L. Bunyavirus-Vector Interactions. Viruses 2014, 6, 4373–4397.
- Ayhan, N.; Prudhomme, J.; Laroche, L.; Bañuls, A.-L.; Charrel, R.N. Broader Geographical Distribution of Toscana Virus in the Mediterranean Region Suggests the Existence of Larger Varieties of Sand Fly Vectors. Microorganisms 2020, 8, 114.
- Spiegel, M.; Plegge, T.; Pöhlmann, S. The Role of Phlebovirus Glycoproteins in Viral Entry, Assembly and Release. Viruses 2016, 8, 202.
- Guardado-Calvo, P.; Rey, F.A. The Envelope Proteins of the Bunyavirales. Adv. Appl. Microbiol. 2017, 98, 83–118.
- Xu, B.; Liu, L.; Huang, X.; Ma, H.; Zhang, Y.; Du, Y.; Wang, P.; Tang, X.; Wang, H.; Kang, K.; et al. Metagenomic Analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a New Bunyavirus. PLoS Pathog. 2011, 7, e1002369.
- Hornak, K.E.; Lanchy, J.-M.; Lodmell, J.S. RNA Encapsidation and Packaging in the Phleboviruses. Viruses 2016, 8, 194.
- Léger, P.; Lozach, P.-Y. Bunyaviruses: From transmission by arthropods to virus entry into the mammalian host first-target cells. Future Virol. 2015, 10, 859–881.
- Freiberg, A.N.; Sherman, M.B.; Morais, M.C.; Holbrook, M.R.; Watowich, S.J. Three-Dimensional Organization of Rift Valley Fever Virus Revealed by Cryoelectron Tomography. J. Virol. 2008, 82, 10341–10348.
- Huiskonen, J.T.; Överby, A.K.; Weber, F.; Grünewald, K. Electron Cryo-Microscopy and Single-Particle Averaging of Rift Valley Fever Virus: Evidence for GN-GC Glycoprotein Heterodimers. J. Virol. 2009, 83, 3762–3769.
- Overby, A.K.; Pettersson, R.F.; Grünewald, K.; Huiskonen, J.T. Insights into bunyavirus architecture from electron cryo-tomography of Uukuniemi virus. Proc. Natl. Acad. Sci. USA 2008, 105, 2375–2379.
- Dessau, M.; Modis, Y. Crystal structure of glycoprotein C from Rift Valley fever virus. Proc. Natl. Acad. Sci. USA 2013, 110, 1696–1701.
- Boulant, S.; Stanifer, M.; Lozach, P.-Y. Dynamics of Virus-Receptor Interactions in Virus Binding, Signaling, and Endocytosis. Viruses 2015, 7, 2794–2815.
- Lozach, P.-Y.; Kühbacher, A.; Meier, R.; Mancini, R.; Bitto, D.; Bouloy, M.; Helenius, A. DC-SIGN as a receptor for phlebo-viruses. Cell Host Microbe 2011, 10, 75–88.
- Hofmann, H.; Li, X.; Zhang, X.; Liu, W.; Kühl, A.; Kaup, F.; Soldan, S.S.; González-Scarano, F.; Weber, F.; He, Y.; et al. Severe fever with thrombocytopenia virus glycoproteins are targeted by neutralizing antibodies and can use DC-SIGN as a receptor for pH-dependent entry into human and animal cell lines. J. Virol. 2013, 87, 4384–4394.
- Tani, H.; Shimojima, M.; Fukushi, S.; Yoshikawa, T.; Fukuma, A.; Taniguchi, S.; Morikawa, S.; Saijo, M. Characterization of Glycoprotein-Mediated Entry of Severe Fever with Thrombocytopenia Syndrome Virus. J. Virol. 2016, 90, 5292–5301.
- Suzuki, T.; Sato, Y.; Sano, K.; Arashiro, T.; Katano, H.; Nakajima, N.; Shimojima, M.; Kataoka, M.; Takahashi, K.; Wada, Y.; et al. Severe fever with thrombocytopenia syndrome virus targets B cells in lethal human infections. J. Clin. Investig. 2020, 130, 799–812.
- Phoenix, I.; Nishiyama, S.; Lokugamage, N.; Hill, T.E.; Huante, M.B.; Slack, O.A.; Carpio, V.H.; Freiberg, A.N.; Ikegami, T. N-Glycans on the Rift Valley Fever Virus Envelope Glycoproteins Gn and Gc Redundantly Support Viral Infection via DC-SIGN. Viruses 2016, 8, 149.
- Shimojima, M.; Sugimoto, S.; Taniguchi, S.; Yoshikawa, T.; Kurosu, T.; Saijo, M. Efficient functional screening of a cellular cDNA library to identify severe fever with thrombocytopenia syndrome virus entry factors. Sci. Rep. 2020, 10, 1–12.
- Léger, P.; Tetard, M.; Youness, B.; Cordes, N.; Rouxel, R.N.; Flamand, M.; Lozach, P.-Y. Differential Use of the C-Type Lectins L-SIGN and DC-SIGN for Phlebovirus Endocytosis. Traffic 2016, 17, 639–656.
- De Boer, S.M.; Kortekaas, J.; De Haan, C.A.M.; Rottier, P.J.M.; Moormann, R.J.M.; Bosch, B.J. Heparan Sulfate Facilitates Rift Valley Fever Virus Entry into the Cell. J. Virol. 2012, 86, 13767–13771.
- Riblett, A.M.; Blomen, V.A.; Jae, L.T.; Altamura, L.A.; Doms, R.W.; Brummelkamp, T.R.; Wojcechowskyj, J.A. A Haploid Genetic Screen Identifies Heparan Sulfate Proteoglycans Supporting Rift Valley Fever Virus Infection. J. Virol. 2016, 90, 1414–1423.
- Pietrantoni, A.; Fortuna, C.; Remoli, M.E.; Ciufolini, M.G.; Superti, F. Bovine Lactoferrin Inhibits Toscana Virus Infection by Binding to Heparan Sulphate. Viruses 2015, 7, 480–495.
- Sun, Y.; Qi, Y.; Liu, C.; Gao, W.; Chen, P.; Fu, L.; Peng, B.; Wang, H.; Jing, Z.; Zhong, G.; et al. Nonmuscle Myosin Heavy Chain IIA Is a Critical Factor Contributing to the Efficiency of Early Infection of Severe Fever with Thrombocytopenia Syndrome Virus. J. Virol. 2013, 88, 237–248.
- Švajger, U.; Anderluh, M.; Jeras, M.; Obermajer, N. C-type lectin DC-SIGN: An adhesion, signalling and antigen-uptake molecule that guides dendritic cells in immunity. Cell. Signal. 2010, 22, 1397–1405.
- Halldorsson, S.; Li, S.; Li, M.; Harlos, K.; Bowden, T.A.; Huiskonen, J.T. Shielding and activation of a viral membrane fusion protein. Nat. Commun. 2018, 9, 1–9.
- Pokidysheva, E.; Zhang, Y.; Battisti, A.J.; Bator-Kelly, C.M.; Chipman, P.R.; Xiao, C.; Gregorio, G.G.; Hendrickson, W.A.; Kuhn, R.J.; Rossmann, M.G. Cryo-EM Reconstruction of Dengue Virus in Complex with the Carbohydrate Recognition Domain of DC-SIGN. Cell 2006, 124, 485–493.
- Pustylnikov, S.; Sagar, D.; Jain, P.; Khan, Z.K. Targeting the C-type lectins-mediated host-pathogen interactions with dextran. J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. 2014, 17, 371–392.
- Silvas, J.A.; Popov, V.L.; Paulucci-Holthauzen, A.; Aguilar, P.V. Extracellular Vesicles Mediate Receptor-Independent Transmission of Novel Tick-Borne Bunyavirus. J. Virol. 2015, 90, 873–886.





