| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gabriel Clarke | + 1854 word(s) | 1854 | 2021-03-02 10:50:40 | | | |
| 2 | Vicky Zhou | Meta information modification | 1854 | 2021-03-15 08:19:43 | | |
Video Upload Options
Organ-on-chip devices have provided the pharmaceutical and tissue engineering worlds much hope since they arrived and began to grow in sophistication. However, limitations for their applicability were soon realized as they lacked real-time monitoring and sensing capabilities. The users of these devices relied solely on endpoint analysis for the results of their tests, which created a chasm in the understanding of life between the lab the natural world. However, this gap is being bridged with sensors that are integrated into organ-on-chip devices.
1. Introduction
Organ-on-chip (OOC) devices first emerged into the scientific world around 1990 under the term “miniaturized total chemical analysis systems (µTAS)” [1], and have improved in sophistication, application, and popularity as time has progressed, with their potential being recognized. In the early stages of the development of these devices, they were limited in their application; however, as research and technology advance, so does the potential for these devices [2][3]. Recent efforts have allowed the potential of these devices to integrate sensors, allowing for real-time analysis of the biological processes—a breakthrough for the experimental world [4][5]. As an example of the progress, the Hashemi Lab worked on a sheathing fabrication technique and microfluidic device with optical sensors that could characterize micrometer-sized phytoplankton for gaining insight into global warming and ocean currents [6][7][8]. Microfluidic microfiber fabrication has made tremendous progress over the past few years, and can now serve as a scaffold for neural stem cells [9][10][11]. Microfluidic systems have started to be used in legitimate, helpful applications, such as in the creation of blood and urine assays, a paper-based microfluidic fuel-cell, and to study caffeine transport through the placenta during pregnancy [12][13][14]. Finally, as an affirmation of the promise of this technology, it is being used for early cancer detection by means of liquid biopsy research, and as an aid in disease diagnosis [15][16].
Among all of these applications, drug testing is perhaps the most important. The current drug testing regimen involves many trials and iterative steps to ensure they are safe, taking between two and ten years, and sometimes costing up to two billion dollars [17][18]. The current, and unfortunately well-vetted method for evaluating a new drug involves a fairly defined sequence of steps in which no steps should be omitted; this makes for a rather painstaking and process, and a low percentage of drugs that make it to being administered to a patient [19]. OOC devices with integrated sensors could potentially solve this long and expensive process by simply allowing for the characterization of a drug without many of the intermediate steps [5][20]. The OOC device resembles human organs or organ systems, so with proper analysis of the “organs” through sensor integration, the toxic effects could potentially be measured quickly and relatively inexpensively [21][22]. Within this ability to skip several time and money-consuming steps in the current process, concerns on animal testing, along with the care and expense to use live animals, could be eliminated [23][24].
OOCs can improve current drug testing methods, shifting away from animal testing, and provide more accurate reports of toxicological effects [25]. Another application of the OOCs with integrated sensors lies within the world of tissue engineering. Sensors, as will be outlined below, integrated into some OOCs, can characterize engineered tissue and tissue interactions with different stimulants in a way that improves the function and applicability of these substances [26]. OOCs can properly and promptly give insight into the interaction of the stress and strain of loading dynamics and drug-induced tissue degradation [5][27]. The nature of the OOCs also necessitate further advancement in tissue engineering as more physiologically relevant tissues are sought after to be used in these devices [5][28][29][30].
The methods and results of many current efforts that incorporate sensors within their OOC devices are outlined. Each study has been evaluated based on its advantages and disadvantages to better understand the current state of this rapidly advancing technology. Future opportunities and overcomings are used to conclude the current devices and methods discussed, portraying an updated vision of the potential of these OOC devices, such as in toxicological studies that can replace animal testing and for tissue engineering insight.
2. Application of OOC Devices with Integrated Sensors
These final study outlines highlight very specific applications that fall out of the previous, more broad classifications. Furthermore, the studies show promise for specific resolution and understanding of a particular issue, namely muscular dystrophy, and inflammatory arthritis. These studies serve as a conclusive hope in the sensor integrated organ-on-chip (OOC) world, as they effectively realize much of the potential discussed for this technology as a while.
Firstly, Electrochemical sensors were utilized in a study on Duchene Muscular Dystrophy (DMD), a neuromuscular disorder that reduces dystrophin [31]. The following study by Caluori et al. was able to gain insight and side effect understanding of Verapamil, showing, for the first time, a distinct relationship of beating-force for Duchene muscular dystrophy cardiac modes [32].
Cardiac clusters were obtained using human embryonic stem cells (hESC) and induced pluripotent stem cells (iPSC) from a patient with DMD. The cells were tested for the presence of sarcomeres with a striated pattern and labeled with cardiac troponin T and the presence of dystrophin in the cytoplasm and then were seeded on mitotically inactivated mouse embryonic fibroblasts, which served as the feeder cells. Embryoid body formation started cardiac differentiation. After further cultivation, beating cardiac clusters (BCCs) were seen on day 14, and then were transferred from a hypoxic atmosphere to a normoxic atmosphere. The BCCs were kept in a medium with 10 µg ml−1 ascorbic acid after day 22. Microelectrode Arrays (MEAs) were prepared before the beating CMs were plated. Through the cleaning and sterilization process prepared the MEAs to be treated with a laminin and fibronectin mixture to promote cell growth after which they were put in an overnight incubator (37 °C, and 5% CO2). BCC samples were manually placed on top of the electrode arrays before being incubated overnight. The BCCs were allowed 3 days to stabilize in order to ensure proper adhesion, spreading, and cell-electrode contact. The MEA electrodes were interfaced with an electronic board with 60 channel unity-gain amplifiers. The thermal noise method was used to calibrate the cantilever spring constant before the cantilever was immersed in a liquid and put in contact with the sample to identify the superficial contraction center. After the center was found, drug trials began with 5 min recording and 10 min settling time intervals. Drugs were incrementally added, but never exceeded 5% of the total volume in the MEA chip (2 mL). All the experimental protocols were repeated on three biological replicates of each cell line. A LabVIEW virtual instrument aided in recording the electromechanical data, which was acquired at a 5 kHz sampling frequency. The data were post-processed with MATLAB R2016b, with statistical analysis performed with Prism 5.0. A 1-way ANOVA with Bonferroni post-test was used to test for statistical significance.
The setup allowed for electromechanical recording through simultaneous visualization, coupled analysis, and synchronous recording. The average noise in force and Z height traces were 18.3 nP and 24.5 nm, respectively. A visible EFT trace was produced by about half of the plated samples during the mechanical beating. This number is fairly low yield and is attributed to scarcely conductive coupling between the BBC and MEA planar electrodes. The samples could be kept without visible contamination for up to 4 days if they were filled with an antibiotic-containing medium, which is particularly useful for several day treatments. Forces of 5 nN were applied and did not produce any spontaneous change in the measured parameters. Beating rate, cardiac cycle duration, contraction speed, and a coupled parameter like that of the electromechanical delay (EMD) were able to be reconstructed within the apparatus and recorded. Baseline differences of beating frequencies were not significant, which allowed for the conclusion that the basal conditions would be sufficient to realize differences between healthy and diseased hPSC-derived BCC. Cardiac parameters on extracellular Ca2+ concentrations were tested as the Ca2+ serves an important role for ECC, especially for hPSC-derived CMs. A 2-way ANOVA test was used to find cell-type dependency, with results of p = 0.007 for contraction rate, p = 0.0003 for contraction duration, p < 0.0001 for time decay, and p = 0.026 for EMD. Ca2+ concentration effects were found to be significant only in relationship with the beating rate (p < 0.0001) [32]. All the cell lines experienced a beating rate decrease proportional to the Ca2+ concentration addition, and the decrease was parallel with an increase in exerted force in all the cell lines (120.3 ± 9.4% for the control group, 110 ± 14% for DORSO, and 112 ± 19% for DMD). Control and DORSO showed no dose-dependent relationships with the Ca2+ concentration, with various ranges of 109–99% and 142–131%, respectively, contrary to the DMD group, which showed a dose-dependent trend up to 257 ± 71% of the basal delay [32]. Ꞵ-adrenergic stimulation was performed with increasing concentration (182 ± 29% and 186 ± 22%, respectively). The DORSO and control groups experienced only about ⅓ and ⅕ of the increase, respectively. Verapamil served as a class IV antiarrhythmic and was shown to improve skeletal muscle force [33][34] in DMD patients, but negative cardiac side effects also resulted [32][35].
The CBB proved to be robust with line-specific variability in differentiation efficiency through obtaining results that no dose-dependence with Ca2+ concentration was observed for the control. The study was able to gain insight and side effect understanding of Verapamil and it was able to show for the first time a distinct relationship for beating force for DMD cardiac modes. While these discoveries are significant, the system lacked in its measurement environment sterility, and only a single MEA electrode and AFM cantilever were used for 3D model probing, leaving the potential for greater variation than what was detected.
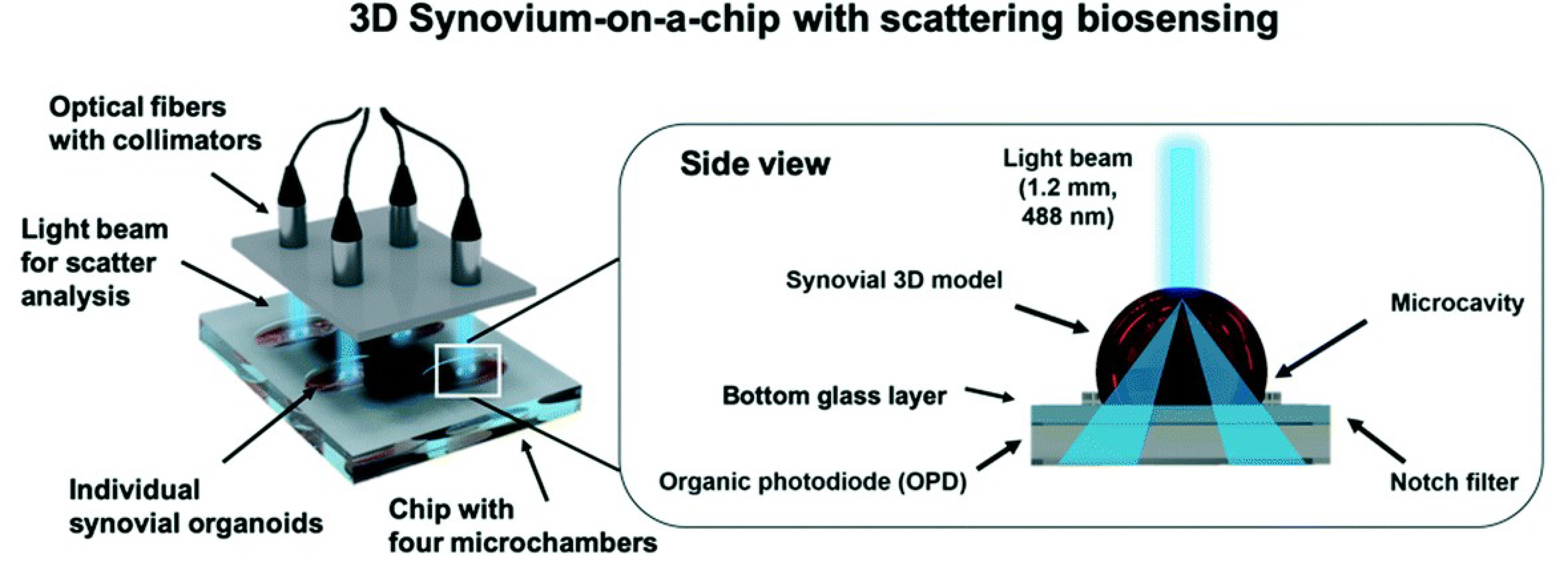
A final study to be outlined in this review was conducted by Rothbauer et al. that used a light scattering platform for synovial organoid development and analysis [36]. The light scattering station consisted of multiplexed 488 nm sapphire laser, split by beam splitters, collimators, and fiber couplers. The light was scattered at an angle greater than 20 degrees to the incident beam. The light passing through the notch filter was detected by an organic photodiode, creating an electrical signal as potential difference,n which can be seen in Figure 1. The potential difference was recorded with LabView GUI. Human synovial tissues from patients with Rheumatoid Arthritis (RA) were used. Human fibroblast-like synoviocytes (FLS) were isolated and cultured for their experiment. After further treatment, the cells were split in a 1:3 ratio, with medium changed every week. Tumor necrosis factor-alpha (TNF-α) was incubated with the arthritic phenotype synoviocytes for biochemical stimulation. The biochip was made from three layers of microscope glass slides bonded by biocompatible pressure-sensitive adhesive tape. Hydrophobic PDMS structures were used to house and hold the three-dimensional synoviocyte organoids. The chips were further treated and then incubated at 37 °C for 35 min for gelation. For analysis of inflammatory stimulation of the synoviocyte cultures, a human IL-6 uncoated ELISA was used. Cell viability was evaluated through live-dead fluorescence staining. Absorbance measurements were performed using a plate reader at 535 nm. Further histological analysis was performed on the data.
Figure 1. Investigation of tissue-level remodeling in regard to inflammatory arthritis, reproduced from open access article Ref. [36]. Graphic showing setup of the chip with integrated biosensors, consisting of four separate microchambers that hold human synovium organoids. Using organic photodiodes below the chip, light scatter measurements are recorded through the separate synovial organoids.
References
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuators B Chem. 1990, 1, 244–248.
- Grayson, A.C.R.; Shawgo, R.S.; Johnson, A.M.; Flynn, N.T.; Li, Y.; Cima, M.J.; Langer, R. A BioMEMS review: MEMS technology for physiologically integrated devices. Proc. IEEE 2014, 92, 6–21.
- Caplin, J.D.; Granados, N.G.; James, M.R.; Montazami, R.; Hashemi, N. Microfluidic Organ-on-a-Chip Technology for Advancement of Drug Development and Toxicology. Adv. Healthc. Mater 2015, 4, 1426–1450.
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754.
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772.
- Hashemi, N.; Howell, P.B., Jr.; Erickson, J.S.; Golden, J.P.; Ligler, F.S. Dynamic reversibility of hydrodynamic focusing for recycling sheath fluid. Lab A Chip 2010, 10, 1952–1959.
- Hashemi, N.; Erickson, J.S.; Golden, J.P.; Jackson, K.M.; Ligler, F.S. Microflow Cytometer for optical analysis of phytoplankton. Biosens. Bioelectron. 2011, 26, 4263–4269.
- Hashemi, N.; Erickson, J.S.; Golden, J.P.; Ligler, F.S. Optofluidic characterization of marine algae using a microflow cytometer. Biomicrofluidics 2011, 5, 032009.
- Bai, Z.; Mendoza, J.M.R.; Montazami, R.; Hashemi, N. On-chip development of hydrogel microfibers from round to square/ribbon shape. J. Mater. Chem. A 2014, 2, 4878.
- Farrokh, S.; Patel, B.B.; McNamara, M.C.; Meis, P.J.; Roghair, M.N.; Lu, M.; Montazami, R.; Sakaguchi, D.S.; Hashemi, N.N. Photo-Cross-Linked Poly(ethylene glycol) Diacrylate Hydrogels: Spherical Microparticles to Bow Tie-Shaped Microfibers. Acs Appl. Mater. Interfaces 2019, 11, 18797–18807.
- Sharifi, F.; Patel, B.B.; Dzuilko, A.K.; Montzami, R.; Sakaguchi, D.S.; Hashemi, N. Polycaprolactone Microfibrous Scaffolds to Navigate Neural Stem Cells. Biomacromolecules 2016, 17, 3287–3297.
- Pemathilaka, R.L.; Caplin, J.D.; Aykar, S.S.; Montazami, R.; Hashemi, N.N. Placenta-on-a-Chip: In Vitro Study of Caffeine Transport across Placental Barrier Using Liquid Chromatography Mass Spectrometry. Glob. Chall. 2019, 3, 1800112.
- Hashemi, N.; Lackore, J.M.; Sharifi, F.; Goodrich, P.J.; Winchell, M.L.; Hashemi, N. A paper-based microbial fuel cell operating under continuous flow condition. TECHNOLOGY 2016, 4, 98–103.
- Sechi, D.; Greer, B.; Johnson, J.; Hashemi, N. Three-dimensional paper-based microfluidic device for assays of protein and glucose in urine. Anal. Chem. 2013, 85, 10733–10737.
- Sierra, J.; Marrugo-Rimeriz, J.; Rodríguez-Trujillo, R.; Mir, M.; Samitier, J. Sensor-Integrated Microfluidic Approaches for Liquid Biopsies Applications in Early Detection of Cancer. Sensors 2020, 20, 1317.
- Guzman, N.A.; Guzman, D.E. A Two-Dimensional Affinity Capture and Separation Mini-Platform for the Isolation, Enrichment, and Quantification of Biomarkers and Its Potential Use for Liquid Biopsy. Biomedicines 2020, 8, 1–4.
- Tonkens, R. An Overview of the Drug Development Process. Physician Exec. 2005, 31, 48–52.
- Bottini, A.A.; Hartung, T. Food for thought… on the economics of animal testing. Altex-Altern. Anim. Exp. 2009, 26, 3–16.
- Lipsky, M.S.; Sharp, L.K. From idea to market: The drug approval process. J. Am. Board Fam. Pract. 2001, 14, 362.
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324.
- Wang, Y.I.; Oleaga, C.; Long, C.J.; Esch, M.B.; McAleer, C.W.; Miller, P.G.; Hickman, J.J.; Shuler, M.L. Self-contained, low-cost Body-on-a-Chip systems for drug development. Exp. Biol. Med. (Maywoodn. J.) 2017, 242, 1701–1713.
- Sun, J.; Warden, A.R.; Ding, X. Recent advances in microfluidics for drug screening. Biomicrofluidics 2019, 13, 061503.
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Universities Federation For Animal Welfare (UFAW): London, UK, 1959.
- Holmes, A.M.; Creton, S.; Chapman, K. Working in partnership to advance the 3Rs in toxicity testing. Toxicology 2010, 267, 14–19.
- Selimović, Š.; Dokmeci, M.R.; Khademhosseini, A. Organs-on-a-chip for drug discovery. Curr. Opin. Pharm. 2013, 13, 829–833.
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781.
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Ridisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278.
- Torisawa, Y.; Spina, C.S.; Mammoto, T.; Mammoto, A.; Weaver, J.C.; Tat, T.; Collins, J.J.; Ingber, D.E. Bone marrow–on–a–chip replicates hematopoietic niche physiology in vitro. Nat. Methods 2014, 11, 663–669.
- Zhang, W.; Zhang, Y.S.; Bakht, S.M.; Aleman, J.; Shin, S.R.; Yue, K.; Sica, M.; Ribas, J.; Duchamp, M.; Ju, J.; et al. Elastomeric free-form blood vessels for interconnecting organs on chip systems. Lab A Chip 2016, 16, 1579–1586.
- Acar, H.; Çinar, S.; Thunga, M.; Kessler, M.R.; Hashemi, N.; Montzami, R. Study of Physically Transient Insulating Materials as a Potential Platform for Transient Electronics and Bioelectronics. Adv. Funct. Mater. 2014, 24, 4135–4143.
- Wilson, K.; Faelan, C.; Patterson-Kane, J.C.; Rudmann, D.G.; Moore, S.A.; Frank, D.; Charleston, J.; Tinsley, J.; Young, D.G.; Milici, A.J. Duchenne and Becker Muscular Dystrophies: A Review of Animal Models, Clinical End Points, and Biomarker Quantification. Toxicol. Pathol. 2017, 45, 961–976.
- Caluori, G.; Pribyl, J.; Pesl, M.; Jelinkova, S.; Rotrekl, V.; Skladal, P.; Raiteri, R. Non-invasive electromechanical cell-based biosensors for improved investigation of 3D cardiac models. Biosens. Bioelectron. 2019, 124–125, 129–135.
- Esau, S.A. Interaction of theophylline, verapamil, and diltiazem on hamster diaphragm muscle force in vitro. Am. J. Physiol. Cell Physiol. 1988, 254, C365–C371.
- Skirboll, L.R.; Howard, R.A.; Dretchen, K.L. The effect of verapamil on the gastrocnemius and soleus muscles of the cat in vivo. Eur. J. Pharm. 1979, 60, 15–21.
- Pennock, G.D.; Dalton, W.S.; Roeske, W.R.; Appleton, C.P.; Mosley, K.; Pleza, P.; Miller, T.P.; Salmon, S.E. Systemic Toxic Effects Associated With High-Dose Verapamil Infusion and Chemotherapy Administration. Jnci J. Natl. Cancer Inst. 1991, 83, 105–110.
- Rothbauer, M.; Höll, G.; Eilenberger, C.; Kratz, S.R.A.; Farooq, B.; Schuller, P.; Calvo, I.O.; Byrne, R.A.; Meyer, B. Monitoring tissue-level remodelling during inflammatory arthritis using a three-dimensional synovium-on-a-chip with non-invasive light scattering biosensing. Lab A Chip 2020, 20, 1461–1471.