
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio Moschetta | + 1357 word(s) | 1357 | 2021-03-04 03:36:09 | | | |
| 2 | Dean Liu | Meta information modification | 1357 | 2021-03-15 07:27:29 | | |
Video Upload Options
The nuclear receptor Liver Receptor Homolog-1 (LRH-1) is widely involved in the complex and balanced biology of the intestine, thus guaranteeing the several functions played by this organ.
1. Introduction
Liver Receptor Homolog 1 (LRH-1) is a nuclear receptor that belongs to NR5A or Ftz-F1 subfamily. Nuclear receptors are transcriptional factors involved in different physiological and pathological processes. They are structurally characterized by a variable N-terminal region, a conserved central DNA-binding domain (DBD), a variable hinge region, a conserved ligand-binding domain (LBD), and a variable C-terminal region. Thanks to these functional domains, nuclear receptors bind to specific region of the DNA and retain an extraordinary capability to be activated by a series of specific regulatory ligands, which finally promote the selective transcription of functional target genes [1].
Essentially expressed in endodermic tissues including intestine, liver, exocrine pancreas, and ovary, LRH-1 is involved in a series of physiological processes ranging from development to metabolism and steroidogenesis [2][3]. Differently from the most of nuclear receptors, which bind DNA as homodimers or heterodimers, LRH-1 can perform its transcriptional function binding to DNA as monomer through its unique and conserved Fitz-F1 DNA-binding domain [4]. Since its discovery in 1993 [5], several studies were conducted to elucidate LRH-1 structure and functions. Originally identified as an orphan nuclear receptor due to the unknown nature of its ligands, a few years ago, the crystal structure analysis revealed that phospholipids, including phosphatidyl inositol, can interact with LRH-1, functioning as its endogenous ligands [6][7][8]. Indeed, mutations to the LRH-1 ligand-binding pocket alter its phospholipid binding capacity, thus reducing its in vivo activity [9]. Besides endogenous ligands, LRH-1 can also be activated by several exogenous compounds. Lee et al. identified both dietary dilauroyl phosphatidylcholine (DLPC) and diundecanoyl phosphatidylcholine (DUPC) as powerful activators of LRH-1 in in vitro and in vivo models. By acting as regulator of lipid and glucose homeostasis, DLPC treatment decreases the amount of glucose, improves insulin sensitivity, and regulates triglycerides levels in two mouse model of insulin resistance [10]. Recently, Whitby et al. have developed a promising class of agonist of LRH-1 showing excellent regulation of target genes, which includes a bicyclic hexahydropentalene core scaffold, named “RJW100” [11][12]. In these years, thanks to the cutting-edge crystal structure studies, remarkable progresses have been made not only in the identification of specific exogenous agonists of LRH-1 but also in the comprehension of the exact interaction that takes place in the lipophilic ligand binding pocket, locking agonists in an aligned consistent orientation and potentiating their effects. In this regard, using differential scanning fluorimetry and cellular luciferase reporter assay, Mays et al. proposed a modification to the LRH-1 agonist scaffold RJW100, which significantly improves the binding mode, thus identifying the first low nanomolar LRH-1 agonist called 6N and providing an astonishing model of structure-guided medicinal chemistry approach [13].
LRH-1 plays a pivotal role in different biological processes, from proliferative, metabolic, and immunoregulatory activities to the early stage of development and cell commitment during differentiation [14]. As suggested from its name, the role of LRH-1 has been mainly elucidated in the liver, where it acts as the promoter-specific activator of cholesterol 7 alpha-hydroxylase (Cyp7a1), the first and rate-limiting enzyme of bile acid biosynthesis and cholesterol homeostasis [15][16][17][18]. Recently, close attention has been paid to the controversial role played by LRH-1 in intestinal homeostasis, regarding intestine cell proliferation and renewal as well as its involvement in intestinal inflammatory pathways.
2. LRH-1 in Stemness: A Boost for Cell Proliferation
LRH-1 performs a vital role in early development and differentiation, as clearly proved by embryonic lethality of LRH-1 null mice [19][20]. However, the LRH-1 activity is not only limited to the primordial phases of life. LRH-1 expression is preserved throughout the entire lifecycle and modulated during the various stages of development especially in tissue characterized by a high proliferative rate, such as those of the enterohepatic axis. Since the epiblast stage of embryonic growth, LRH-1 is essential to maintain Oct4 gene expression, the master regulator of pluripotency during the early stage of embryonic development, thus assuring the pluripotency of inner cell mass and embryonic stem cells [21][22][23]. Interestingly, LRH-1 can replace Oct4 in the derivation of induced pluripotent stem cells (iPSC) from murine somatic cells [24], underlying the pivotal role of LRH-1 in the control of development processes and cellular proliferation. In the primordial phase of organogenesis, LRH-1 is detectable in the yolk sac endoderm, branchial arch, and neural crest. Later, LRH-1 expression is preserved in the liver, in the pancreas, and in the intestinal crypts of Lieberkühn [25], contributing to define the enterohepatic phenotype.
Considering that homozygous LRH-1 knockout mice die before birth [19], the precise regulatory mechanism by which LRH-1 promotes cell proliferation and expansion growth of digestive organs has always been problematic to analyze. For this reason, Zhai et al. conducted a study on zebrafish, a useful model organism of developmental biology, demonstrating that the inactivation of LRH-1 results in hypoplastic endoderm organs. Indeed, the liver, the intestine, the endocrine, and the exocrine pancreas were significantly smaller in homozygous LRH-1 knockout (LRH-1−/−) larvae compared with control siblings. Notably, LRH-1−/− fish showed that an increased number of cells stopped in G1 phase, while only a few cells were progressing in G2 phase, thus demonstrating that LRH-1 promotes G1 to S phase transition during cell proliferation. Specifically, LRH-1 induces the transcription of cyclin E1 (CcnE1) and acts as co-activator of WNT/β-catenin pathway, to promote the expression of cyclin D (CcnD1), thus regulating the critical G1-S phase transition. Furthermore, different genes that activate lipid metabolism were highly expressed in endoderm organs, suggesting that phosphatidylcholine signaling may activate LRH-1 to modulate the gene expression profile and the growth of digestive organs during zebrafish embryonic development [26].
Besides embryogenesis, LRH-1 also plays a pivotal role in the perpetual renewal of the intestinal epithelium, consistently with its expression in the intestinal crypts [14]. Given the lethality of homozygous LRH-1 knockout mice [19], Botrugno et al. conducted a study in heterozygous LRH-1 knockout mice (LRH-1+/−) demonstrating that LRH-1 regulates intestinal cell renewal by controlling cell proliferation synergistically with β-catenin pathway [19]. Indeed, LRH-1 promotes cell cycle progression through two major mechanism: in a DNA binding-independent manner, by acting as a coactivator for β-catenin/Tcf4 to promote CcnD1 and c-Myc transcription, and in a DNA binding-dependent manner, in which LRH-1 itself directly binds the DNA and sustains CcnE1 transcription [19]. This dual transcription factor and co-activator role of LRH-1 convergently contributes to induce G1 cyclins synthesis, strengthening the LRH-1 involvement in cell cycle progression. More evidence highlighting the key importance of LRH-1 expression in the crypt compartment in order to maintain the homeostasis of the intestinal epithelium have been recently provided. Knocking out LRH-1 in the mouse intestine resulted in decreased Notch signaling, which, in turn, lowers the stem cell marker LGR5 and triggers apoptosis in the crypt [27]. This indicates that LRH-1 may play a role in epithelial cell differentiation in the intestine, behind ISC maintenance (Figure 2A). However, a detailed investigation of the exacted mechanism of action of LRH-1 in intestinal stemness is still far to be accomplished, but it would certainly shed a light into its contribution to physiological and pathological state.
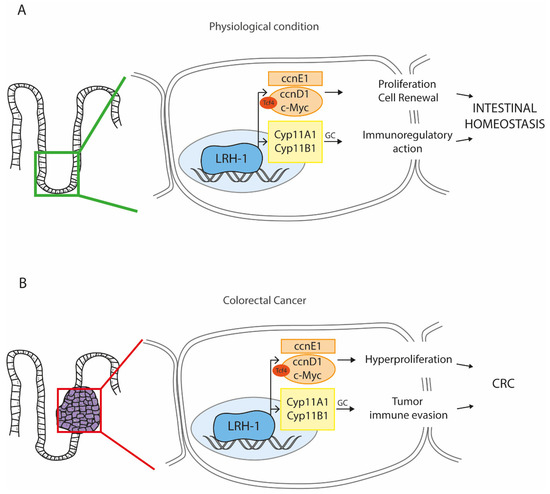
Figure 2. The multifaceted role of the Liver Receptor Homolog-1 (LRH-1) in the intestine. LRH-1 is nuclear receptor that can be endogenously activated by phospholipids present in the bile. (A) In physiological condition, LRH-1 is mainly expressed in the proliferative compartment of the intestine, where it drives the expression of genes involved in cell renewal and proliferation by regulating the WNT and NOTCH pathways, thus supporting the proper functions of stem cells. At the same time, LRH-1 can also regulate the intestinal glucocorticoids (GC) production, providing an overall immunoregulatory action. These two complementary functions of LRH-1 guarantee the intestinal homeostasis and counteract the onset of the inflammatory bowel disease (IBD). (B) The activation of LRH-1 in overt condition of colorectal cancer (CRC) may be deleterious. Indeed, the expression of the LRH-1 target genes results in hyperproliferation of cancer cells and tumor immune invasion, which collectively sustain the CRC progression (Abbreviations: ccnE1, cyclin E1; ccnD1, cyclin D1; Cyp11A1/B1, cytochrome P450 family 11 subfamily A/B member 1).
References
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schutz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839.
- Fayard, E.; Auwerx, J.; Schoonjans, K. LRH-1: An orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004, 14, 250–260.
- Falender, A.E.; Lanz, R.; Malenfant, D.; Belanger, L.; Richards, J.S. Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology 2003, 144, 3598–3610.
- Lee, Y.K.; Moore, D.D. Liver receptor homolog-1, an emerging metabolic modulator. Front. Biosci. A J. Virtual Libr. 2008, 13, 5950–5958.
- Becker-Andre, M.; Andre, E.; DeLamarter, J.F. Identification of nuclear receptor mRNAs by RT-PCR amplification of conserved zinc-finger motif sequences. Biochem. Biophys. Res. Commun. 1993, 194, 1371–1379.
- Sablin, E.P.; Krylova, I.N.; Fletterick, R.J.; Ingraham, H.A. Structural basis for ligand-independent activation of the orphan nuclear receptor LRH-1. Mol. Cell 2003, 11, 1575–1585.
- Krylova, I.N.; Sablin, E.P.; Moore, J.; Xu, R.X.; Waitt, G.M.; MacKay, J.A.; Juzumiene, D.; Bynum, J.M.; Madauss, K.; Montana, V.; et al. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 2005, 120, 343–355.
- Wang, W.; Zhang, C.; Marimuthu, A.; Krupka, H.I.; Tabrizizad, M.; Shelloe, R.; Mehra, U.; Eng, K.; Nguyen, H.; Settachatgul, C.; et al. The crystal structures of human steroidogenic factor-1 and liver receptor homologue-1. Proc. Natl. Acad. Sci. USA 2005, 102, 7505–7510.
- Ortlund, E.A.; Lee, Y.; Solomon, I.H.; Hager, J.M.; Safi, R.; Choi, Y.; Guan, Z.; Tripathy, A.; Raetz, C.R.; McDonnell, D.P.; et al. Modulation of human nuclear receptor LRH-1 activity by phospholipids and SHP. Nat. Struct. Mol. Biol. 2005, 12, 357–363.
- Lee, J.M.; Lee, Y.K.; Mamrosh, J.L.; Busby, S.A.; Griffin, P.R.; Pathak, M.C.; Ortlund, E.A.; Moore, D.D. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature 2011, 474, 506–510.
- Whitby, R.J.; Dixon, S.; Maloney, P.R.; Delerive, P.; Goodwin, B.J.; Parks, D.J.; Willson, T.M. Identification of small molecule agonists of the orphan nuclear receptors liver receptor homolog-1 and steroidogenic factor-1. J. Med. Chem. 2006, 49, 6652–6655.
- Whitby, R.J.; Stec, J.; Blind, R.D.; Dixon, S.; Leesnitzer, L.M.; Orband-Miller, L.A.; Williams, S.P.; Willson, T.M.; Xu, R.; Zuercher, W.J.; et al. Small molecule agonists of the orphan nuclear receptors steroidogenic factor-1 (SF-1, NR5A1) and liver receptor homologue-1 (LRH-1, NR5A2). J. Med. Chem. 2011, 54, 2266–2281.
- Mays, S.G.; Flynn, A.R.; Cornelison, J.L.; Okafor, C.D.; Wang, H.; Wang, G.; Huang, X.; Donaldson, H.N.; Millings, E.J.; Polavarapu, R.; et al. Development of the First Low Nanomolar Liver Receptor Homolog-1 Agonist through Structure-guided Design. J. Med. Chem. 2019, 62, 11022–11034.
- Fernandez-Marcos, P.J.; Auwerx, J.; Schoonjans, K. Emerging actions of the nuclear receptor LRH-1 in the gut. Biochim. Biophys. Acta 2011, 1812, 947–955.
- Nitta, M.; Ku, S.; Brown, C.; Okamoto, A.Y.; Shan, B. CPF: An orphan nuclear receptor that regulates liver-specific expression of the human cholesterol 7alpha-hydroxylase gene. Proc. Natl. Acad. Sci. USA 1999, 96, 6660–6665.
- Lu, T.T.; Makishima, M.; Repa, J.J.; Schoonjans, K.; Kerr, T.A.; Auwerx, J.; Mangelsdorf, D.J. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 2000, 6, 507–515.
- Goodwin, B.; Jones, S.A.; Price, R.R.; Watson, M.A.; McKee, D.D.; Moore, L.B.; Galardi, C.; Wilson, J.G.; Lewis, M.C.; Roth, M.E.; et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 2000, 6, 517–526.
- Schoonjans, K.; Annicotte, J.S.; Huby, T.; Botrugno, O.A.; Fayard, E.; Ueda, Y.; Chapman, J.; Auwerx, J. Liver receptor homolog 1 controls the expression of the scavenger receptor class B type I. EMBO Rep. 2002, 3, 1181–1187.
- Botrugno, O.A.; Fayard, E.; Annicotte, J.S.; Haby, C.; Brennan, T.; Wendling, O.; Tanaka, T.; Kodama, T.; Thomas, W.; Auwerx, J.; et al. Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation. Mol. Cell 2004, 15, 499–509.
- Pare, J.F.; Malenfant, D.; Courtemanche, C.; Jacob-Wagner, M.; Roy, S.; Allard, D.; Belanger, L. The fetoprotein transcription factor (FTF) gene is essential to embryogenesis and cholesterol homeostasis and is regulated by a DR4 element. J. Biol. Chem. 2004, 279, 21206–21216.
- Marikawa, Y.; Alarcon, V.B. Establishment of trophectoderm and inner cell mass lineages in the mouse embryo. Mol. Reprod. Dev. 2009, 76, 1019–1032.
- Sung, B.; Do, H.J.; Park, S.W.; Huh, S.H.; Oh, J.H.; Chung, H.J.; Kang, M.J.; Kim, J.H.; Kim, N.H.; Kim, J.H. Regulation of OCT4 gene expression by liver receptor homolog-1 in human embryonic carcinoma cells. Biochem. Biophys. Res. Commun. 2012, 427, 315–320.
- Gu, P.; Goodwin, B.; Chung, A.C.; Xu, X.; Wheeler, D.A.; Price, R.R.; Galardi, C.; Peng, L.; Latour, A.M.; Koller, B.H.; et al. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol. Cell. Biol. 2005, 25, 3492–3505.
- Heng, J.C.; Feng, B.; Han, J.; Jiang, J.; Kraus, P.; Ng, J.H.; Orlov, Y.L.; Huss, M.; Yang, L.; Lufkin, T.; et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell 2010, 6, 167–174.
- Rausa, F.M.; Galarneau, L.; Belanger, L.; Costa, R.H. The nuclear receptor fetoprotein transcription factor is coexpressed with its target gene HNF-3beta in the developing murine liver, intestine and pancreas. Mech. Dev. 1999, 89, 185–188.
- Zhai, G.; Song, J.; Shu, T.; Yan, J.; Jin, X.; He, J.; Yin, Z. LRH-1 senses signaling from phosphatidylcholine to regulate the expansion growth of digestive organs via synergy with Wnt/beta-catenin signaling in zebrafish. J. Genet. Genom. Yi Chuan Xue Bao 2017, 44, 307–317.
- Bayrer, J.R.; Wang, H.; Nattiv, R.; Suzawa, M.; Escusa, H.S.; Fletterick, R.J.; Klein, O.D.; Moore, D.D.; Ingraham, H.A. LRH-1 mitigates intestinal inflammatory disease by maintaining epithelial homeostasis and cell survival. Nat. Commun. 2018, 9, 4055.




