| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sameera Nallanthighal | + 1988 word(s) | 1988 | 2021-03-03 08:56:46 | | | |
| 2 | Rita Xu | -833 word(s) | 1155 | 2021-03-15 05:00:18 | | |
Video Upload Options
Collagen type XI alpha 1 (COL11A1) is one of the alpha chains of type XI collagen, which is important for bone development. Interestingly, COL11A1 levels are frequently upregulated in various cancers and high levels of COL11A1 are correlated with poor clinical outcome in many solid cancers. Increasing evidence shows that COL11A1 promotes tumor cell aggressiveness through multiple mechanisms.
1. Introduction
Collagens are the most abundant proteins (~30% mass) in mammals and the main component of extracellular matrix (ECM) [1]. Collagens comprise 28 subtypes (type I through XXVIII) and type I collagen is the most abundant type (~90%) in the body [1]. Each collagen can form a homotrimer or heterotrimer consisting of three alpha chains. Each alpha chain is synthesized as a procollagen containing N-terminal and C-terminal propeptides and forms a triple helix in the cytoplasm. Once secreted, both N- and C-terminal propeptides are cleaved by proteinases, crosslinked, and assembled into collagen fibrils [2][3].
COL11A1 encodes one of three alpha chains of type XI collagen, a minor fibrillar collagen mainly expressed in the cartilage [3][4]. In the cartilage, COL11A1 forms a heterotrimer with COL11A2 and COL2A1 to assemble type XI collagen [3][4]. Mutations in COL11A1 gene are associated with type II Stickler syndrome and Marshall syndrome, two autosomal dominant disorders showing varying degrees of facial dysmorphism, nearsightedness, and hearing loss [5][6][7][8]. A single-nucleotide polymorphism in COL11A1 gene is also associated with susceptibility to lumbar disc herniation [9]. The homozygous chondrodysplasia (cho/cho) mice harboring a point mutation in COL11A1 gene die at birth due to severe skeletal defects [10]. Furthermore, collagens in the cartilage of cho/cho mice form abnormally thick and fragmented fibers [11][12], demonstrating crucial roles of COL11A1 in nucleation and initial assembly of collagen fibers.
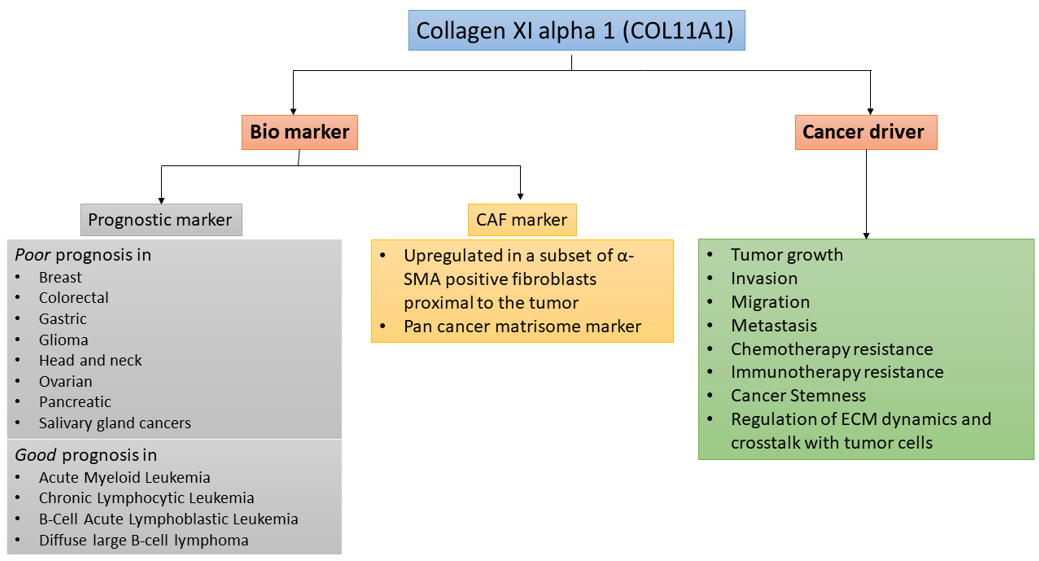
Although COL11A1 expression in normal tissues is very low, COL11A1 expression is significantly upregulated in many types of cancer [2][13] (Figure 1). High levels of COL11A1 are often associated with aggressive tumor phenotype and poor prognosis in multiple solid tumors types such as ovarian, breast, pancreas, and colorectal cancer [2][13]. In stark contrast, it has been shown in hematological malignancies including Acute Myeloid Leukemia (AML), Chronic Lymphocytic Leukemia (CLL), B-Cell Acute Lymphoblastic Leukemia (B-ALL), and Diffuse Large B-cell Lymphoma (DLBCL) that COL11A1 overexpression is associated with better prognosis [14]. In solid tumors, although a small number of cancer cells overexpress COL11A1, COL11A1 is predominantly overexpressed by a subset of cancer-associated fibroblasts (CAFs) adjacent to cancer cells [2], suggesting COL11A1 as a specific marker for CAFs. However, despite the importance of COL11A1 in skeletal development and fibrillogenesis, its biological functions in cancer remain poorly understood.
Figure 1. COL11A1 is a biomarker and is driver of aggressiveness in cancer.
2. COL11A1 Biology and Binding Partners
COL11A1 binds to COL11A2 and COL11A3 to form a heterotrimeric complex of collagen type XI [15]. More recent studies on type XI collagen show that the COL11A3 subunit is actually the product of the COL2A1 gene [16]. It is now accepted that collagen type XI is a triple helical heterotrimer made up of COL11A1, COL11A2, and COL2A1. It should be noted that to date, no study has confirmed the existence of a triple helical homotrimer version of type XI collagen, and the composition of collagen type XI might change in a tissue specific manner. Collagen type XI has been known to regulate collagen type II fibrillogenesis across different mammalian model organisms [3][12][17] and is usually associated with thin collagen type II fibers in cartilage. Collagen type XI has also been shown to regulate collagen type I fibrillogenesis in chick embryo sternal chondrocytes as well [18]. However, COL11A2 and COL2A1 are not the only collagen subunits that can bind to COL11A1. A study by Yoshioka et al. [19] shows high expression of COL11A1 in tongue, the intestine, and the optic vesicle of the developing mouse embryo, in which COL5A2 is also highly expressed. COL11A1 is expressed in equal ratios to COL5A1 and COL5A2 in bovine bone as demonstrated by Niyibizi and Eyre [20]. The authors of this study suggest that COL11A1 can form a collagen heterotrimer with COL5A1 and COL5A2 while also noting the possibility that other subunit combinations can be possible (such as one COL11A1 and two COL5A1 subunits, for example). In agreement with these studies, Kleman et al. [21] demonstrate that COL11A1 may bind to COL5A2 in a cancer context as well. This groups show that A204 cells (a human rhabdomyosarcoma cell line) deposit a collagen matrix made up of COL11A1 and COL5A2 at a 2:1 ratio, suggesting that these alpha chains form a heterotrimer at those ratios. Although the authors hypothesize that COL11A1 forms a heterotrimer with COL5A2 at a 2:1, other combinations of these collagen alpha chains might be possible in a cancer type-specific manner.
COL11A1 may also bind to many other proteins outside of collagens. Using mass spectrometry, Brown et al. [22] identified several proteins that interact with the amino terminal domain (NTD) of COL11A1 in fetal bovine cartilage, which is displayed at the surface of collagen fibrils in cartilage. These proteins include ECM proteoglycans (such as biglycan, fibromodulin, chondroadherin) and other ECM components (Thrombospondin-1, matrilin-1/3, chondrocalcin) in addition to other collagens (collagens type II, XI, XIV, XII, and IX). Some cellular proteins also associate with COL11A1’s NTD, including cell surface proteins (Annexins I, II, V) and protein fate and modification proteins (Heat shock proteins HSPA5 and HSP90B1), and metabolic and cytoskeletal proteins (Fructose bisphosphate aldolase, lactate dehydrogenase A, β-actin). Oncostatin M (OSM), an inflammatory cytokine, has also been shown to bind to collagen type XI after being deposited by neutrophils to MDA-MB-231 breast cancer cell-derived matrices in vitro [23]. Although this interaction is not known to regulate COL11A1 function, binding, or signaling, OSM immobilized to type XI collagen induced Signal Transducer and Activator of Transcription (STAT) signaling in the breast cancer cell line T47D.
Taken together, COL11A1 can bind to COL11A2 and COL2A1 to form collagen type XI, which is known to regulate fibrillogenesis of collagen types I and II. There is also evidence that COL11A1 can also bind to COL5A1 and COL5A2 to form its own unique heterotrimer, although it is unclear at what ratios COL11A1 may associate with these two collagen V subunits. In addition to other collagens, COL11A1 may also bind to other proteins, such as proteoglycans, other components of the ECM, and potentially even some cellular proteins, including the cytokine OSM.
Overall, it is unclear how the composition of type XI collagen varies in the context of cancer compared to that in normal tissues. Given that the TME undergoes extensive reorganization and alteration in collagen composition, it is possible that COL11A1 can partner with unconventional binding partners to promote malignancy, which, at this point, remains unexplored.
References
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978.
- Vazquez-Villa, F.; Garcia-Ocana, M.; Galvan, J.A.; Garcia-Martinez, J.; Garcia-Pravia, C.; Menedez-Rodriguez, P.; Gonzalez-del Rey, C.; Barneo-Serra, L.; de los Toyos, J.R. COL11A1/(pro)collagen 11A1 expression is a remarkable biomarker of human invasive carcinoma-associated stromal cells and carcinoma progression. Tumor Biol. 2015, 36, 2213–2222.
- Kadler, K.E.; Hill, A.; Canty-Laird, E.G. Collagen fibrillogenesis: Fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 2008, 20, 495–501.
- Eyre, D.R. Collagens and cartilage matrix homeostasis. Clin. Orthop. Relat. Res. 2004.
- Vogiatzi, M.G.; Li, D.; Tian, L.; Garifallou, J.P.; Kim, C.E.; Hakonarson, H.; Levine, M.A. A novel dominant COL11A1 mutation in a child with Stickler syndrome type II is associated with recurrent fractures. Osteoporos. Int. 2018, 29, 247–251.
- Vijzelaar, R.; Waller, S.; Errami, A.; Donaldson, A.; Lourenco, T.; Rodrigues, M.; McConnell, V.; Fincham, G.; Snead, M.; Richards, A. Deletions within COL11A1 in Type 2 stickler syndrome detected by multiplex ligation-dependent probe amplification (MLPA). BMC Med. Genet. 2013, 14.
- Richards, A.J.; Fincham, G.S.; McNinch, A.; Hill, D.; Poulson, A.V.; Castle, B.; Lees, M.M.; Moore, A.T.; Scott, J.D.; Snead, M.P. Alternative splicing modifies the effect of mutations in COL11A1 and results in recessive type 2 Stickler syndrome with profound hearing loss. J. Med. Genet. 2013, 50, 765–771.
- Griffith, A.J.; Sprunger, L.K.; Sirko-Osadsa, D.A.; Tiller, G.E.; Meisler, M.H.; Warman, M.L. Marshall syndrome associated with a splicing defect at the COL11A1 locus. Am. J. Hum. Genet. 1998, 62, 816–823.
- Liu, W.J.; Sun, G.S.; Guo, L.S.; Wang, L.L.; Fan, W.Q.; Lang, M.L.; Chen, D.; Yi, X.H. A genetic variant in COL11A1 is functionally associated with lumbar disc herniation in Chinese population. J. Genet. 2017, 96, 867–872.
- Li, Y.; Lacerda, D.A.; Warman, M.L.; Beier, D.R.; Yoshioka, H.; Ninomiya, Y.; Oxford, J.T.; Morris, N.P.; Andrikopoulos, K.; Ramirez, F.; et al. A Fibrillar Collagen Gene, Col11a1, Is Essential for Skeletal Morphogenesis. Cell 1995, 80, 423–430.
- Wenstrup, R.J.; Smith, S.M.; Florer, J.B.; Zhang, G.Y.; Beason, D.P.; Seegmiller, R.E.; Soslowsky, L.J.; Birk, D.E. Regulation of Collagen Fibril Nucleation and Initial Fibril Assembly Involves Coordinate Interactions with Collagens V and XI in Developing Tendon. J. Biol. Chem. 2011, 286, 20455–20465.
- Fernandes, R.J.; Weis, M.; Scott, M.A.; Seegmiller, R.E.; Eyre, D.R. Collagen XI chain misassembly in cartilage of the chondrodysplasia (cho) mouse. Matrix Biol. 2007, 26, 597–603.
- Raglow, Z.; Thomas, S.M. Tumor matrix protein collagen XI alpha 1 in cancer. Cancer Lett. 2015, 357, 448–453.
- Jia, D.Y.; Liu, Z.Q.; Deng, N.; Tan, T.Z.; Huang, R.Y.J.; Taylor-Harding, B.; Cheon, D.J.; Lawrenson, K.; Wiedemeyer, W.R.; Walts, A.E.; et al. A COL11A1-correlated pan-cancer gene signature of activated fibroblasts for the prioritization of therapeutic targets. Cancer Lett. 2016, 382, 203–214.
- Morris, N.P.; Bachinger, H.P. Type XI collagen is a heterotrimer with the composition (1 alpha, 2 alpha, 3 alpha) retaining non-triple-helical domains. J. Biol. Chem. 1987, 262, 11345–11350.
- Sirko-Osadsa, D.; Murray, M.A.; Scott, J.A.; Lavery, M.A.; Warman, M.L.; Robin, N.H. Stickler syndrome without eye involvement is caused by mutations in COL11A2, the gene encoding the alpha(2)(XI) chain of type XI collagen. J. Pediatrics 1998, 132, 368–371.
- Keene, D.R.; Oxford, J.T.; Morris, N.P. Ultrastructural localization of collagen types II, IX, and XI in the growth plate of human rib and fetal bovine epiphyseal cartilage: Type XI collagen is restricted to thin fibrils. J. Histochem. Cytochem. 1995, 43, 967–979.
- Hansen, U.; Bruckner, P. Macromolecular specificity of collagen fibrillogenesis—Fibrils of collagens I and XI contain a heterotypic alloyed core and a collagen I sheath. J. Biol. Chem. 2003, 278, 37352–37359.
- Yoshioka, H.; Iyama, K.; Inoguchi, K.; Khaleduzzaman, M.; Ninomiya, Y.; Ramirez, F. Developmental pattern of expression of the mouse alpha 1 (XI) collagen gene (Col11a1). Dev. Dyn. 1995, 204, 41–47.
- Niyibizi, C.; Eyre, D.R. Identification of the Cartilage-Alpha-1 (Xi) Chain in Type-V Collagen from Bovine Bone. FEBS Lett. 1989, 242, 314–318.
- Kleman, J.P.; Hartmann, D.J.; Ramirez, F.; van der Rest, M. The human rhabdomyosarcoma cell line A204 lays down a highly insoluble matrix composed mainly of alpha 1 type-XI and alpha 2 type-V collagen chains. Eur. J. Biochem. 1992, 210, 329–335.
- Brown, R.J.; Mallory, C.; McDougal, O.M.; Oxford, J.T. Proteomic analysis of Col11a1-associated protein complexes. Proteomics 2011, 11, 4660.
- Ryan, R.E.; Martin, B.; Mellor, L.; Jacob, R.B.; Tawara, K.; McDougal, O.M.; Oxford, J.T.; Jorcyk, C.L. Oncostatin M binds to extracellular matrix in a bioactive conformation: Implications for inflammation and metastasis. Cytokine 2015, 72, 71–85.